Introduction
The in vitro production (IVP) system of porcine embryos is an important technique for production of transgenic pigs used as human disease and xenograft models (Jeon et al., Reference Jeon, Yoon, Cai, Hwang, Kim, Zheng, Lee, Kim and Hyun2014). Because embryo development and fertility are influenced by oocyte maturation state, in vitro maturation (IVM) is the most important step in IVP for the successful production of embryos. However, during IVM, oocytes are exposed to in vitro environmental conditions such as light (Bedford and Dobrenis, Reference Bedford and Dobrenis1989), high oxygen concentration (Kitagawa et al., Reference Kitagawa, Suzuki, Yoneda and Watanabe2004), risk of contamination (Sutton et al., Reference Sutton, Gilchrist and Thompson2003) and handling (Fleming et al., Reference Fleming, Evans, Walton and Armstrong1985), which may result in a reduction in the maturation rate and developmental competence of in vitro matured oocytes. Therefore, many studies have been conducted in the attempt to improve the low efficiency of oocyte IVM (Im et al., Reference Im, Lai, Liu, Hao, Wax, Bonk and Prather2004; Iwamoto et al., Reference Iwamoto, Onishi, Fuchimoto, Somfai, Takeda, Tagami, Hanada, Noguchi, Kaneko, Nagai and Kikuchi2005; Yoshioka, Reference Yoshioka2011), however the quality of in vitro matured oocytes is still lower than in vivo matured oocytes.
Maturation mediums used in IVM of mammalian oocytes are optimized to culture a variety of cell types and contain different amino acids, inorganic salts, vitamins and other components. Generally, growth factors and hormones are supplied in the culture medium to stimulate cytoplasmic and nuclear maturation of oocytes (Guler et al., Reference Guler, Poulin, Mermillod, Terqui and Cognié2000), however these substances are insufficient for efficient oocyte maturation in the in vitro environment. To improve the low efficiency of IVM, follicular fluid (FF), which supplies nutrients, essential amino acids, and other unknown factors and thereby supports oocyte growth in follicles, is commonly added to the culture medium. Naito et al. (Reference Naito, Fukuda and Toyoda1988) reported that porcine FF (pFF) had a stimulatory effect on male pronucleus formation and promoted cytoplasmic maturation in porcine oocytes. Furthermore, cumulus expansion and nuclear maturation compared with non-treatment groups during IVM were increased by supplementation with pFF (Algriany et al., Reference Algriany, Bevers, Schoevers, Colenbrander and Dieleman2004) and mitochondrial DNA copy numbers were reduced (Mao et al., Reference Mao, Whitworth, Spate, Walters, Zhao and Prather2012).
Oocyte growth in follicles is closely associated with FF at different stages in the oestrous cycle. During follicular development, FF composition changes and influences oocyte growth, maturation state, developmental competence and cumulus expansion (Gérard et al., Reference Gérard, Loiseau, Duchamp and Seguin2002). Generally, porcine oocytes from 3–6 mm size follicles are used for IVP of embryos, because oocytes from smaller-sized follicles have lower meiotic and developmental competence. Marchal et al. (Reference Marchal, Vigneron, Perreau, Bali-Papp and Mermillod2002) reported that oocytes from follicles <3 mm in diameter developed low numbers at metaphase-II stage, and had lower penetration rate and reduced blastocyst formation compared with oocytes collected from 3–6 mm diameter follicles. In addition, porcine oocytes harvested from <3.1 mm diameter follicles gave a lower proportion of oocytes with normal ooplasm morphology and had more than three cumulus layers compared with oocytes collected from 3.1–8.0 mm follicles (Yoon et al., Reference Yoon, Shin, Park, Roh, Lim, Lee, Hwang and Lee2000). Porcine oocytes from follicles >8 mm in diameter matured after 18 h IVM showed complete oocyte maturation and greater developmental competence compared with oocytes collected from 3–7 mm follicles and in vitro matured for 44 h (Kwak et al., Reference Kwak, Yoon, Cheong, Jeon, Lee and Hyun2014).
