Introduction
Several polyphagous leaf-feeding caterpillar species (Lepidoptera: Tortricidae) form an important pest complex in commercial plantings of apple, Malus domestica Borkhausen (Rosaceae), and pear, Pyrus communis (Linnaeus) (Rosaceae), in the Pacific Northwest of North America (Madsen and Procter Reference Madsen and Procter1985; Beers et al. Reference Beers, Brunner, Willett and Warner1993). Summer-generation larvae of bivoltine leafroller species like Pandemis limitata (Robinson), P. pyrusana Kearfott, and Choristoneura rosaceana Harris are the most important species that damage apples when larvae construct leaf shelters and feed on adjacent fruits (Brunner and Beers Reference Brunner and Beers1990). Conventional apple producers usually manage these leafrollers with synthetic insecticides (Beers et al. Reference Beers, Brunner, Willett and Warner1993) but organic apple producers have few effective insecticides (Edwards Reference Edwards1998). Pheromone-based mating disruption is an established method for managing tortricid species (Witzgall et al. Reference Witzgall, Kirsch and Cork2010). Some organic apple growers in the Pacific Northwest are using multispecies sex pheromone mating disruption to manage a complex of leafroller pests including Pandemis Hübner species (Knight et al. Reference Knight, Thomson and Cockfield1998; Knight and Turner Reference Knight and Turner1999; Judd and Gardiner Reference Judd and Gardiner2004, Reference Judd and Gardiner2008).
The efficacy of these pheromone-disruption technologies can be threatened by high female moth density within orchards and the immigration of mated females from alternative host plants surrounding orchards (Cardé and Minks Reference Cardé and Minks1995). Our ability to easily measure these threats is limited, but experience with codling moth shows semiochemical-based monitoring of female moths can be helpful in this situation (Knight Reference Knight2010; Hári et al. Reference Hári, Pénzes, Jósvai, Holb, Szarukán and Szólláth2011). Monitoring female moth populations provides producers the information needed to apply supplemental controls when and where needed. Unfortunately, identified chemical attractants for female moths are rare among tortricid species (El-Sayed Reference El-Sayed2016). Nevertheless, recent research has shown that acetic acid in combination with some caterpillar-induced apple-leaf volatiles having a benzenoid structure can attract several tortricid species (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016; Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016).
European studies have thoroughly characterised the volatiles released by apple leaves in response to larval feeding by Pandemis heparana Denis and Schiffermüller (Giacomuzzi Reference Giacomuzzi2010; Giacomuzzi et al. Reference Giacomuzzi, Abraham and Angeli2013, Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016). Among the many compounds emitted by apple leaves, six were considered unique and only released in response to herbivory. In total, 14 compounds, including two benzenoids, phenylacetonitrile, and 2-phenylethanol, elicited antennal responses from male and female moths (Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016). Subsequent field trials revealed that none of the individual apple-leaf volatiles was attractive to a mixed population of P. heparana and P. cerasana (Hübner) (Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016). Yet, when phenylacetonitrile and 2-phenylethanol were individually combined with acetic acid, each binary blend caught a similar number of moths and each caught significantly more moths than their individual components (Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016). In a similar study focussed on the North American tortricid species, Spilonota ocellana (Denis and Schiffermüller) and C. rosaceana, El-Sayed et al. (Reference El-Sayed, Knight, Byers, Judd and Suckling2016) recorded incidental catches of two nontarget species, P. limitata and P. pyrusana, using binary blends of acetic acid and these same aromatic benzenoids. Collectively, these studies suggest it may be feasible to develop kairomone-based lures for trapping female P. limitata that could be beneficial for organic apple producers.
The overall objective of the present study was to gather information to facilitate the development of kairomone-based trapping of female P. limitata. Specific objectives of the current study are: (1) to confirm the relative attraction of P. limitata by acetic acid, phenylacetonitrile, 2-phenylethanol, and various combinations, (2) to test some prototype commercial dispensers, (3) to measure the attraction of these kairomones relative to a sex pheromone, and (4) to examine the impact of combining sex pheromone and kairomone lures in the same trap on relative catches of male and female P. limitata.
