INTRODUCTION
Mild cognitive impairment (MCI) is a heterogeneous clinical syndrome due to multiple pathologies (Petersen, Reference Petersen2004). Amnesic MCI (aMCI) subjects with old age (usually >70), selective episodic memory impairment, and stable or very slowly progressing cognitive decline have been described as a phenotypical expression of focal medial temporal lobe dysfunction, possibly due to Alzheimer’s disease (AD) pathology (Marra et al., Reference Marra, Villa, Quaranta, Valenza, Vita and Gainotti2012). However, their clinical profile significantly differs from that of typical aMCI due to AD, characterized by temporo-parietal neurodegeneration and associated to an annual rate of conversion to dementia of approximately 17% (Landau et al., Reference Landau, Harvey, Madison, Reiman, Foster and Aisen2010).
Growing literature suggests that aMCI with medial temporal lobe dysfunction (aMCI-mTLD) in aged population is largely associated with non-AD neuropathology (Crary et al., Reference Crary, Trojanowski, Schneider, Abisambra, Abner, Alafuzoff and Nelson2014; Ferrer, Santpere, & van Leeuwen, Reference Ferrer, Santpere and van Leeuwen2008; Nelson et al., Reference Nelson, Dickson, Trojanowski, Jack, Boyle, Arfanakis and Schneider2019). In particular, limbic-predominant age-related TDP-43 encephalopathy (LATE) has been recently described as one of the main pathological substrates of mTLD phenotypes (Nelson et al., Reference Nelson, Dickson, Trojanowski, Jack, Boyle, Arfanakis and Schneider2019).
18F-fluorodeoxyglucose positron emission tomography (FDG-PET) studies in aMCI-mTLD reported selective medial temporal and limbic hypometabolism without the typical AD pattern (Cerami et al., Reference Cerami, Dodich, Iannaccone, Magnani, Santangelo, Presotto and Perani2018). Combined FDG- and amyloid-PET evidence suggests comorbid brain pathologies, including β-amyloid plaques and tauopathy (Cerami et al., Reference Cerami, Dodich, Iannaccone, Magnani, Santangelo, Presotto and Perani2018). The clinical-neuropsychological features of this aMCI-mTLD cohort show several features of confirmed LATE cases: advanced age, predominant amnestic syndrome of hippocampal type mimicking AD dementia, selective temporo-limbic neurodegeneration, and low risk of cognitive decline (Nelson et al., Reference Nelson, Dickson, Trojanowski, Jack, Boyle, Arfanakis and Schneider2019).
Available evidence on cognitive deficits in mTLD patients reported a slow decline in episodic memory, language, and global cognitive status, with other cognitive domains being later affected (e.g., (Nag et al., Reference Nag, Yu, Boyle, Leurgans, Bennett and Schneider2018; Nag et al., Reference Nag, Yu, Wilson, Chen, Bennett and Schneider2017)). Interestingly, even though temporo-limbic dysfunction is a main feature of aMCI-mTLD, no researcher has yet investigated social cognition skills in these patients.
Social cognition is an ample cognitive domain, including processes aimed at recognizing and interpreting social information, understanding behaviors and modulating social response (Frith, Reference Frith2008). Social processing thus includes components of social perception (e.g., emotion recognition), empathy, and theory of mind abilities (ToM) (Frith, Reference Frith2007). This labyrinth of cognitive processes is orchestrated by a complex array of low- and high-level connections (Frith, Reference Frith2007; Van Overwalle & Baetens, Reference Van Overwalle and Baetens2009). While mentalizing relies on a large-scale anterior–posterior network, the limbic system is involved in emotion recognition and processing (Dalgleish, Reference Dalgleish2004).
Deficits of social cognition have been heterogeneously reported in MCI (see for review (Bora & Yener, Reference Bora and Yener2017)), with reports of moderate impairments as well as negative findings. Notably, no attempt to investigate social cognition in MCI with different pathological substrates has been done yet.
