Introduction
Schizophrenia (SZ) and bipolar disorders (BD) are severe mental disorders with overlapping clinical characteristics and often regarded as part of a clinical continuum (Vieta et al., Reference Vieta, Berk, Schulze, Carvalho, Suppes, Calabrese and Grande2018). They share complex pathophysiology, involving genetic and environmental factors. A key pathophysiological hypothesis for SZ and BD is abnormal neurodevelopment, which seems to fit with several observations of genetic susceptibility (Smeland et al., Reference Smeland, Wang, Frei, Li, Hibar, Franke and Andreassen2018) and early life stressors such as birth complications, infections, migration, neglect and trauma (Radua et al., Reference Radua, Ramella-Cravaro, Ioannidis, Reichenberg, Phiphopthatsanee, Amir and Fusar-Poli2018).
Accounts of obstetric complications (OCs) often describe the pre- and perinatal environment and comprise a broad range of adversities that can occur during the fetal, perinatal and the neonatal period (McNeil, Cantor-Graae, & Sjostrom, Reference McNeil, Cantor-Graae and Sjostrom1994; McNeil & Sjöström, Reference McNeil and Sjöström1995). Although frequent in both healthy and psychiatric populations (Nicodemus et al., Reference Nicodemus, Marenco, Batten, Vakkalanka, Egan, Straub and Weinberger2008; Ursini et al., Reference Ursini, Punzi, Chen, Marenco, Robinson, Porcelli and Weinberger2018; Wortinger et al., Reference Wortinger, Engen, Barth, Lonning, Jørgensen, Andreassen and Agartz2019), OCs have been found to increase the risk of SZ with an odds ratio of 1.5–5 (Cannon, Jones, & Murray, Reference Cannon, Jones and Murray2002a; Geddes & Lawrie, Reference Geddes and Lawrie1995; Nosarti et al., Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe, Yin and Hultman2012; Pugliese et al., Reference Pugliese, Bruni, Carbone, Calabro, Cerminara, Sampogna and De Fazio2019), but the relationship between OCs and BD is unclear (Nosarti et al., Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe, Yin and Hultman2012; Pugliese et al., Reference Pugliese, Bruni, Carbone, Calabro, Cerminara, Sampogna and De Fazio2019; Scott, McNeill, Cavanagh, Cannon, & Murray, Reference Scott, McNeill, Cavanagh, Cannon and Murray2006). Genetic risk for developing SZ multiplies by a factor of 5 in the context of severe OCs (Ursini et al., Reference Ursini, Punzi, Chen, Marenco, Robinson, Porcelli and Weinberger2018), which suggest a connection between a genetic liability for SZ and vulnerability for adverse events during pregnancy and birth.
Asphyxia is a condition defined as a deficient supply of oxygen to the body. Within labor and delivery complications, perinatal asphyxia is a severe complication with the potential to cause great harm in the offspring, such as neonatal mortality or morbidity with long-term consequences (McNeil & Sjöström, Reference McNeil and Sjöström1995). In a previous study, a history of more than one co-occurring OC was found to be associated with lower cognitive abilities among adult patients with SZ and BD, as well as in healthy controls (Wortinger et al., Reference Wortinger, Engen, Barth, Lonning, Jørgensen, Andreassen and Agartz2019). Interestingly, asphyxia was prevalent across groups and very common among participants who had experienced several OCs, with a prevalence of 81%. This raised the possibility that neural insult related to asphyxia is consequential for adult brain function and, possibly, structure.
During early childhood, total brain volume (TBV) and intracranial volume (ICV) increase in parallel until early adolescence, wherein TBV appears to be the driving factor for ICV growth (Kiesler & Ricer, Reference Kiesler and Ricer2003). From early adolescence, the development of ICV and TBV diverge; ICV appears to remain static, but TBV decreases during adolescence into adulthood (Tamnes et al., Reference Tamnes, Herting, Goddings, Meuwese, Blakemore, Dahl and Mills2017). Childhood head circumference measures are strongly correlated with magnetic resonance imaging (MRI) derived ICV estimates (Hshieh et al., Reference Hshieh, Fox, Kosar, Cavallari, Guttmann, Alsop and Inouye2016), and ICV might be used as a proxy of early brain development. One longitudinal study found that the rate of cranial growth, measured as head circumference from birth to age 4, predicted later cognitive abilities (Jaekel, Sorg, Baeuml, Bartmann, & Wolke, Reference Jaekel, Sorg, Baeuml, Bartmann and Wolke2019). These findings substantiate the idea that ICV can inform on the development of brain structure and function.
The discrepancy between ICV and TBV may refer to the normal process of age-related brain maturation and atrophy (Storsve et al., Reference Storsve, Fjell, Tamnes, Westlye, Overbye, Aasland and Walhovd2014), as well as pathological processes beginning later in life. Several studies have assessed ICV and TBV in relation to cognitive phenotypes in patients with SZ, BD and healthy control participants (Czepielewski, Wang, Gama, & Barch, Reference Czepielewski, Wang, Gama and Barch2017; Van Rheenen et al., Reference Van Rheenen, Cropley, Zalesky, Bousman, Wells, Bruggemann and Pantelis2018; Woodward & Heckers, Reference Woodward and Heckers2015). These studies subdivided patients into groups based on current cognitive abilities and an estimate of cognitive abilities preceding the occurrence of disease symptoms. The abnormal cognitive profiles were defined as the neurodevelopmental phenotype, which had low premorbid and current cognitive abilities and exhibited smaller ICV and smaller absolute TBV – indicative of early cerebral disruption or hypoplasia (Czepielewski et al., Reference Czepielewski, Wang, Gama and Barch2017; Woodward & Heckers, Reference Woodward and Heckers2015), and the neuroprogressive phenotype, which had only low current cognitive abilities and showed only proportionally smaller TBV in relation to ICV – indicative of excessive brain tissue loss and progressive atrophy (Czepielewski et al., Reference Czepielewski, Wang, Gama and Barch2017; Van Rheenen et al., Reference Van Rheenen, Cropley, Zalesky, Bousman, Wells, Bruggemann and Pantelis2018; Woodward & Heckers, Reference Woodward and Heckers2015). The rationale behind these measures is that in the neurodevelopmental phenotype low cognitive abilities and small ICV are consistently present due to halted brain growth, which suggests abnormalities in brain growth and function. Whereas, the neuroprogressive group had only low current cognitive abilities and an ICV that was not different from healthy controls, indicating normal brain growth. Cognitive impairment occurred later and was accompanied by progressive brain tissue loss that is only apparent when adjusting for ICV.