Materials and methods
All trapping experiments were conducted in a 30-ha commercial organic apple orchard several kilometres south of Cawston (latitude 49.0142°N, 119.6974°W, elevation 400 m) in the Similkameen Valley, British Columbia, Canada. This orchard contained several varietal plantings or orchard blocks (Ambrosia, Gala, Granny Smith, and Spartan), of dwarfing, high-density superspindle trees with an average height of 3–4 m and 1200–5444 apple trees/ha.
Glacial acetic acid, phenylacetonitrile, 2-phenylethanol, and dichloromethane solvent were purchased in 99% purity from Sigma-Aldrich (St. Louis, Missouri, United States of America). Two P. limitata pheromone components (Roelofs et al. Reference Roelofs, Cardé, Hill and Cardé1976), Z11-tetradecenyl acetate (Z11-14:OAc) and Z-9-tetradecenyl alcohol (Z9-14:OH) were purchased in 99% isomeric purity from Pherobank (Wageningen, The Netherlands). Acetic acid was stored at ambient laboratory temperature (20 °C) while all other compounds were stored at −20 °C until used.
In most experiments acetic acid was dispensed from an 8-mL polypropylene vial (Nalg-Nunc International, Rochester, New York, United States of America). Each vial contained 3 mL of glacial acetic acid applied to two cotton balls. Volatilised acetic acid was emitted through a 3-mm-diameter hole drilled in the lid of each vial. This acetic acid release device, hereafter referred to as our standard acetic-acid co-lure, was modified after Landolt et al. (Reference Landolt, Suckling and Judd2007) and used by El-Sayed et al. (Reference El-Sayed, Knight, Byers, Judd and Suckling2016). Red natural rubber septa (VWR International, Mississauga, Ontario, Canada) were used to release phenylacetonitrile, 2-phenylethanol, and pheromone alone and in combination in various experiments (Judd et al. Reference Judd, Knight and El-Sayed2017). All rubber septa were extracted with dichloromethane for 24 hours and air dried in a fume hood overnight before use. To construct composite binary or ternary lures containing acetic acid+phenylacetonitrile, acetic acid+2-phenylethanol, acetic acid+phenylacetonitrile+2-phenylethanol, or acetic acid+2-phenylethanol+sex pheromone, we drilled one or two additional holes (5 mm diameter) in the lid of our standard acetic-acid co-lure into which we inserted the narrow end of a rubber septum. Phenylacetonitrile, 2-phenylethanol, and sex pheromone were dissolved in dichloromethane and loaded into and volatilised from the large wells of these rubber septa. Proprietary polymeric cup membrane dispensers (Trécé Incorporated, Adair, Oklahoma, United States of America) having a 1.8-cm diameter membrane release surface were used in two experiments to release acetic acid (TRE-1468), phenylacetonitrile (TRE-1381), 2-phenylethanol (TRE-1256), and phenylacetonitrile+2-phenylethanol (TRE-1379). The physical properties and loadings of these prototype commercial membrane dispensers are proprietary.
Pherocon® VI style, white plastic delta traps with polybutene sticky liners (Trécé Incorporated) were used in all experiments. Individual experiments were conducted in separate varietal blocks separated by over 50 m within the 30-ha orchard. All traps were hung from wires within apple trees at ~1.7 m above ground with 20 m between the treatment traps within each replicate (=statistical block) and all statistical blocks of traps were separated by at least 30 m. Treatment traps were assigned randomly to positions within each statistical block using a random number table. All traps were at least 30 m from the border of any orchard varietal block. Sticky-trap liners were replaced at least weekly and returned to the laboratory where moths were counted and sexed.