This study aimed at acquiring new data about social cognition disorders in specific MCI subtypes. In details, by using in vivo imaging biomarkers for the diagnostic classification, this study aims at comparing emotion recognition abilities in aMCI due to AD or with mTLD, under the hypothesis that selective medial temporal lobe and limbic neurodegeneration might be associated to significantly lower performance in negative emotions recognition.
MATERIALS AND METHODS
Subjects
The sample included 30 subjects fulfilling criteria for aMCI (Petersen, Reference Petersen2004) consecutively enrolled at the San Raffaele Hospital (Milan, Italy) from 2011 and 2017. Sixteen were classified as aMCI due to AD (aMCI-AD) according to McKhann criteria (Albert et al., Reference Albert, DeKosky, Dickson, Dubois, Feldman, Fox and Phelps2011), while 14 showed medial temporal lobe dysfunction (aMCI-mTLD). AMCI-AD inclusion criteria were amnestic subtype of MCI; typical AD temporo-parietal hypometabolic pattern at FDG-PET and/or atrophy at MRI imaging. Inclusion criteria for aMCI-mTLD were amnestic subtype of MCI; medial temporal lobe and limbic-predominant FDG-PET pattern without the typical AD hypometabolism. Individual FDG-PET data have been assessed with a semi-quantitative standardized procedure including a dementia-specific FDG-PET template for the normalization phase (Della Rosa et al., Reference Della Rosa, Cerami, Gallivanone, Prestia, Caroli, Castiglioni and Consortium2014) and a large image database for the statistical comparison on a voxel-by-voxel basis (i.e., 1 patient vs 112 control subjects). The procedure results in an individual FDG-PET hypometabolic pattern (Perani et al., Reference Perani, Della Rosa, Cerami, Gallivanone, Fallanca, Vanoli and Consortium2014). According to previous findings on single subjects and patient groups (Cerami et al., Reference Cerami, Della Rosa, Magnani, Santangelo, Marcone, Cappa and Perani2015; Reference Cerami, Dodich, Iannaccone, Magnani, Santangelo, Presotto and Perani2018), individual FDG-PET hypometabolic pattern of aMCI-AD patients included bilateral temporo-parietal hypometabolism, with precuneus and/or posterior cingulate cortex involvement, while FDG-PET pattern of aMCI-mTLD included hippocampal and medial temporal lobe structures, together with insula, fronto-medial, and anterior superior temporal cortices in some cases.
All subjects underwent a standard neurological examination and neuropsychological assessment including language, memory, attention and executive functions, and visuo-spatial abilities. All subjects had a three-to-five-year clinical follow-up. Clinical classification has been performed by neurologists blinded to the study aims.
Twenty healthy controls (HC) were recruited at community centers for statistical comparisons. Exclusion criteria were positive neuropsychiatric history or neurologic examination, Clinical Dementia Rating (CDR) scale > 0 and Mini Mental State Examination (MMSE) < 28. No HC subject was taking any medication interfering with neurobehavioral functioning.
All subjects, or their informants/caregivers, gave informed consent to the experimental procedure that had been approved by the local ethical committee.
Demographic and clinical characteristics of the study participants are presented in Table 1.