Longitudinal studies (Ducharme et al., Reference Ducharme, Albaugh, Nguyen, Hudziak, Mateos-Perez and Labbe2015; Mills, Lalonde, Clasen, Giedd, & Blakemore, Reference Mills, Lalonde, Clasen, Giedd and Blakemore2014; Raznahan et al., Reference Raznahan, Shaw, Lalonde, Stockman, Wallace, Greenstein and Giedd2011; Tamnes et al., Reference Tamnes, Herting, Goddings, Meuwese, Blakemore, Dahl and Mills2017; Wierenga, Langen, Oranje, & Durston, Reference Wierenga, Langen, Oranje and Durston2014) have shown that cortical surface area (SA) increases during childhood followed by subtle decreases during adolescence, which supports the idea that SA is established early in human development. Whereas, cortical thickness (CT) decreases during adolescence and appears to be the main contributor to TBV reductions (Storsve et al., Reference Storsve, Fjell, Tamnes, Westlye, Overbye, Aasland and Walhovd2014; Tamnes et al., Reference Tamnes, Herting, Goddings, Meuwese, Blakemore, Dahl and Mills2017). Fetal hypoxia, caused by an inadequate supply of oxygen to the fetus due to several reasons during pregnancy and labor (e.g. birth or neonatal asphyxia, umbilical cord abnormalities, placental infarcts, third-trimester bleeding, preeclampsia, etc.), was associated with smaller SA, but not with CT in a first-episode psychosis study (Smith et al., Reference Smith, Thornton, Lang, MacEwan, Kopala, Su and Honer2015). The relationship between fetal hypoxia and regional SA and CT has not been previously reported. Putative associations between fetal hypoxia and subcortical structures have shown mixed results with smaller hippocampal and amygdala volumes reported in SZ and BD (Haukvik et al., Reference Haukvik, McNeil, Lange, Melle, Dale, Andreassen and Agartz2014a; Van Erp et al., Reference Van Erp, Saleh, Rosso, Huttunen, Lonnqvist, Pirkola and Cannon2002) and larger hippocampal volumes reported in SZ (Haukvik et al., Reference Haukvik, Saetre, McNeil, Bjerkan, Andreassen, Werge and Agartz2010). A study comparing SZ patients with their siblings and unrelated healthy controls showed a significant relationship between fetal hypoxia and smaller gray matter volume (but not white matter volume) in both patients and their relatives, which was not found in the healthy controls (Cannon et al., Reference Cannon, van Erp, Rosso, Huttunen, Lonnqvist, Pirkola and Standertskjold-Nordenstam2002b). Extensive studies on hypoxic-ischemic injury have reported neuronal death, reduced neural growth processes and white matter damage in infants (Rees & Inder, Reference Rees and Inder2005; Volpe, Reference Volpe2012), but altered white matter volume associated with fetal hypoxia has not been identified in SZ.
Using prospective birth registry data, we were able to show a connection between OCs and a neurodevelopmental phenotype characterized by lower premorbid and current cognitive abilities (Wortinger et al., Reference Wortinger, Engen, Barth, Lonning, Jørgensen, Andreassen and Agartz2019). Here we investigated whether the relationship extends to neuroimaging measures of brain structure. Specifically, we explored whether a history of asphyxia-related OCs (ASP) is associated with adult brain measures in patients with severe mental illness and healthy controls. The aims of this study were to: (1) investigate the prevalence of ASP across diagnoses; (2) examine the effect of a history of ASP on ICV, TBV, global brain structural volumes, total cortical surface area and mean cortical thickness and regional brain measures in ASP defined subgroups; and (3) determine if effects of ASP are more pronounced in SZ or BD.
Methods and Materials
Participants
The Thematically Organized Psychosis (TOP) study is a thematic research effort focused on the disease mechanisms of psychotic disorders and is the main study protocol at the Norwegian Centre for Mental Disorders Research (NORMENT, Oslo, Norway; www.med.uio.no/norment/english). Adult patients with schizophrenia or bipolar spectrum disorders were recruited consecutively from out- and inpatient psychiatric units of public hospitals in the Oslo region. The hospitals are located in different parts of the city and are representative of the city's variation in sociodemographic characteristics. The healthy controls (HC) were randomly selected from the national population register and were residents in the same catchment area as the patients. After a complete description of the study, all participants gave written informed consent. The Regional Committee for Research Ethics and the Norwegian Data Inspectorate approved the study. Participant inclusion for this study was carried out between 2002 and 2012 in accordance with the Declaration of Helsinki.
Exclusion criteria for both patients and HC were hospitalization for previous moderate or severe head injury, neurological disorder, medical conditions thought to interfere with brain function and age outside the range of 18–65 years. Additional exclusion criteria for HC were current or previous somatic illness and substance misuse disorders or dependency within the last 6 months. HC were also excluded if they or a first-degree relative had a lifetime history of severe psychiatric disorder.