In addition, the effect of follicle size on oocyte maturation and developmental competence of supplementation with FF derived from follicles of different sizes during IVM differently affected the quality of in vitro matured oocytes and their subsequent development. Meiotic maturation, cumulus expansion and embryo development were enhanced by treatment with pFF isolated from 5–8 mm follicles compared with pFF collected from 2–4 mm follicles, whereas pFF from 2–4 mm follicles did not influence oocyte maturation and subsequent development (Algriany et al., Reference Algriany, Bevers, Schoevers, Colenbrander and Dieleman2004). Similarly, blastocyst formation in oocytes collected from follicles <3 mm was lower than oocytes collected from follicles between 3.1–8 mm after supplementation of both groups with pFF (Yoon et al., Reference Yoon, Shin, Park, Roh, Lim, Lee, Hwang and Lee2000). Despite the change in quality of in vitro matured oocytes following treatment with pFF derived from follicles of different sizes, the effect of pFF from follicles >8 mm in diameter on IVM of porcine oocytes has not yet been reported. We hypothesized that treatment with pFF collected from large-sized follicles (>8 mm) during IVM would be more beneficial compared with conventional treatment with pFF aspirated from medium-sized follicles (3–6 mm). Therefore, the aim of present study was to compare the effects of pFF from large- and medium-sized follicles on nuclear maturation, cumulus expansion and intracellular glutathione (GSH) and reactive oxygen species (ROS) levels, and on the subsequent development of porcine oocytes.
Materials and methods
Materials
Medium-199, CellTrackerTM Red, and 2′,7′-dichlorodihydrofluorescein diacetate (Carboxy-DCFDA) were purchased from Invitrogen (Invitrogen, Carlsbad, CA, USA). Luteinizing hormone (LH), follicle-stimulating hormone (FSH), epidermal growth factor (EGF), bovine serum albumin (BSA), and bisbenzimide H 33342 trihydrochloride (Hoechst 33342) were obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA).
Preparation of pFF
Ovaries were classified morphologically as medium- or large-sized follicles based on diameter (medium, 3–6 mm; large, >8 mm). A syringe attached to an 18-gauge needle was used to aspirate pFF from follicles of different sizes. The collected pFFs from medium- and large-sized follicles were named MFF and LFF, respectively. To remove cellular debris, the collected pFF was centrifuged three times at 1660 g for 45 min at 4°C, then filtered using a syringe filter (0.45-μm pore size), and stored at −20°C until use.
In vitro maturation
Ovaries were collected from pre-pubertal gilts in a local slaughterhouse and transported to the laboratory within 2 h in 0.9% (w/v) NaCl saline at 32–35°C. An 18-gauge needle connected to a 10-ml syringe was used to aspirate cumulus–oocyte complexes (COCs) from antral follicles (3–6 mm in diameter). Immature COCs with up to three layers of cumulus cells and compact, homogeneous cytoplasm were selected under a stereo-microscope, and washed in medium-199 supplemented with 10 ng/ml EGF, 10 µg/ml LH, 0.5 µg/ml FSH, 10 IU/ml human chorionic gonadotropin (hCG) and 10% (v/v) pFF. Then, 50–55 COCs were matured in 4-well plates with 650 μl medium-199 containing hormones, EGF and 10% (v/v) MFF or LFF for 22 h at 38.5°C and 5% CO2 in air conditions (IVM-I), then subsequently maturated in hormone-free medium-199 containing 10% MFF or LFF with 10 ng/ml EGF for 22 h (IVM-II).
Assessment of cumulus expansion
To evaluate the effect of pFF from differently sized follicles on cumulus expansion, 30 COCs in each group were used to assess cumulus expansion after the IVM-1 procedure. ImageJ software (v.1.46; National Institutes of Health, Bethesda, MD, USA) was used to measure the area of each COCs, and all cumulus expansion data were normalized to the MFF treatment group.