Table 1. Demographic and clinical features of the sample
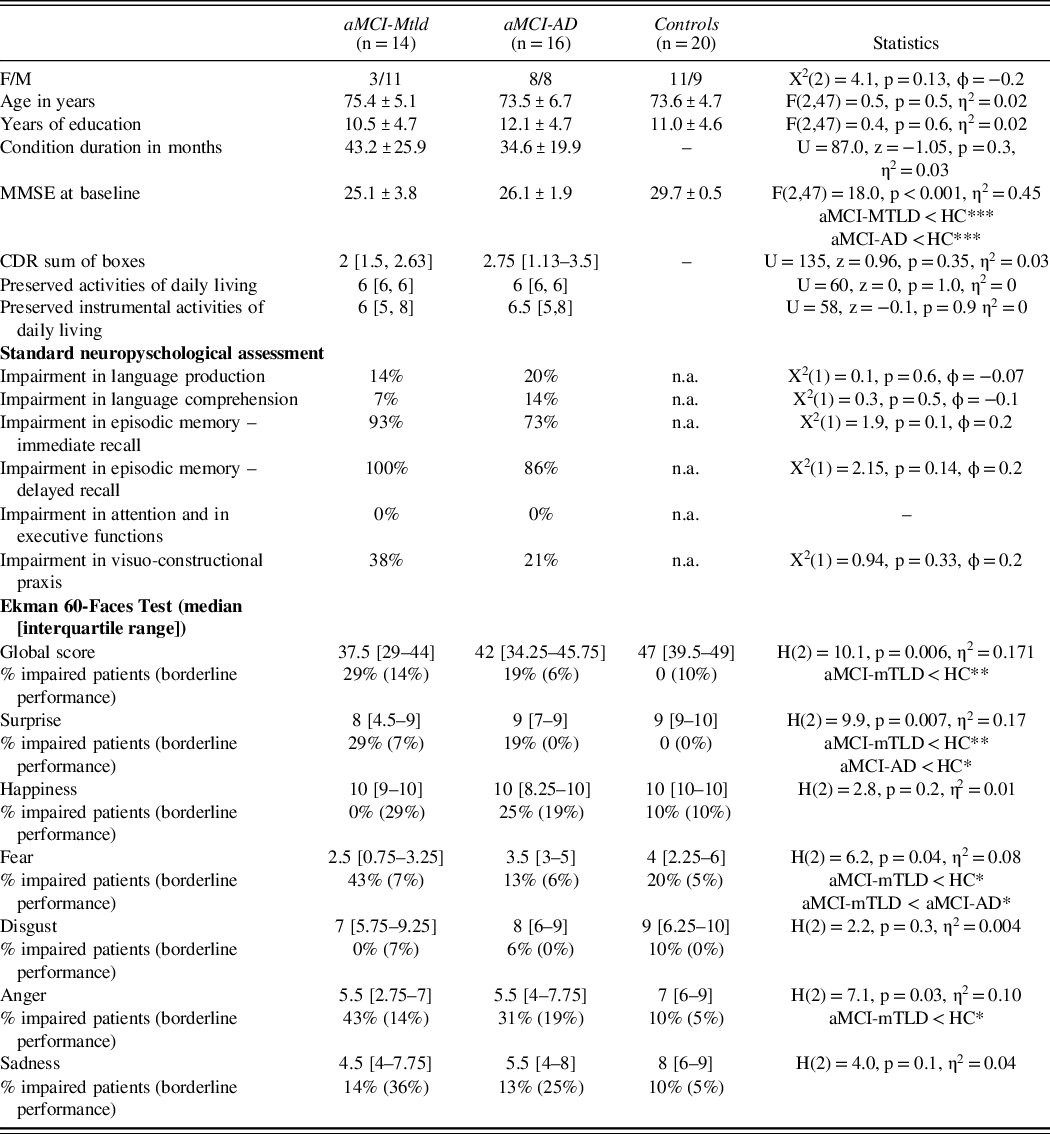
aMCI-AD = amnestic mild cognitive impairment due to Alzheimer’s disease; aMCI-mTLD = amnestic mild cognitive impairment with medio-temporal lobe dysfunction; HC = healthy controls; CDR = clinical dementia rating scale; MMSE = mini mental state examination; * p < 0.05, **p < 0.01, *** p < 0.001, n.a. not available.
Emotion Recognition Assessment
Emotion recognition was assessed with the Italian version of the Ekman 60-Faces Test – Ek-60F adapted using as reference the computer software available on CD-ROM for the American version (Dodich et al., Reference Dodich, Cerami, Canessa, Crespi, Marcone, Arpone and Cappa2014) and including 60 pictures depicting 10 actors’ faces, each one expressing one of the basic emotions (i.e., surprise, happiness, fear, disgust, anger, and sadness). Each image was shown for 5 s, the whole task lasted about 8–10 min and was performed in a stand-alone session by an experimenter blinded to the aMCI classification.