Experiment 1 was conducted to measure the relative attraction of acetic acid, phenylacetonitrile, and 2-phenylethanol alone and in binary and ternary combination. In total, eight treatments were included in this experiment: blank control, acetic acid, phenylacetonitrile, 2-phenylethanol, phenylacetonitrile+2-phenylethanol, acetic acid+phenylacetonitrile, acetic acid+2-phenylethanol, and acetic acid+phenylacetonitrile+2-phenylethanol. Traps with the blank, phenylacetonitrile, 2-phenylethanol, and phenylacetonitrile+2-phenylethanol treatments all contained an empty 8 mL vial+a blank rubber septum or one containing 10 mg of the corresponding benzenoid compounds, respectively. All traps baited with treatments that included acetic acid contained our standard acetic-acid co-lure. All of these composite lures were attached at the centre and on the side, inner surface, of a delta trap using a 2.5-cm length of Velcro® industrial sticky-back tape (Canadian Tire, Penticton, British Columbia, Canada). This experiment had five replicates and was conducted from 8–22 August 2016.
Experiment 2 was conducted to measure and compare the relative attraction of acetic acid+phenylacetonitrile, acetic acid+2-phenylethanol, and acetic acid+phenylacetonitrile+2-phenylethanol when the aromatic compounds were released from rubber septa or commercial prototype membrane dispensers (Trécé Incorporated). These rubber septa and membrane dispensers provide markedly different chemical release rates (Knight et al. Reference Knight, El-Sayed, Judd and Basoalto2017). For example, red rubber septa loaded with 10 mg of 2-phenylethanol and the TRE-1256 membrane lure released 2-phenylethanol at rates of 0.62±0.01 and 1.01±0.03 mg/day, when held at 25 °C, respectively (Knight et al. Reference Knight, El-Sayed, Judd and Basoalto2017). There were six treatment combinations (three aromatic blends×two release devices) in this experiment: (1) phenylacetonitrile (TRE-1381), (2) 2-phenylethanol (TRE-1256), (3) phenylacetonitrile+2-phenylethanol (TRE-1379), (4) one septum loaded with 10 mg of phenylacetonitrile, (5) one septum loaded with 10 mg of 2-phenylethanol, and (6) one septum loaded with 10 mg of phenylacetonitrile and one septum loaded with 10 mg of 2-phenylethanol. All lure treatments contained our standard acetic-acid co-lure that was combined with a septum as necessary and attached to delta traps with Velcro as described before. All membrane lures were pinned inside each delta trap at the centre apex. This experiment was conducted with none replicates from 10–24 August 2016.
Experiment 3 was conducted to measure the relative attraction of acetic acid+2-phenylethanol lures when 2-phenylethanol was released from either rubber septa or membrane dispensers (see experiment 2) in combination with acetic acid released from either of two co-lures having markedly different release rates. Laboratory gravimetric analyses at 25 °C indicates that our standard acetic-acid co-lure and a TRE-1468 acetic-acid-membrane co-lure release acetic acid at rates of 62.65±2.39 and 27.77±0.48 mg/day, respectively (Knight et al. Reference Knight, El-Sayed, Judd and Basoalto2017). There were four treatment combinations (two acetic acid sources×two 2-phenylethanol release devices) in this experiment: (1) a standard acetic-acid co-lure+septum loaded with 10 mg of 2-phenylethanol; (2) a standard acetic-acid co-lure+membrane 2-phenylethanol lure (TRE-1256); (3) a membrane acetic-acid co-lure (TRE-1468)+septum loaded with 10 mg of 2-phenylethanol; and (4) a membrane acetic-acid co-lure (TRE-1468)+membrane 2-phenylethanol lure (TRE-1256). All standard acetic-acid co-lures were combined with a septum as necessary and attached to delta traps with Velcro as described before. All membrane lures were pinned inside each delta trap at the centre apex. This experiment was conducted with six replicates from 12–24 August 2016.