Measurement of intracellular GSH and ROS levels
After IVM-I and IVM-II procedures, 15 mature COCs from each group were selected at random for measurement of intracellular GSH and ROS levels. Cumulus cells were denuded by gently pipetting in maturation medium containing 0.1% (w/v) hyaluronidase and denuded oocytes were fixed in 4% (v/v) paraformaldehyde solution at room temperature for 7 min, then stained with 5 μM CellTracker™ Red or 20 μM carboxy-DCFDA for 30 min in a dark room. Stained oocytes were washed three times with PBS-PVA and observed under an epifluorescence microscope with an optical filter (GSH, excitation: 510–560 nm, emission: 590 nm; ROS, excitation: 450–490 nm, emission: 520 nm). Multi Gauge 3.0 software (Fuji photo film Co, Ltd, Japan) was used to measure the band intensities of GSH and ROS.
Assessment of nuclear maturation
After the IVM-II procedure, cumulus cells around mature COCs were removed by gently pipetting in IVM medium containing 0.1% hyaluronidase. The denuded oocytes were mounted onto slides and fixed in acetic alcohol solution (acetic acid:ethanol, 1:3 v/v) for 48 h at room temperature, then stained with 1% (w/v) aceto-orcein solution at room temperature for 7 min and examined under a light microscope. Oocytes at the germinal vesicle, germinal vesicle breakdown, metaphase-I or telophase-I stages were classed as immature, and metaphase-II stage oocytes were classified as mature.
In vitro fertilization
To weaken the adhesion between cumulus cells, mature COCs were placed for 5 min in IVM medium containing 0.1% (w/v) hyaluronidase, then 15–20 COCs were transferred to a 50-μl droplet of modified Tris-buffered medium (mTBM) supplemented with 0.4% (w/v) BSA without caffeine. Fresh boar semen was washed twice using Modena B and resuspended with mTBM containing 0.2% (w/v) BSA and caffeine to a final concentration of 6 × 105 spermatozoa/ml; 50 μl sperm suspension was added to a droplet of COCs, which was co-incubated at 38.5°C in 5% CO2 in air for 6 h.
In vitro development and assessment of embryo development
After 6 h of fertilization, spermatozoa and cumulus cells binding to the zona pellucida were removed by gentle pipetting. Putative zygotes were placed in 4-well plates containing 650 μl porcine zygote medium-3 (PZM-3) supplemented with 0.3% (w/v) BSA and were cultured for 48 h at 38.5°C in 5% CO2 in air. The culture medium was then exchanged for fresh medium and putative zygotes were incubated for 5 days. At 7 days after fertilization, embryo development was evaluated and the number of cells in expanded blastocysts was determined. Blastocysts were fixed in 4% (v/v) paraformaldehyde solution for 15 min, then stained with 1 ng/ml Hoechst 33342, and observed under an epifluorescence microscope.
Experimental designs
To investigate the effect of LFF during maturation on oocyte maturation and developmental competence, MFF or LFF was added during the IVM-I and IVM-II procedures, respectively. We assessed cumulus expansion, intracellular GSH and ROS levels, nuclear maturation and embryo development. Cumulus expansion and GSH and ROS levels were measured as maturation parameters after IVM-I; intracellular GSH and ROS levels and nuclear maturation were measured after IVM-II, and cleavage rate after 48 h in vitro culture, whereas blastocyst formation and total number of cells in blastocysts were evaluated at 7 days after fertilization.
Statistical analysis
Data were presented as the mean ± standard error of the mean (SEM) and Statistical Analysis System Software (SAS, v.9.3) was used to analyze all numerical data representing each parameter. All percentage values were transformed into arcsine to obtain a normal distribution. Duncan’s multiple range test was used to assess relative cumulus expansion, GSH and ROS levels, nuclear maturation and embryonic development, and a generalized linear model (GLM) was used to compare parameters. A P-value < 0.05 was considered to indicate a statistically significant difference.