After a preliminary trial run consisting of an example of each emotion performed by an actor who did not appear in the test phase, pictures were serially presented on a computer screen. Participants were asked to report verbally the emotion expressed in each of them selecting one out of the six available options (i.e., emotion words) displayed on the bottom of the screen. The scoring procedure resulted in a maximum score of 60 for global performance and a maximum of 10 for single emotion subscores.
Statistical Analysis
Subjects were classified as impaired/unimpaired in basic cognitive domains based on the specific Italian normative data. In addition, according to the Ek-60F Italian standardization (Dodich et al., Reference Dodich, Cerami, Canessa, Crespi, Marcone, Arpone and Cappa2014), we calculated frequencies of globally impaired Ek-60F performances and of impaired scores for single emotions.
Group comparisons among demographic and experimental variables were analyzed using either parametric or nonparametric tests, according to data distribution. Demographic and clinical variables were compared between groups using one-way ANOVA (Bonferroni post hoc test). Due to the nonnormal distribution of Ek-60F scores, Kruskal−Wallis test has been used to compare performances between aMCI-mTLD, aMCI-AD, and HC. Post hoc Mann−Whitney tests were used to compare all pairs of groups.
Statistical analyses were performed using SPSS for Windows (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.)
RESULTS
Basic Cognitive Profiles in aMCI-mTLD vs. aMCI-AD
No difference in the demographic and clinical features was found in the two aMCI groups (Table 1). At the enrollment, 21 patients showed a single domain cognitive profile (13 aMCI-AD and 8 aMCI-mTLD), while the rest (i.e., 3 aMCI-AD and 6 aMCI-mTLD) had a multiple domain aMCI. Cognitive assessment proved severe episodic memory impairments in both aMCI groups, together with visuo-constructive or language deficits in about a quarter of the sample. No subject showed impaired executive functioning. No patients fulfilled criteria for other neurodegenerative disease (e.g., frontotemporal dementia) within the follow-up period. At the follow-up, while 3 aMCI-mTLD patients progressed from single to multiple domain aMCI, the rest of aMCI-mTLD did not change clinical classification. In the aMCI-AD group, 2 patients progressed to dementia and 6 patients were reclassified as multiple domain aMCI.
Differential Profile of Emotion Recognition Deficits in aMCI-mTLD vs aMCI-AD
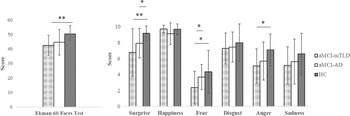
Based on the Italian normative values, the Ek-60F global score was impaired in the 29% of aMCI-mTLD subjects and 19% of aMCI-AD. More than 40% of aMCI-mTLD subjects showed impaired fear recognition according to the normative cutoff score (Table 1 and Figure 1). A small number of HC showed low performance in single emotions without being overall impaired according to the normative reference (Dodich et al., Reference Dodich, Cerami, Canessa, Crespi, Marcone, Arpone and Cappa2014).