Experiment 4 was conducted to measure the attraction of an acetic acid+2-phenylethanol lure relative to a sex pheromone lure, and to test the hypothesis that combining this kairomone with a sex pheromone might increase catches of male P. limitata. We compared three treatment lures in this experiment: (1) a 1-mg sex-pheromone septum lure, (2) a standard acetic-acid co-lure+10 mg 2-phenylethanol septum lure, and (3) a standard acetic-acid co-lure+10 mg 2-phenylethanol septum lure+1 mg sex pheromone septum lure. The latter treatment tested the hypothesis that combining these semiochemicals in the same trap could lead to a more attractive male lure. Sex pheromone and 2-phenylethanol were loaded on separate septa to avoid any chemical interaction on the release substrate. The sex pheromone lure contained 1 mg of a 94:6 blend of Z11-14:OAc and Z9-14:OH (Roelofs et al. Reference Roelofs, Cardé, Hill and Cardé1976). The sex-pheromone septum was pinned to the 2-phenylethanol septum. This experiment had seven replicates and was conducted from 17–31 August 2016.
All insect count data were tested for normality (Kolmogorov–Smirnov test) and equality of variances (Levine’s Median test) to ensure they met the assumptions of an analysis of variance (ANOVA). Any non-normal data sets were normalised and variances stabilised using a √(x+0.5) transformation (Zar Reference Zar1984). Mean treatment catches were analysed post hoc using Tukey’s honest significant difference multiple-comparison test following significant ANOVA. In experiments 1–3 we performed preliminary ANOVA analyses to test for various interactions and if none were present we analysed experiments as randomised complete block designs using two-way ANOVA with no interaction (Zar Reference Zar1984). All statistical analyses were performed with experimental error rates set at α=0.05 using SigmaPlot® 12.5 (SYSTAT Software, San Jose, California, United States of America).
Results
In experiment 1 the treatment lures had a significant effect (F(6,52)=48.714, P<0.001) on moth catch but the ranking of the seven treatment lures (blank excluded) was independent of moth sex because the sex×lure interaction was nonsignificant (F(6,52)=0.288, P=0.492). Therefore, we pooled male (52.4%) and female (47.6%) catches and compared treatment lures using total moth catch (F(6,24)=55.721, P<0.001) (Fig. 1). Neither of the individual benzenoid compounds, nor a binary combination, caught many P. limitata (Fig. 1). Moth catches with acetic acid were small but greater than zero (one-tailed test, t=2.059, df=4, P=0.06), suggesting acetic acid is a weak attractant. More importantly, moth catches increased significantly when acetic acid was added as a co-lure to 2-phenylethanol, phenylacetonitrile, and 2-phenylethanol+phenylacetonitrile combined (Fig. 1). Traps baited with acetic acid+2-phenylethanol caught significantly more moths than traps baited with acetic acid+phenylacetonitrile, but catches with acetic acid+2-phenylethanol were not significantly different than those in traps baited with the ternary blend including phenylacetonitrile (Fig. 1).