Results
Effect of FF on cumulus expansion and GSH and ROS levels after IVM-I
Treatment of LFF during IVM-I significantly affected cumulus expansion and intracellular GSH levels in porcine oocytes (Table 1). The relative expansion rates of cumulus cells were higher in the LFF-treated groups compared with in the MFF-treated groups (P < 0.05). Intracellular GSH levels were increased by LFF treatment compared with MFF treatment (P < 0.05), however there were no significant differences in ROS levels between the MFF and LFF groups.
Table 1. Effects of porcine follicular fluid from large follicles (>8 mm in diameter) on cumulus expansion and intracellular glutathione (GSH) and reactive oxygen species (ROS) levels at 22 h after in vitro maturation of porcine oocytes

* Data are presented as the mean ± standard error of the mean (SEM) of three replicates.
† MFF : porcine follicular fluids from follicles 3–6 mm in diameter.
‡ LFF : porcine follicular fluids from follicles >8 mm in diameter.
a,b Superscript letters indicate significant differences (P < 0.05) in the same column.
Effect of LFF on nuclear maturation and GSH and ROS levels after IVM-II
Regarding meiotic maturation, intracellular GSH and ROS levels in porcine oocytes were influenced by LFF treatment during IVM-I and/or IVM-II (Table 2). Regardless of FF treatment during IVM-II, LFF treatment during IVM-I enhanced the nuclear maturation of oocytes relative to the treatment group matured with MFF at both the IVM-I and IVM-II stages (P < 0.05). However, LFF treatment during IVM-II did not affect meiotic maturation in oocytes that were incubated with MFF for the IVM-I step. Intracellular GSH levels were significantly higher in all LFF treatment groups compared with MFF groups and at both stages of IVM (P < 0.05). ROS levels were reduced in all LFF treatment groups compared with MFF groups (P < 0.05). In particular, ROS levels were lower in the LFF treatment groups during IVM-I compared with MFF treatment groups, regardless of IVM-II treatment.
Table 2. Effects of porcine follicular fluid from large follicles (>8 mm in diameter) on nuclear maturation and intracellular glutathione (GSH) and reactive oxygen species (ROS) levels at 44 h after in vitro maturation of porcine oocytes
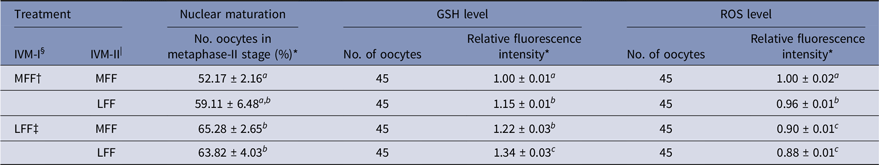
* Data are presented as the mean ± standard error of the mean (SEM) of three replicates.
§ IVM-I: in vitro maturation at 22 h after incubation.
| IVM-II: in vitro maturation at 44 h after incubation.
† MFF: porcine follicular fluids from follicles 3–6 mm in diameter.
‡ LFF: porcine follicular fluids from follicles >8 mm in diameter.
a–c Superscript letters indicate significant differences (P < 0.05) in the same column.
Effect of LFF during IVM on embryonic development
Treatment of LFF during IVM-I or IVM-II enhanced developmental competence in porcine embryos (Table 3). Both blastocyst formation and the number of cells in blastocysts were increased by LFF treatment, regardless of IVM stage (P < 0.05). However, zygote cleavage rates were not influenced by LFF treatment.