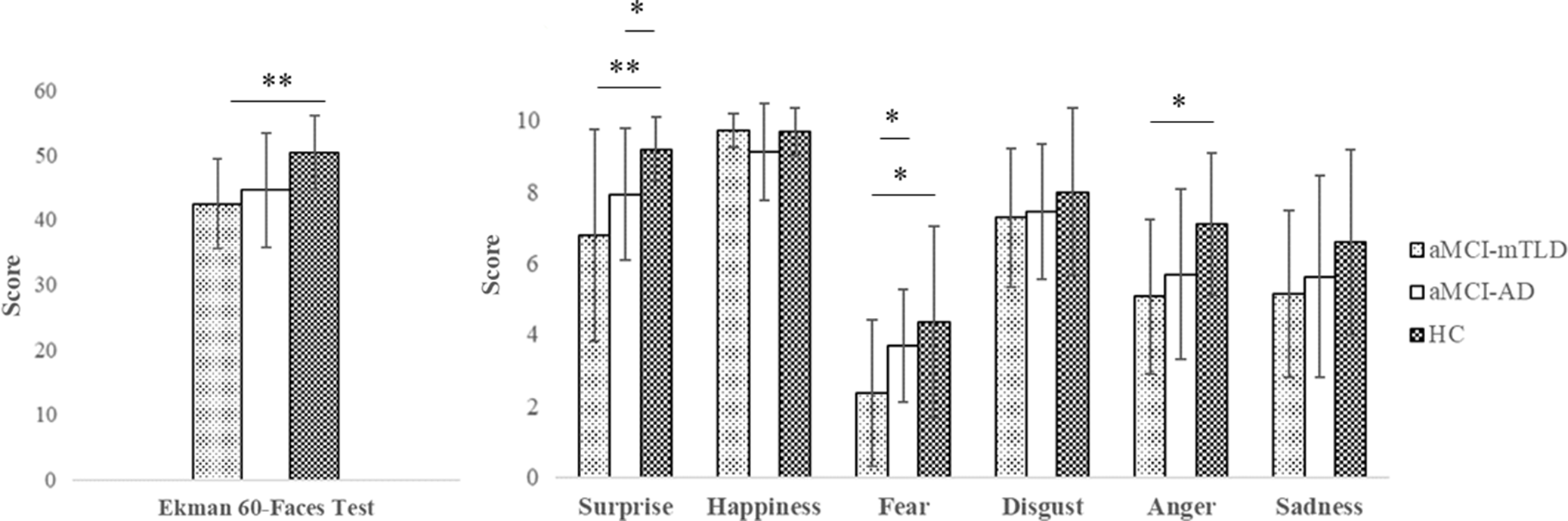
Fig. 1. Differences at Ekman-60Faces test between aMCI-AD, aMCI-mTLD, and controls. * p < 0.05, **p < 0.01.
Kruskal−Wallis analysis showed significant differences in the Ek-60F performances of the two aMCI groups compared to HC (Figure 1). Post hoc analysis proved that aMCI-AD had comparable global scores to HC (U(NaMCI-AD = 16, NHC = 20), =221.0, z = 1.49, p = 0.05, η2 = 0.06), whereas they had significantly lower scores in surprise recognition compared to HC (U(NaMCI-AD = 16, NHC = 20), =230.5, z = 2.35, p = 0.02, η2 = 0.15). The aMCI-mTLD subjects had significantly lower fear recognition scores compared to both aMCI-AD (U(NaMCI-mTLD = 14, NaMCI-AD = 16), = 159.5, z = 2.01, p = 0.04, η2 = 0.13) and HC (U(NaMCI-mTLD = 14, NHC = 20), =204.0, z = 2.26, p = 0.02, η2 = 0.14). Surprise (U(NaMCI-mTLD = 14, NHC = 20), =218.5, z = 2.84, p = 0.005, η2 = 0.24) and anger (U(NaMCI-mTLD = 14, NHC = 20), =212.0, z = 2.54, p = 0.01, η2 = 0.19) recognition was also impaired in aMCI-mTLD compared to HC. Consistently, Ek-60F global score was also reduced in aMCI-mTLD (U(NaMCI-mTLD = 14, NHC = 20), =229.5, z = 3.14, p = 0.002, η2 = 0.29).