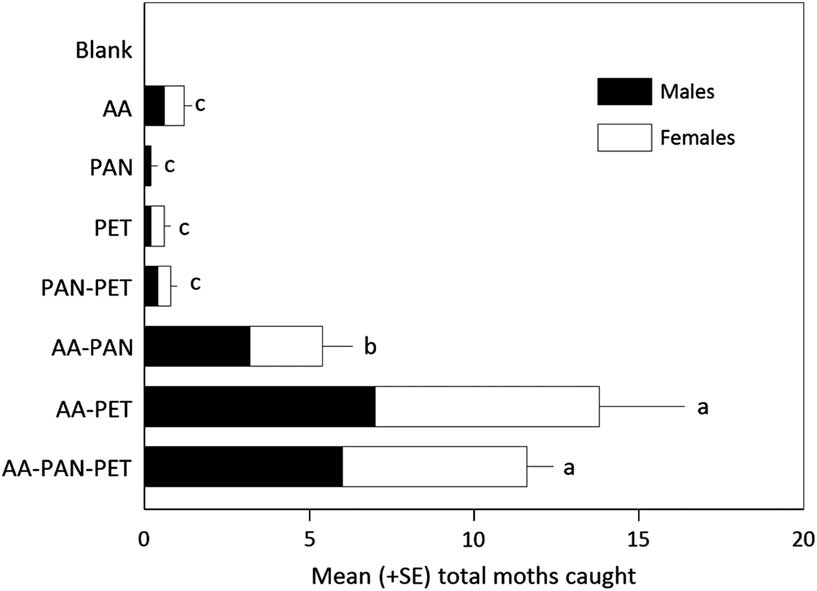
Fig. 1 Mean (±standard error (SE)) total number of Pandemis limitata moths caught during experiment 1 (8–22 August 2016) in sticky white delta traps baited with red rubber septa loaded with 10 mg of phenylacetonitrile (PAN) or 2-phenylethanol (PET) alone, and in binary or ternary combination with an acetic-acid (AA) co-lure (3 mL AA/3 mm open 8-mL vial) relative to blank traps. Bars followed by a common letter indicate mean total catches are not significantly different (Tukey’s honest significant difference test, α=0.05) following significant analysis of variance (P<0.05). Blank traps not included in statistical analysis.
In experiment 2, total moth catch was significantly affected by the aromatic volatile treatment (F(2,48)=11.862, P<0.001) and the type of device used to release it (F(1,48)=36.994, P<0.001). Because there was no significant interaction of these factors (F(2,48)=0.143, P=0.868), we compared the six combination treatment lures and found significant differences for male (F(5,40)=17.114, P<0.001), female (F(5,40)=20.643, P<0.001), and total moth catch (F(5,40)=22.249, P<0.001) using randomised block ANOVAs (Table 1). In general, membrane dispensers caught more moths than septum dispensers, and acetic acid+2-phenylethanol, and acetic acid+2-phenylethanol+phenylacetonitrile lures caught similar numbers of moths, but significantly more moths than the acetic acid+phenylacetonitrile lure (Table 1).
Table 1 Catches of Pandemis limitata moths during experiment 2 (10–24 August 2016) in sticky white delta traps baited with the aromatic compounds phenylacetonitrile (PAN) or 2-phenylethanol (PET) when released from red rubber septa or membrane dispensers in combination with acetic-acid (AA) co-lures (3 mL AA/3 mm open 8-mL vial).
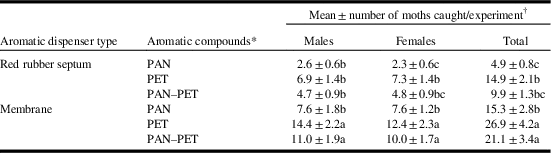
* Each red rubber septum was loaded with 10 mg of each compound. Trécé Incorporated membrane dispensers are loaded with proprietary amounts of PAN (TRE-1381), PET (TRE-1256), and PAN–PET (TRE-1379).
† Means within a column followed by the same letter are not significantly different (Tukey’s honest significant difference test, α=0.05) following significant ANOVA (P<0.05).
In experiment 3, the amount of acetic acid released by two different co-lures had no significant effect on male (F(1,15)=0.195, P=0.762), female (F(1,15)=0.421, P=0.74), or total moth catch (F(1,15)=0.396, P=0.758) when combined with the two different 2-phenylethanol dispensers (Table 2). The type of 2-phenylethanol dispenser also had no significant effect on male (F(1,15)=0.781, P=0.421), female (F(1,15)=0.714, P=0.438), or total moth catch (F(1,15)=0.936, P=0.371) in experiment 3 (Table 2).
Table 2 Influence of different acetic-acid co-lures on catches of Pandemis limitata moths during experiment 3 (12–24 August 2016) in sticky white delta traps baited with different 2-phenylethanol dispensers.