Table 3. Effects of porcine follicular fluid from large follicles (>8 mm in diameter) on embryonic development and total number of cells in blastocysts of in vitro matured oocytes during in vitro maturation
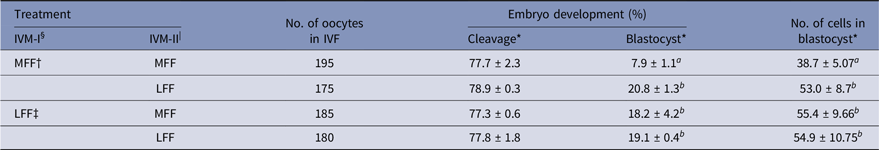
* Data are presented as the mean ± standard error of the mean (SEM) of three replicates.
§ IVM-I: In vitro maturation at 22 h after incubation.
| IVM-II: In vitro maturation at 44 h after incubation.
† MFF : porcine follicular fluids from follicles 3–6 mm in diameter.
‡ LFF : porcine follicular fluids from follicles >8 mm in diameter.
a,b Superscript letters indicate significant differences (P < 0.05) in the same column.
Discussion
Follicular fluid is an important supplement during IVM of porcine oocytes, used to improve the low efficiency of meiotic and cytoplasmic maturation. Various media have been developed for culture of cells in in vitro environments without body fluids. However, cultured cells still require supplements derived from body fluids such as serum, uterine and oviductal fluid and FF that contain undefined factors necessary for cell growth, differentiation, maturation and proliferation. Complete maturation of oocytes is the most important process for successful fertilization and subsequent embryonic development. Therefore, FF is usually added to maturation medium for improvement of maturation efficiency during IVM of mammalian oocytes.
During follicle development, the biochemical composition of FF, such as ions, metabolites, hormones and enzymes, changes. Numerous factors are increased or decreased in accordance with follicle size and these could be used as biochemical markers for assessment of oocyte quality (Revelli et al., Reference Revelli, Piane, Casano, Molinari, Massobrio and Rinaudo2009). Different components in FF, including oestradiol, insulin-like growth factor-1 (IGF-1), activin and inhibin, act as regulators of FSH responsiveness (Ginther et al., Reference Ginther, Beg, Bergfelt, Donadeu and Kot2001). Administration of 17β-estradiol to ovariectomized rats has been shown to enhance the responsiveness of FSH (Cooper et al., Reference Cooper, Fawcett and McCann1974). Expression of FSH receptors was increased by oestradiol treatment of granulosa cells of hypophysectomized rats, whereas expression of LH receptors was decreased. However, LH receptor expression was increased by the administration of FSH after pre-treatment with oestradiol (Richards et al., Reference Richards, Ireland, Rao, Bernath, Midgley and Reichert1976). This difference suggests that oestrogen in follicles enhanced FSH responsiveness and gonadotropin activity by increasing the expression of their specific receptors.
In the present study, relative cumulus expansion rate and intracellular GSH level were increased by LFF treatment during the first 22 h of maturation. Gonadotropins are closely associated with maturation parameters, including cumulus expansion and nuclear and cytoplasmic maturation. Interestingly, the effects of FSH on oocyte maturation and fertilization were enhanced in the presence of FF. In porcine COCs, when maturation medium was supplemented with FSH and FF from follicles of various sizes, cumulus expansion and male pronucleus formation were increased compared with FSH only supplementation. In contrast, embryo development in larger follicle (3–5 mm in diameter) FF groups was higher compared with that in smaller follicle (2–4 mm in diameter) FF groups (Algriany et al., Reference Algriany, Bevers, Schoevers, Colenbrander and Dieleman2004). Oestradiol concentration was higher in bovine FF from the largest follicles compared with the second- and third-largest follicles and increased in accordance with follicle diameter (Beg et al., Reference Beg, Bergfelt, Kot and Ginther2002). These results suggested that increased oestradiol concentration in LFF could enhance the stimulatory effect of FSH present in IVM medium and influence cumulus expansion in porcine COCs.