All patients underwent a thorough clinical investigation by trained psychologists and physicians. Clinical diagnoses were assessed using the Structured Clinical Interview for DSM-IV axis 1 disorder (SCID-I) module A-E (Spitzer, Williams, Gibbon, & First, Reference Spitzer, Williams, Gibbon and First1988). Psychosocial function was assessed with the Global Assessment of Function scale, split version [GAF; (Pedersen, Hagtvet, & Karterud, Reference Pedersen, Hagtvet and Karterud2007)]. Current psychotic symptoms were rated by the use of the Positive and Negative Syndrome Scale [PANSS; (Kay, Fiszbein, & Opler, Reference Kay, Fiszbein and Opler1987)].
HC were interviewed by trained research assistants and examined with the Primary Care Evaluation of Mental Disorders (Prime-MD) to ensure no current or previous psychiatric disorders (Spitzer et al., Reference Spitzer, Williams, Kroenke, Linzer, deGruy, Hahn and Johnson1994).
For the current study, participants were included if they had both OC and MRI data. The total subject sample (n = 529) consisted of patients with a DSM-IV diagnosis within the schizophrenia spectrum (SZ): schizophrenia (DSM-IV 295.1, 295.3, 295.6 and 295.9; n = 106), schizophreniform disorder (DSM-IV 295.4; n = 16), schizoaffective disorder (DSM-IV 295.7; n = 14) or psychosis not otherwise specified (DSM-IV 298.9; n = 60); or within the bipolar disorder spectrum (BD): Bipolar I disorder (DSM-IV 296.0–7; n = 72), Bipolar II disorder (DSM-IV 296.89; n = 36) or bipolar disorder not otherwise specified (DSM-IV 296.80; n = 7); and HC (n = 218).
Obstetric complications
Birth data were collected from the Medical Birth Registry of Norway (MBRN). In Norway, there is mandatory reporting on all births after gestational week 16. MBRN data were scored for the presence and severity of OCs (McNeil & Sjöström, Reference McNeil and Sjöström1995) by KE.
The validated McNeil–Sjöström scale (McNeil et al., Reference McNeil, Cantor-Graae and Sjostrom1994; McNeil & Sjöström, Reference McNeil and Sjöström1995) includes several hundred events of potential harm to the fetus/offspring, each classified according to the severity on an ordinal scale from 1 to 6. As in other reports (Nicodemus et al., Reference Nicodemus, Marenco, Batten, Vakkalanka, Egan, Straub and Weinberger2008; Ursini et al., Reference Ursini, Punzi, Chen, Marenco, Robinson, Porcelli and Weinberger2018; Wortinger et al., Reference Wortinger, Engen, Barth, Lonning, Jørgensen, Andreassen and Agartz2019), an incidence of severe OCs was reported in those participants who had experienced one or more complications of a grade 5 or 6. Complications below grade 5 have the potential to cause harm, but to a lesser extent (McNeil et al., Reference McNeil, Cantor-Graae and Sjostrom1994). Examples of grade 3 complications are conditions like non-preeclamptic hypertension, polyhydramnios and hyperemesis and examples of grade 4 complication are conditions like mild preeclampsia, oligohydramnios and breech delivery (McNeil et al., Reference McNeil, Cantor-Graae and Sjostrom1994). We classified participants with complications of grade 4 and below as having an absence of severe OCs. A complication of grade 5 is defined as an event that is ‘potentially clearly greatly relevant/harmful’ to the central nervous system of the developing fetus/offspring and a complication of grade 6 is defined as an event that causes ‘very great harm to or deviation in offspring’ (McNeil & Sjöström, Reference McNeil and Sjöström1995). Examples of grade 5 or 6 are as follows: severe preeclampsia, bleeding before 28 weeks, asphyxia, discolored placenta/amniotic fluid, emergency caesarean delivery, preterm birth ⩽35 weeks, low Apgar score (0–3 at 1 min or 0–7 at 5 min), bleeding during labor, low birth weight ⩽2000 g and eclampsia.
The ASP variable was defined as having had a report of a complication coded AS53 (asphyxia without other signs), AS54 (asphyxia with poor fetal sound), AS55 (asphyxia and discolored amniotic fluid), AS56 (asphyxia with poor fetal sound and discolored amniotic fluid) or AS61 (asphyxia) on the MBRN registry form, all of which state the presence of ASP at birth. See Wortinger et al. (Reference Wortinger, Engen, Barth, Lonning, Jørgensen, Andreassen and Agartz2019) for MBRN form. OC co-occurrence was defined as having experienced more than one severe OC during pregnancy and birth.
Image acquisition and processing
All participants underwent MRI scanning on a 1.5 T Siemens Magnetom Sonata scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with an 8-channel head coil. After a conventional 3-plane localizer, two sagittal T1-weighted magnetization prepared rapid gradient echo (MPRAGE) volumes were acquired with the Siemens tfl3d1_ns pulse sequence (TE = 3.93 ms, TR = 2730 ms, TI = 1000 ms, flip angle = 7°; FOV = 24 cm, voxel size = 1.33 × 0.94 × 1 mm3, number of partitions = 160).
The Freesurfer software package (version 5.3) was used to process images with volume and surface-based streams (Dale, Fischl, & Sereno, Reference Dale, Fischl and Sereno1999; Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich, Haselgrove and Dale2002). Post-processed images received a quality control of surface reconstruction and subcortical segmentations, and if deemed necessary, manual editing was performed by trained assistants following standard FreeSurfer procedures (McCarthy et al., Reference McCarthy, Ramprashad, Thompson, Botti, Coman and Kates2015). Volume-based analyses were used to extract mean volume estimates for intracranial volume (ICV; estimate based on the Talairach transform), total brain volume (TBV; brain segmentation volume, without ventricles), white and gray matter volumes, subcortical volumes and cortical surface area across the whole brain. Surface-based analyses were used to extract cortical thickness measurements by reconstructing a 3-dimensional cortical surface model.