* Acetic acid emission at 25 °C based on weight loss is 62.65±2.39 mg/day from vials and 27.77±0.48 mg/day from TRE-1468 membrane (Knight et al. Reference Knight, El-Sayed, Judd and Basoalto2017).
† 2-phenylethanol emission at 25 °C based on weight loss is 0.62±0.01 mg/day for a septum and 1.01±0.03 mg/day from TREC-1256 membrane (Knight et al. Reference Knight, El-Sayed, Judd and Basoalto2017).
‡ Means within a column followed by the same letter are not significantly different by ANOVA (P>0.05).
In experiment 4, catches of male P. limitata in traps baited with a sex pheromone lure were eight times greater (F(2,12)=44.8, P<0.001) than male catches in traps baited with the acetic acid+2-phenylethanol kairomone lure (Table 3). Traps baited with a sex pheromone and kairomone combined caught 0.5 times fewer male moths than traps baited with sex pheromone alone, but four times more male P. limitata than the acetic acid+2-phenylethanol kairomone lure alone. Significantly (F(1,6)=6.568, P=0.025) fewer females (53%) were caught by the kairomone lure when a sex pheromone lure was placed in the same trap (Table 3). Total moth catches with sex pheromone were significantly (F(2,12)=4.463, P=0.036) greater than total catches with kairomone or kairomone plus sex pheromone (Table 3).
Table 3 Comparative catches of Pandemis limitata moths during experiment 4 (17–31 August 2016) in sticky white delta traps baited with sex pheromone and kairomone alone and combined.

* Sex pheromone lures contained 1 mg of a 94:6 blend of Z11-14:OAc and Z9-14:OH (Roelofs et al. Reference Roelofs, Cardé, Hill and Cardé1976). Kairomone lures contained 10 mg of 2-phenylthanol on a red septum and 3 mL of acetic acid in a 3-mm open 8-mL polypropylene vial. Pheromone and 2-phenylethanol were on separate septa in combined treatment (++).
† Means within a column followed by the same letter are not significantly different by Tukey’s honest significant difference test (α=0.05) following a significant ANOVA (P<0.05).
Discussion
Our studies have confirmed that a binary lure releasing acetic acid and 2-phenylethanol attracts both male and female P. limitata and is a more attractive lure than either component alone. The experiment with rubber septa lures clearly showed that 2-phenylethanol alone is an ineffective attractant of P. limitata, whereas acetic acid appeared weakly attractive as it is for several tortricid species (Knight et al. Reference Knight, Hilton, Basoalto and Stelinski2014). These results are consistent with European studies showing that 2-phenylethanol was not attractive to Pandemis species when used alone (Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016). However, unlike Pandemis species in the European study (Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016), P. limitata moths were caught significantly more often with acetic acid+2-phenylethanol than they were with acetic acid+phenylacetonitrile. Given the relatedness of these Nearctic Pandemis species (Dombroskie and Sperling Reference Dombroskie and Sperling2012) it seems likely that the differences among species, in terms of catches with these two caterpillar-induced benzenoid compounds, probably has more to do with experimental details than differences in olfactory preference. Mean moth catches in the European study were several times lower than ours, and the authors reported using fewer replicates, therefore, population density and dispersion differences may have led to more variation and a lack of statistical separation among lure treatments (Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016).