DISCUSSION
Recent clinical-neuropathological evidences support the need for a revision in the study of cognitive neurodegenerative disorders in the elderly population. The high prevalence of non-AD brain diseases, including α-synucleinopathies, non-AD tauopathies, hippocampal sclerosis, and cerebrovascular disease, that can all mimic AD clinically may account for discrepancies between clinical and pathology-based AD diagnoses, especially in older people (Nelson et al., Reference Nelson, Trojanowski, Abner, Al-Janabi, Jicha, Schmitt and Ighodaro2016). The prevalence of AD pathology decreases in advanced old age, and approximately 25% of MCI from large case series shows cognitive impairments almost exclusively resulting from non-AD pathology (Nelson et al., Reference Nelson, Trojanowski, Abner, Al-Janabi, Jicha, Schmitt and Ighodaro2016). Given that subjects of advanced age constitute a rapidly growing demographic group in many countries, old-aged aMCI with non-AD pathology can be expected to have an expanding but probably underrecognized impact on clinical neurology. Although no disease-modifying treatment is currently available for AD and non-AD cognitive neurodegenerative syndromes, their differential diagnosis at clinical level might have important implications in terms of prognosis, patient management, and clinical trial participation.
Observational studies investigating neuropsychological profile combined with biomarker evidence in aMCI-mTLD may represent a chance to better characterize in vivo the phenotype of suspected non-AD pathology MCI cases. Standard neuropsychological assessment in suspected MCI subjects is generally focused on the amnestic syndrome of hippocampal type in order to early detect prodromal AD (Dubois et al., Reference Dubois, Feldman, Jacova, Hampel, Molinuevo, Blennow and Cummings2014). However, growing evidence shows that subjects affected by non-AD pathology (e.g. LATE, argyrophilic grain disease, or primary age-related tauopathy (Crary et al., Reference Crary, Trojanowski, Schneider, Abisambra, Abner, Alafuzoff and Nelson2014; Ferrer et al., Reference Ferrer, Santpere and van Leeuwen2008; Nelson et al., Reference Nelson, Dickson, Trojanowski, Jack, Boyle, Arfanakis and Schneider2019)) may also be characterized by mild cognitive deficits with a predominant amnestic phenotype. Accordingly, misdiagnosis is very frequent and, as reported in LATE case series, a high number of subjects receives in vivo a clinical diagnosis of AD (Besser, Teylan, & Nelson, Reference Besser, Teylan and Nelson2020).
Our findings suggest that, while standard neuropsychological assessment may fail in successfully distinguishing non-AD aMCI subjects from aMCI due to AD pathology, social cognition deficits may represent useful cognitive markers able to add decisive information for the diagnosis of aMCI-mTLD subjects. This result seems to be also corroborated by the high percentage of aMCI-mTLD patients showing impaired Ek-60F global score (43%), as well as anger (57%), fear (50%), and surprise (36%), based on the normative data (Dodich et al., Reference Dodich, Cerami, Canessa, Crespi, Marcone, Arpone and Cappa2014). Overall, emotion recognition deficit could represent a useful cognitive marker in improving the diagnostic accuracy of this group of patients. Basic emotion recognition crucially depends on temporo-limbic integrity, as previously demonstrated in neurological conditions specifically affecting these brain regions (Adams, Gordon, Baird, Ambady, & Kleck, Reference Adams, Gordon, Baird, Ambady and Kleck2003; Dodich et al., Reference Dodich, Cerami, Iannaccone, Marcone, Alongi, Crespi and Perani2016). Interestingly, also a small rate of HC poorly performed in single emotion recognition in the context of preserved global performance. This result is in line with previous evidence showing that aging significantly affects emotion recognition abilities, mainly in the recognition of negative stimuli (Ruffman, Henry, Livingstone, & Phillips, Reference Ruffman, Henry, Livingstone and Phillips2008).