Statistical analysis
Statistical analyses were performed within the Statistical Package of Social Sciences (SPSS), version 25 (IBM, USA; www.spss.com). Demographic and clinical characteristics were compared between groups using χ2 tests for categorical variables and analysis of variance (ANOVA) or covariance (ANCOVA) for continuous variables. Subjects with missing data on any particular variable were omitted from analyses involving that variable but were included in analyses for which all required variables were present.
To evaluate the contribution of OCs other than ASP on global brain measures, we assessed ICV, TBV, global brain structural volumes, total cortical surface area and mean cortical thickness in a full factorial 3 × 3 ANCOVA with OCs [OCs with ASP (OC, ASP+); OCs other than ASP (OC, ASP−); no OCs present (no OC)] and the group included as between-group factors, covarying for age and sex. Since OC, ASP− was not associated with global brain measures, the variable OCs, ASP− was not included in further analyses.
We assessed ICV, TBV, white and gray matter volumes, total subcortical volume, total cortical surface area and mean cortical thickness using a full factorial 2 × 3 ANCOVA with ASP (ASP+/ASP−) and group included as between-group factors, covarying for age and sex. A false discovery rate (FDR) of 5% was used to correct for multiple testing of the following comparisons, separately: global brain measures – ICV, TBV, total subcortical gray matter volume, total gray matter volume, left, right and whole cortical gray and white matter volumes, total cortical surface area and thickness (12 comparisons); subcortical volumes were assessed for the left and right hippocampus, amygdala, thalamus, caudate, putamen, pallidum, accumbens and cerebellum (16 comparisons); and regional cortical surface area was evaluated on all 34 regions of the left and right hemispheres based on the Desikan-Killiany brain atlas (34 comparisons per hemisphere). In the statistical analysis, ICV was subsequently added to each full factorial model to assess relative differences in those measures showing main effects of ASP. Effect sizes are reported as Cohen's d (Nakagawa & Cuthill, Reference Nakagawa and Cuthill2007).
Results
Demographic and clinical variables
Demographic and clinical data are presented in Table 1.
Table 1. Demographic and clinical characteristics

s.d., standard deviation; HC, healthy controls; BD, bipolar spectrum; SZ, schizophrenia spectrum; PANSS, Positive and Negative Syndrome Scale; GAF-S, Global Assessment of Functioning- symptoms; GAF-F, Global Assessment of Functioning- functioning; APD, antipsychotic drug; CPZ, chlorpromazine. Bold values denote statistical significance at the p < 0.05 level.
¥ Means are adjusted for sex.
† Means are adjusted for age and sex.
* p < 0.05; ** p < 0.01; *** p < 0.001.
a Total sample size (HC: ASP− n = 186, ASP+ n = 32; BD: ASP− n = 93, ASP+ n = 22; SZ: ASP− n = 174, ASP+ n = 21).
b Total sample size (HC: ASP− n = 175, ASP+ n = 30; BD: ASP− n = 88, ASP+ n = 21; SZ: ASP− n = 167, ASP+ n = 18).
c Total sample size (HC: ASP− n = 89, ASP+ n = 22; BD: ASP− n = 53, ASP+ n = 14; SZ: ASP− n = 99, ASP+ n = 11).
d Total sample size (HC: ASP− n = 184, ASP+ n = 32; BD: ASP− n = 90, ASP+ n = 21; SZ: ASP− n = 167, ASP+ n = 18).
e Total sample size (HC: ASP− n = 186, ASP+ n = 32; BD: ASP− n = 93, ASP+ n = 22; SZ: ASP− n = 175, ASP+ n = 21).
f Total sample size (HC: ASP− n = 183, ASP+ n = 31; BD: ASP− n = 92, ASP+ n = 22; SZ: ASP− n = 170, ASP+ n = 20).
Obstetric complications
There were no significant differences in the frequency of OCs between patient groups and HC, but it did differ between the two patient groups (Table 2). ASP was significantly more prevalent in the BD group compared to the SZ group. Low birth weight was more frequent in the SZ group compared to the HC. Bleeding during labor occurred more often in the HC in comparison to the SZ group. The most frequent complications across groups were ASP (⩾44%) and discolored placenta/amniotic fluid (⩾41%). There were significantly more cases of OC co-occurrence in the presence of ASP than when ASP was not present (Table 1). Discolored placenta/amniotic fluid was the most common co-occurring severe OC with ASP, present in 31 out of 41 cases. Low birthweight co-occurred with ASP in three out of 41 cases.
Table 2. Frequency and type of obstetric complications using χ2 tests
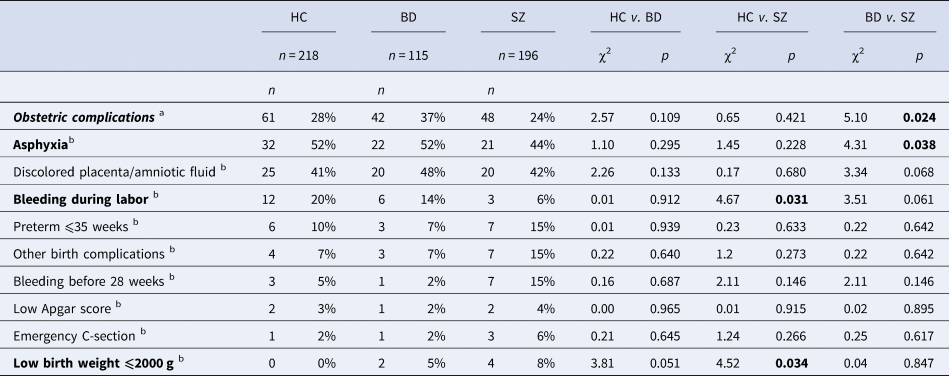
HC, healthy controls; BD, bipolar spectrum; SZ, schizophrenia spectrum. Bold values denote statistical significance at the p < 0.05 level.
a % refers to the number of cases with obstetric complications in relation to the group sample size.
b % refers to the number of cases with the specific complication in relation to the total number of obstetric complications within each group.