Pandemis limitata, like C. rosaceana (Knight et al. Reference Knight, El-Sayed, Judd and Basoalto2017), was caught significantly more often in traps baited with acetic acid+2-phenylethanol than in traps baited with acetic acid+phenylacetonitrile, whereas catches of another sympatric leafroller, S. ocellana, were greatest with acetic acid+phenylacetonitrile (Judd et al. Reference Judd, Knight and El-Sayed2017). These results prompted us to question whether the species differences in adult response are related to the relative amounts of these two benzenoids in the apple-leaf volatiles that the caterpillar of each species induces. Our review of the relevant literature (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016; Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016, Reference Giacomuzzi, Matthias, Basoalto and Knight2017b) suggests that the headspace volatiles described for the various caterpillar species do little to explain these results. 2-phenylethanol is no more prevalent in volatiles induced by species that respond strongly to it (Pandemis and C. rosaceana) than it is in the volatiles induced by S. ocellana. The ratio of 2-phenylethanol:phenylacetonitrile in headspace volatiles induced by P. limitata is unknown, but the ratios induced in response to feeding by P. heparana (Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016), P. pyrusana (Giacomuzzi et al. Reference Giacomuzzi, Matthias, Basoalto and Knight2017b), and S. ocellana (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016) are 0.15, 0.29, and 1.04, respectively. These ratios do not explain the differences in the catches of these various tortricid species with acetic acid+2-phenylethanol or phenylacetonitrile. Although the apple-leaf volatiles induced by these various tortricid species act as conspecific attractants (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016) they also appear to act as broad tortricid attractants. Several papers have now shown that benzenoids are common herbivore-induced plant volatiles that attract adult tortricids when presented in combination with acetic acid. The behavioural differences among adults of each species do not appear related to the caterpillar-induced specific ratios. This suggests that these compounds or their blends are not species-specific and a herbivore-induced plant volatile blend induced by one caterpillar might attract heterospecific adults. The overall ecological significance of these adult responses is currently unknown, but purported to be related to host-plant finding (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016) or attraction to food-based cues associated with microbial growth or host suitability (Giacomuzzi et al. Reference Giacomuzzi, Matthias, Basoalto and Knight2017b).
Given the general attraction of several tortricid species to acetic acid+2-phenylethanol it seems premature to assign any species-specific behavioural significance to catches of P. limitata with this lure. The behavioural “motivation” of captured insects is almost always unknown and these two compounds are found together in other natural contexts. While it is true both acetic acid and 2-phenylethanol are present in volatiles from caterpillar-infested apple leaves (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016; Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Khomenko, Biasioli, Schutz and Tasin2016) they are also emitted by undamaged apple leaves (Giacomuzzi et al. Reference Giacomuzzi, Cappellin, Nones, Khomenko, Knight, Biasioli and Angeli2017a, Reference Giacomuzzi, Matthias, Basoalto and Knight2017b). Furthermore, 2-phenylethanol is found in fermenting sweet baits, floral odours, and fruit aromas (El-Sayed et al. Reference El-Sayed, Heppelthwaite, Manning, Gibb and Suckling2005; Knudsen et al. Reference Knudsen, Eriksson, Gershenzon and Ståhl2006; Negre-Zakharov et al. Reference Negre-Zakharov, Long and Dudareva2009). A wide range of moths are attracted to these odours (El-Sayed Reference El-Sayed2016). More precise work on feeding and host-selection behaviour in P. limitata will be needed to fully understand the behavioural significance of its response to acetic acid and 2-phenylethanol.