Despite the processing of emotional faces that engages a widely distributed brain network (Fusar-Poli et al., Reference Fusar-Poli, Placentino, Carletti, Landi, Allen, Surguladze and Politi2009; Phan, Wager, Taylor, & Liberzon, Reference Phan, Wager, Taylor and Liberzon2002), functional imaging studies on emotion recognition consistently found a pivotal role of the amygdala in the detection and generation of fear-related emotions (see (Phan et al., Reference Phan, Wager, Taylor and Liberzon2002) for review). On the other hand, anger recognition has been associated to a broader network of paralimbic and frontal regions (Fusar-Poli et al., Reference Fusar-Poli, Placentino, Carletti, Landi, Allen, Surguladze and Politi2009). In particular, human amygdala is believed to track the valence of external cues, classifying the level of emotional arousal of a given emotion, to distinguish among specific emotion categories (Fusar-Poli et al., Reference Fusar-Poli, Placentino, Carletti, Landi, Allen, Surguladze and Politi2009) and to process salient and aversive stimuli (Fusar-Poli et al., Reference Fusar-Poli, Placentino, Carletti, Landi, Allen, Surguladze and Politi2009). Since both fear and anger signal an increase in the probable presence of a threat, it is not unexpected that the recognition of these basic emotions is particularly affected in aMCI-mTLD subjects presenting with a selective medio-temporal hypometabolism extended to limbic structures (Cerami et al., Reference Cerami, Dodich, Iannaccone, Magnani, Santangelo, Presotto and Perani2018). This result is consistent with a solid literature on neurological patients affected by amygdala damage, who systematically showed impaired recognition for both emotions (e.g., (Adams et al., Reference Adams, Gordon, Baird, Ambady and Kleck2003)).
Conversely to the well-studied neural correlates of negative emotions (Fusar-Poli et al., Reference Fusar-Poli, Placentino, Carletti, Landi, Allen, Surguladze and Politi2009; Phan et al., Reference Phan, Wager, Taylor and Liberzon2002), the presence of a specific brain network specialized in the detection of surprise is still a matter of debate. In our sample, a common pattern of surprise recognition deficit emerged in aMCI. Since literature supports a broad neural system including amygdala and insula, as well as parahippocampal, fusiform, and postcentral gyri, to mediate the recognition of surprise (Zhao, Zhao, Zhang, Cui, & Fu, Reference Zhao, Zhao, Zhang, Cui and Fu2017), different neural substrates may underlie this result. Further studies exploring possible distinctive neural correlates of basic emotion recognition deficits in aMCI-mTLD and aMCI-AD groups are certainly needed to clarify the issue.
MCI performances (Bora & Yener, Reference Bora and Yener2017) significantly vary according to inclusion criteria and cognitive profile, with subjects with multiple-domain and visuo-spatial impairments showing worse performance. Nevertheless, previous MCI studies mainly include heterogeneous samples classified according to clinical criteria (Bora & Yener, Reference Bora and Yener2017) without information about in vivo AD biomarkers. Thus, no conclusive findings on the impairment of emotion recognition ability can be drawn in these samples. Comprehensive studies of social cognition in MCI may thus help in clarifying the relationship between possible underlying pathology and interindividual phenotypical variation and in improving the awareness of this neuropathological condition.
A main limitation of this study is represented by the small sample size of aMCI subgroups, due to the restrictive inclusion criteria and the clinical setting. Neuropsychological studies with larger MCI samples classified according to a biological definition through first- and second-level biomarkers (e.g. MRI, amyloid/tau PET, CSF, plasma) are needed. Future studies should then be focused on the investigation of the clinical validity of socio-affective tasks in aMCI with different pathological substrates. The study of emotion recognition and processing in MCI patients would also benefit of multiple and more ecological measures and of a longitudinal design to evaluate socio-affective disorders in relationship to disease progression. Subtle differences in demographics and clinical features characterize the two aMCI subgroups, which are however related to intrinsic features of the two clinical syndromes (Nelson et al., Reference Nelson, Dickson, Trojanowski, Jack, Boyle, Arfanakis and Schneider2019) and to the clinical design of the study. In conclusion, this study suggests that social cognition impairments significantly characterize aMCI-mTLD, possibly representing a useful cognitive marker for its early recognition.
FINANCIAL SUPPORT
Mondino Foundation IRCCS was founded by the Italian Ministry of Health Ricerca Corrente 2020.
CONFLICTS OF INTEREST
None.