ASP effects on global brain measures
We found that OC, ASP+ was specifically related to ICV and other brain measure differences (see online Supplementary materials Table S1) and OC, ASP− was not related to any of the brain measures. In the full factorial model, there was a significant main effect of OCs on ICV after controlling for the effect of age and sex, F (2,518) = 4.32, p = 0.014. Pairwise comparisons revealed that adult ICV after having experienced OC, ASP+ was significantly smaller than when no OCs were present (p = 0.006) and ICV was smaller with OC, ASP+ than having experienced severe OC, ASP− (p = 0.011). The presence of an OC, ASP− did not show a significant difference in ICV compared to those with no OCs (p = 0.601). The covariate, sex, was significantly related to ICV, F (1,518) = 361.24, p < 0.001, wherein females had smaller ICV than males. There were no significant OCs by group interactions on any of the brain measures. Since OC, ASP− was not associated with ICV or other global brain measures, the variable OCs, ASP− was not included in further analyses.
There was a significant main effect of ASP that survived the FDR correction rate of 5% for all brain measures, except total cortical thickness (Fig. 1 and online Supplementary materials Table S2). ICV and absolute values of TBV; total subcortical gray matter volume; total gray matter volume; left, right and whole cortical gray and white matter volumes; total cortical surface area was smaller in the three ASP+ subgroups within SZ, BD and HC. There were no significant ASP by group interactions on any of the global brain measures. There was no effect of ASP on any of these global measures after adjusting for ICV.

Fig. 1. ICV, TBV, global brain structural volumes and total surface area estimate in health controls (HC) and patients within the schizophrenia spectrum (SZ) and bipolar disorder spectrum (BD), unadjusted for ICV. Top panels: Absolute ICV and TBV corrected means were significantly decreased in all adult participant subgroups that had experienced asphyxia at birth. Middle panels: Cortical and subcortical gray matter corrected volumes were significantly decreased in all adult participant subgroups that had experienced asphyxia at birth. Bottom panel: Cortical white matter volume and total surface area values were significantly decreased in all adult participant subgroups that had experienced asphyxia at birth. Main effect of asphyxia was significant at p < 0.05 for cortical white matter volume, and all other values had a significance of p < 0.005. Measures were corrected for age and sex. Error bars = standard error of mean. ** p < 0.005, * p < 0.05.
Regional cortical surface area estimates
There was a significant main effect of ASP that survived the FDR correction rate of 5% for 20 regional surface area measures (Fig. 2 and online Supplementary materials Table S3). Absolute regional values of the left and right superior frontal; left and right caudal middle frontal; left pars opercularis; left and right lateral orbitofrontal; right rostral anterior cingulate; right superior parietal; left and right supramarginal; right precuneus; left isthmus cingulate; right entorhinal; right lingual; left and right cuneus; right pericalcarine; and left and right insulae surface area estimates were smaller in the ASP+ subgroups. There were no significant ASP by group interactions on any of the surface area estimates. There was no significant effect of ASP on any surface area after adjusting for ICV.
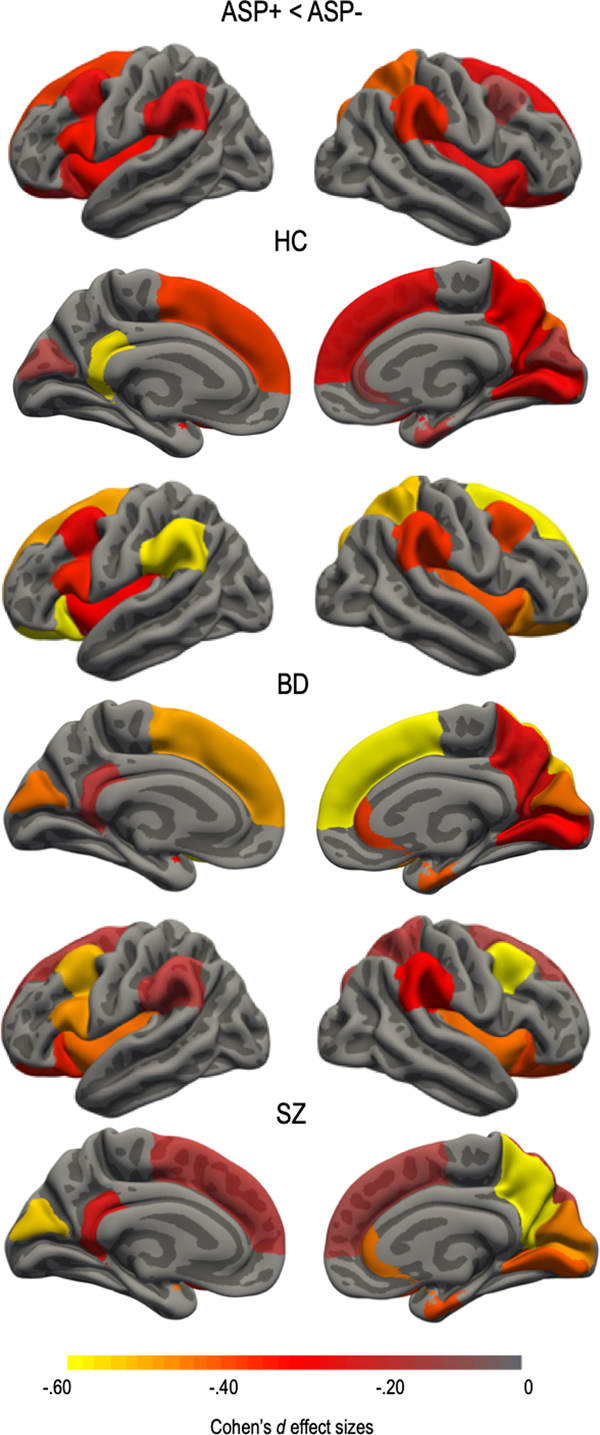
Fig. 2. Asphyxia (ASP+) effect size differences for regions surviving false discovery rate (FDR) correction of 5% in health controls (HC) and patients within the schizophrenia spectrum (SZ) and bipolar disorder spectrum (BD). Regional cortical surface area was smaller in all ASP+ subgroups. Regions that did not survive correction were assigned a value of 0. Effect sizes were corrected for age and sex. Color bar represents Cohen's d values with ASP+ subgroups showing smaller surface area compared to the ASP− subgroups.