Larval host plants can influence mating behaviour in many Lepidoptera (Landolt and Phillips Reference Landolt and Phillips1997) and there is good evidence that host-plant volatiles increase the sex pheromone responses of male Lepidoptera including tortricids (Dickens et al. Reference Dickens, Smith and Light1993; Landolt and Phillips Reference Landolt and Phillips1997; Reddy and Guerrero Reference Reddy and Guerrero2004; Yang et al. Reference Yang, Bengtsson and Witzgall2004; Schmidt-Büsser et al. Reference Schmidt-Büsser, von Arx and Guerin2009; Varela et al. Reference Varela, Avilla, Anton and Gemeno2011; von Arx et al. Reference von Arx, Schmidt-Büsser and Guerin2012). El-Sayed et al. (Reference El-Sayed, Knight, Byers, Judd and Suckling2016) suggested that combining caterpillar-induced benzenoid-based kairomones with sex pheromone might increase catches of male tortricid moths. However, we found that adding acetic acid+2-phenylathanol to the sex pheromone of P. limitata significantly reduced male moth catch. Likewise, both 2-phenylethanol and phenylacetonitrile without acetic acid (Knight et al. Reference Knight, El-Sayed, Judd and Basoalto2017) and phenylacetonitrile with acetic acid (Judd et al. Reference Judd, Knight and El-Sayed2017), significantly reduced catches of C. rosaceana and S. ocellana in pheromone-baited traps, respectively. Hatano et al. (Reference Hatano, Saveer, Borrero-Echeverry, Strauch, Zakir and Bengtsson2015) demonstrated that some herbivore-induced plant volatiles can suppress olfactory neuronal signalling pathways and inhibit sexual behaviours in both male and female Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae). Our negative result combining the caterpillar-induced volatiles acetic acid and 2-phenylethanol with sex pheromone supports a large body of literature suggesting herbivore-induced volatiles identify plants of poor quality that should be detected and avoided (De-Moraes et al. Reference De-Moraes, Mescher and Tumlinson2001). Experiments that examine mating behaviour of male and female Pandemis leafrollers on conspecific-infested and clean plants are needed to critically examine the role of benzenoids in finding mates or host plants (El-Sayed et al. Reference El-Sayed, Knight, Byers, Judd and Suckling2016).
Combining 2-phenylethanol and sex pheromone not only reduced catches of male P. limitata, but somewhat surprisingly, it also reduced catches of females. Female P. limitata are capable of detecting their own pheromone and exposure to high doses, as might be experienced near pheromone dispensers, causes females to move more frequently (DeLury et al. Reference DeLury, Judd and Gardiner2005). It seems possible that the detection of sex pheromone near sources of caterpillar-induced kairomones might cause female moths to be repelled and avoid trap entry. Auto-detection of sex pheromone by female moths and subsequent changes in flight and dispersal behaviour has been reported in other tortricid species (Palaniswamy and Seabrook Reference Palaniswamy and Seabrook1978; Barnes et al. Reference Barnes, Millar, Kirsch and Hawks1992; Weissling and Knight Reference Weissling and Knight1996). However, not all tortricids respond this way because catches of female S. ocellana were not reduced when phenylacetonitrile and sex pheromone were combined in a single trap or dispenser (Judd et al. Reference Judd, Knight and El-Sayed2017). Given these species differences it will be necessary to examine the semiochemical interactions for each target species separately before deciding whether combining semiochemicals is worthwhile.
Development of multispecies kairomone-based trapping to manage a suite of sympatric tortricid moths is a logical extension of the approach being used to develop mating disruption for leafroller complexes (Judd and Gardiner Reference Judd and Gardiner2004, Reference Judd and Gardiner2008). With S. ocellana, a ternary blend of acetic acid+phenylacetonitrile+2-phenylethanol caught as many female moths as did the optimal binary blend, acetic acid+phenylacetonitrile (Judd et al. Reference Judd, Knight and El-Sayed2017). Similarly, this same ternary blend caught as many female P. limitata as did its optimal binary blend, acetic acid+2-phenylethanol. Therefore, using this single ternary blend it should be possible to simultaneously trap a suite of tortricid moths. This lure may allow development of multispecies mass trapping as a supplement to multispecies pheromone disruption or as a stand-alone organic control. This emergent technology will require development of long-lasting commercial release devices, certified organic trapping systems, and a full understanding of the various semiochemical interactions of the important and unique species complex within each growing region.
Acknowledgements
The authors thank Mark Gardiner and Kandace Zurowski-Tiffin for their technical assistance and most especially their preparation of lures. The authors thank Godfrey Sellmer for allowing us to conduct research trials in his orchard. The authors also thank Bill Lingren, Trécé Incorporated (Adair, Oklahoma, United States of America) for donating some of the dispensers used in our studies. This project was supported with partial funding from the Washington Tree Fruit Research Commission (Wenatchee, Washington, United States of America).