Subcortical volumes
There was a significant main effect of asphyxia that survived the FDR correction rate of 5% for 7 of the 16 subcortical volumes (Fig. 3 and online Supplementary materials Table S4). Absolute values of the left and right hippocampus; left amygdala; right thalamus; left and right caudate; and right putamen volumes were smaller in the ASP+ subgroups compared to the ASP− subgroups, except for the caudate volumes in HC compared to both patient groups. An interaction effect indicated that within both patient groups, patients who experienced ASP at birth had smaller left and right caudate volumes compared to patients who did not experience ASP, which was not the case for the HC group, F (2,521) = 7.78, p < 0.001 and F (2,521) = 4.78, p = 0.009, respectively. There were no significant ASP by group interactions on the remaining subcortical volumes. Smaller subcortical volumes seem to be explained by smaller ICV, as almost no subgroup differences remained after correcting for ICV. Volumes of the left and right caudate remained significantly smaller in both patient ASP+ subgroups, even after ICV correction, F (2,520) = 8.29, p < 0.001 and F(2,520) = 4.82, p = 0.008, respectively.

Fig. 3. Cohen's d effect sizes for ICV and subcortical volume differences between asphyxia-defined subgroups of health controls (HC) and patients within the schizophrenia spectrum (SZ) and bipolar disorder spectrum (BD). Within each patient and HC group, ASP+ v. ASP− effect size differences are shown for ICV and subcortical volumes surviving false discovery rate (FDR) correction of 5%. Smaller ASP+ volumes were observed in all groups, except for left and right caudate in the HC. *An interaction effect indicated that within both patient groups, ASP+ subgroups had smaller left and right caudate compared to ASP− subgroups, but this was not the case for the HC group. Effect sizes were corrected for age and sex.
Discussion
Participants with birth asphyxia showed smaller head size (ICV) as well as smaller volume/surface area across a range of global and regional brain structures. The pattern of differences between ASP+ and ASP− subgroups was similar across patient and HC groups, but effects were larger in the patient groups. Of note, this effect was also found when comparing the ASP+ subgroup with participants who had experienced severe OCs other than ASP. Smaller head size in the ASP+ compared to ASP− subgroups explained all but one of the differences found in global and regional brain measures (as findings did not remain significant after adjustment for ICV). The notable exception was the smaller caudate nuclei, where the findings remained significant among patients, also after adjustment for ICV, which may suggest an ASP by illness vulnerability-interaction.
ICV has been postulated as a biomarker of neurodevelopmental origin, and TBV as an indicator of neurodegenerative processes (Woodward & Heckers, Reference Woodward and Heckers2015). Reports in psychosis research (Czepielewski et al., Reference Czepielewski, Wang, Gama and Barch2017; Woodward & Heckers, Reference Woodward and Heckers2015) found smaller ICV and absolute TBV present in patients with low cognitive abilities on both assessments of current and estimated premorbid functioning, signifying hypoplasia and a neurodevelopmental phenotype. While smaller relative TBV (corrected for ICV) was apparent in patients with only low current cognitive abilities (i.e. not premorbid estimates), implying atrophy and a neuroprogressive phenotype (Czepielewski et al., Reference Czepielewski, Wang, Gama and Barch2017; Van Rheenen et al., Reference Van Rheenen, Cropley, Zalesky, Bousman, Wells, Bruggemann and Pantelis2018). Our previous work on this sample found lower current and premorbid cognitive abilities in the subgroup of SZ patients with OCs, but having experienced more than one severe OC was related to lower cognitive abilities in all groups, with 81% having had experienced ASP (Wortinger et al., Reference Wortinger, Engen, Barth, Lonning, Jørgensen, Andreassen and Agartz2019). The results here provide evidence that the relationship between having had experienced ASP extends to smaller ICV and absolute TBV and further supports the neurodevelopmental profile in ASP+ subgroups. We did not observe a neuroprogressive phenotype in the ASP- subgroups, as the TBV results did not survive ICV correction.
Small ICV found in SZ is regarded as an indication of impaired neurodevelopment (Haijma et al., Reference Haijma, Van Haren, Cahn, Koolschijn, Hulshoff Pol and Kahn2013; Hulshoff Pol et al., Reference Hulshoff Pol, van Baal, Schnack, Brans, van der Schot, Brouwer and Kahn2012; Okada et al., Reference Okada, Fukunaga, Yamashita, Koshiyama, Yamamori, Ohi and Hashimoto2016; van Erp et al., Reference van Erp, Hibar, Rasmussen, Glahn, Pearlson, Andreassen and Turner2016). We found a lower birth weight in the ASP+ subgroup of SZ patients, which might denote that more vulnerable patients present an aberrant development already before experiencing ASP. Additionally, we report that adult height, but not length at birth, was significantly lower in both ASP+ subgroups of HC and SZ, which could indicate an altered developmental trajectory after experiencing ASP. We cannot rule out the possibility that ASP contributes to the smaller ICV and abnormal development commonly reported in SZ, but since smaller ICV and lower adult height was also found in the ASP+ subgroup of HC, differences cannot be explained by genetic risk for disease development, alone.
ASP is one of the most severe forms of OCs (McNeil & Sjöström, Reference McNeil and Sjöström1995), due to the blatant neural harm in offspring. It is well established that ASP causes the neurological sequelae in cerebral palsy (Volpe, Reference Volpe2012). Recurring themes from MRI studies of term newborns with neonatal encephalopathy presumed to be hypoxic-ischemic are the combinations of abnormalities of basal ganglia/thalamus, cerebral cortex, parasagittal (watershed) cortex, white matter and brainstem (Volpe, Reference Volpe2012). In our study, ASP effects were directly related to smaller adult ICV and global brain volumes but only certain regional cortical and subcortical differences. Deficient blood oxygen supply to cortical vascular zones of the three major cerebral vessels might explain the smaller surface areas of the caudal middle frontal, supramarginal and insular regions and to subcortical end zones of short penetrating blood vessels might explain the smaller caudate, putamen and thalamus volumes reported here, making these regions more susceptible to brain structure abnormalities.
We found smaller TBV, white and gray matter volumes in ASP+ subgroups, which are reported in both BD and SZ with larger effect sizes in SZ (Arnone et al., Reference Arnone, Cavanagh, Gerber, Lawrie, Ebmeier and McIntosh2009; de Zwarte et al., Reference de Zwarte, Brouwer, Agartz, Alda, Aleman, Alpert and van Haren2019; Ellison-Wright & Bullmore, Reference Ellison-Wright and Bullmore2010; Haijma et al., Reference Haijma, Van Haren, Cahn, Koolschijn, Hulshoff Pol and Kahn2013; Hibar et al., Reference Hibar, Westlye, Doan, Jahanshad, Cheung, Ching and Andreassen2018; Hulshoff Pol et al., Reference Hulshoff Pol, van Baal, Schnack, Brans, van der Schot, Brouwer and Kahn2012; Kempton, Geddes, Ettinger, Williams, & Grasby, Reference Kempton, Geddes, Ettinger, Williams and Grasby2008; McDonald et al., Reference McDonald, Bullmore, Sham, Chitnis, Wickham, Bramon and Murray2004; van Erp et al., Reference van Erp, Walton, Hibar, Schmaal, Jiang, Glahn and Turner2018). In a large twin study, there was an association between small TBV and genetic risk of developing SZ, wherein lower white matter volume accounted for 94% of significant portions of the phenotypic correlations, and a significant environmental correlation was found for gray matter (van Haren et al., Reference van Haren, Rijsdijk, Schnack, Picchioni, Toulopoulou, Weisbrod and Kahn2012). We found greater differences in gray matter than white matter volumes between the ASP+ and ASP− subgroups. The added effect of ASP might render these subgroups vulnerable to further neurodevelopmental abnormalities that amplify once illness processes have begun, for example, with the putative negative effects of antipsychotic medication on gray matter (Haijma et al., Reference Haijma, Van Haren, Cahn, Koolschijn, Hulshoff Pol and Kahn2013). ASP+ might be an additive risk to a genetic predisposition that operates by impairing both size development (Smeland et al., Reference Smeland, Wang, Frei, Li, Hibar, Franke and Andreassen2018) and resilience of the fetal brain (Murray, Bhavsar, Tripoli, & Howes, Reference Murray, Bhavsar, Tripoli and Howes2017; Nicodemus et al., Reference Nicodemus, Marenco, Batten, Vakkalanka, Egan, Straub and Weinberger2008).
Our finding of ASP being related to total cortical surface area, as opposed to cortical thickness, is consistent with previous OC and brain structure studies in both psychoses (Haukvik et al., Reference Haukvik, Rimol, Roddey, Hartberg, Lange, Vaskinn and Agartz2014b; Smith et al., Reference Smith, Thornton, Lang, MacEwan, Kopala, Su and Honer2015) and healthy groups (De Bie et al., Reference De Bie, Oostrom, Boersma, Veltman, Barkhof, Delemarre-van de Waal and van den Heuvel2011; Dubois et al., Reference Dubois, Benders, Borradori-Tolsa, Cachia, Lazeyras, Ha-Vinh Leuchter and Huppi2008; Neilson et al., Reference Neilson, Shen, Cox, Clarke, Wigmore, Gibson and Lawrie2019; Walhovd et al., Reference Walhovd, Fjell, Brown, Kuperman, Chung and Hagler2012), which together supports cortical surface area measures as the appropriate neuroimaging phenotype related to a neurodevelopmental profile. Our results of regionally smaller surface areas across frontal, parietal, occipital, temporal and insular lobes are also among regions that represent important nodes in large-scale brain networks, which have been associated with cognitive and affective dysfunction in psychiatric and neurological disorders (Menon, Reference Menon2011). The insulae, key nodes of the salience network (SN), play a crucial role in the dynamic interactions and regulations of two other core networks (i.e. central executive network; default mode network) important in human cognition; specifically, cognitive control over external stimuli and internal mental processes (Menon, Reference Menon2011). Impaired SN interactions found in SZ might contribute to psychosis (Supekar, Cai, Krishnadas, Palaniyappan, & Menon, Reference Supekar, Cai, Krishnadas, Palaniyappan and Menon2019).
ASP+ subgroups revealed differences in hippocampal, amygdala, thalamus, caudate and putamen volumes, from seven out of the 16 subcortical comparisons, which are commonly reported different in SZ and BD studies (de Zwarte et al., Reference de Zwarte, Brouwer, Agartz, Alda, Aleman, Alpert and van Haren2019; Haijma et al., Reference Haijma, Van Haren, Cahn, Koolschijn, Hulshoff Pol and Kahn2013; Hibar et al., Reference Hibar, Westlye, van Erp, Rasmussen, Leonardo, Faskowitz and Andreassen2016; Okada et al., Reference Okada, Fukunaga, Yamashita, Koshiyama, Yamamori, Ohi and Hashimoto2016; van Erp et al., Reference van Erp, Walton, Hibar, Schmaal, Jiang, Glahn and Turner2018). Caudate volumes were smaller in both ASP+ patient subgroups, which was not the case for HC. The caudate is of particular interest in SZ pathology because it is highly innervated by dopamine neurons and mediates a range of cognitive, motor and language functions impaired in the disorder (Zampieri, Bellani, Crespo-Facorro, & Brambilla, Reference Zampieri, Bellani, Crespo-Facorro and Brambilla2014). Specifically, hemispheric specialization of the caudate nucleus and cortical regions with connections to the caudate nucleus was reported to be significantly altered in SZ with disrupted hemispheric coordination (Mueller, Wang, Pan, Holt, & Liu, Reference Mueller, Wang, Pan, Holt and Liu2015). This finding suggests that the caudate is central in how efficient the brain is in organizing function to specific hemispheres, which starts early in development (Chi, Dooling, & Gilles, Reference Chi, Dooling and Gilles1977; Sun et al., Reference Sun, Patoine, Abu-Khalil, Visvader, Sum, Cherry and Walsh2005).
We did not observe differences in the number of patients taking lithium, antipsychotic medication or dose equivalents between the ASP patient subgroups. Even though uncertainty exists about what causes brain structure alterations associated with SZ and BD, we cannot rule out an influence of medication; especially, given that previous research has shown that lithium and antipsychotic medications enlarge thalamic (Haijma et al., Reference Haijma, Van Haren, Cahn, Koolschijn, Hulshoff Pol and Kahn2013; Hibar et al., Reference Hibar, Westlye, van Erp, Rasmussen, Leonardo, Faskowitz and Andreassen2016) and basal ganglia volumes (caudate, putamen and pallidum) (Chakos, Lieberman, Alvir, Bilder, & Ashtari, Reference Chakos, Lieberman, Alvir, Bilder and Ashtari1995; Di Sero et al., Reference Di Sero, Jorgensen, Nerland, Melle, Andreassen, Jovicich and Agartz2019; Haijma et al., Reference Haijma, Van Haren, Cahn, Koolschijn, Hulshoff Pol and Kahn2013). Taken together, our data suggest that smaller subcortical volumes in ASP+ patients are present early in development and persist with later use of medication. This might be consistent with the notion that smaller basal ganglia volumes could be a marker of medication non-response, a possibility which was suggested by some studies (Buchsbaum et al., Reference Buchsbaum, Shihabuddin, Brickman, Miozzo, Prikryl, Shaw and Davis2003; Di Sero et al., Reference Di Sero, Jorgensen, Nerland, Melle, Andreassen, Jovicich and Agartz2019; Li et al., Reference Li, Chen, Deng, He, Wang, Jiang and Li2012).
The use of the Medical Birth Registry of Norway is a major strength of this study, which allows for a precise and prospective assessment of OCs in individuals who later develop psychiatric disorders. Our study shows clinical evidence of early life factors and their possible impact on the developing brains of adult participants, including HC, which is currently missing in the field. Including severe OCs as a component of developmental risk may advance our ability to make prognoses in psychiatric disorders, which might complement genetic risk.
Our findings should be considered in the context of limitations to the exclusion criteria of the TOP study, which precluded the recruitment of HC if they had a first-degree relative with a lifetime history of severe psychiatric disorder. If more genetically varied HC had been included in the study, it is possible that even more pronounced differences in brain structure would have been evident, especially ICV. The BD group had half the sample size of the HC and SZ groups, but they had a higher rate of ASP compared to the SZ group. This disproportion of ASP+ BD patients to the ASP+ SZ patients might have contributed to the greater effects found in caudate volumes.
Conclusions
Our study raises the question of the role of early-life risk factors in the etiology of brain development in BD and SZ. When we examined the differences in brain structure between ASP subgroups of participants, we found that the greatest disparity in measures was between ASP− and ASP+ patient subgroups, and that similar differences were present in HC. This suggests that ASP+ have contributed to smaller brain structures in all groups, which is a finding not easily explained by genetic loading for psychiatric disorders alone. Specifically, our findings give support for the ICV as a marker of aberrant neurodevelopment and the caudate nuclei as a marker for disease development. A better understanding of ASP as a context to brain development could advance the role of perinatal care for reducing the burden of severe mental illness. New strategies for neuroprotective therapies during pregnancy and birth, particularly in women with or genetic risk for mental illness, can be a major form of primary prevention of schizophrenia.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720002779
Acknowledgements
We appreciate the support from the Research Council of Norway (grant number 223273) and the South-Eastern Norway Regional Health Authority (grant number 2017–093). We thank the participants of the TOP study for their time and effort used this study. We express gratitude to the clinicians and research assistants at NORMENT for this extensive data collection and a special thanks to all referring hospital units. We appreciate the help of Thomas McNeil for valuable discussions and free use of the McNeil-Sjöström Scale. We also thank Stener Nerland for data extraction and Vera Lonning for valuable discussions in planning this study.
Conflict of interest
The authors report no financial relationships with commercial interest, other than Dr Andreassen, who received speaker's honoraria from Lundbeck.