During cytoplasmic maturation of oocytes, intracellular GSH levels play an important role in protecting the cells from oxidative stress and are closely associated with cumulus cells surrounding the oocytes. These cumulus cells are reported to interact with oocytes through gap junctions and regulate the synthesis and accumulation of GSH in ooplasm. Yamauchi and Nagai (Reference Yamauchi and Nagai1999) reported that GSH content in porcine oocytes after IVM was lower in denuded oocytes compared with COCs, and that lower GSH levels negatively affected fertilization parameters. Similarly, GSH concentration in partially denuded oocytes was higher compared with completely denuded oocytes groups after 44 h IVM, while it was lower compared with concentrations in COC groups (Maedomari et al., Reference Maedomari, Kikuchi, Ozawa, Noguchi, Kaneko, Ohnuma, Nakai, Shino, Nagai and Kashiwazaki2007). In addition, treatment with heptanol, an inhibitor of gap junctional coupling between cumulus cells and the oocytes, reduced the amount of GSH in porcine oocytes after maturation (Mori et al., Reference Mori, Amano and Shimizu2000). Therefore, LFF treatment of porcine oocytes during IVM could increase intracellular GSH levels via enhancement of cumulus expansion.
Meiotic maturation of mammalian oocytes is important for fertilization and developmental competence, and is influenced by various factors including oxidative stress, gonadotropins and growth factors. In pigs, the presence of FSH during maturation increased the proportion of mature oocytes that reach metaphase-II (Bing et al., Reference Bing, Nagai and Rodrìguez-Martinez2001). Treatment with FSH and LH during IVM also enhanced cortical granule migration and blastocyst formation in porcine COCs (Sun et al., Reference Sun, Lai, Bonk, Prather and Schatten2001). The present study showed that the proportion of oocytes at metaphase-II stage was significantly higher in the LFF treatment groups during IVM-I compared with the MFF treatment groups at both stages of IVM. Meiotic maturation was promoted by FSH supplied to IVM-I medium. In a previous study by Algriany et al. (Reference Algriany, Bevers, Schoevers, Colenbrander and Dieleman2004), nuclear maturation of porcine oocytes was increased by FSH, and supplementation of FF with FSH enhanced meiotic maturation compared with treatment with FSH alone. These results demonstrated that supplementation of LFF during IVM-I stimulated meiotic maturation more effectively compared with MFF treatment, and that this effect may be related to an increase in FSH responsiveness due to FF.
The state of oxidative stress in mammalian oocytes is a fundamental parameter for assessment of oocyte quality and its further development. Because porcine oocytes have a higher lipid content compared with those of other species, they are particularly sensitive to ROS-induced damage responsible for DNA fragmentation, apoptosis and mitochondrial damage (Kowaltowski and Vercesi, Reference Kowaltowski and Vercesi1999; Fujihira et al., Reference Fujihira, Kishida and Fukui2004; Kitagawa et al., Reference Kitagawa, Suzuki, Yoneda and Watanabe2004; Sovernigo et al., Reference Sovernigo, Adona, Monzani, Guemra, Barros, Lopes and Leal2017). Therefore a reduction in oxidative stress during the IVM stage of porcine oocytes is important to improve maturation and further embryonic development. Various supplements, including vitamins, hormones and chemical elements, may be added to maturation medium to prevent oxidative stress (Kere et al., Reference Kere, Siriboon, Lo, Nguyen and Ju2013; Jeon et al., Reference Jeon, Yoon, Cai, Hwang, Kim, Zheng, Lee, Kim and Hyun2014; Li et al., Reference Li, Zhang, He, Zhu, Xu, Ma, Tao and Liu2015). In the present study, as found previously for oocyte nuclear maturation and GSH content, blastocyst, blastocyst formation and the number of cells in blastocysts were increased in both LFF groups and this corresponded to decreased intracellular ROS levels. During follicle development in cows, catalase activity and ROS levels decreased, whereas total antioxidant capacity was enhanced (Gupta et al., Reference Gupta, Choi, Yu, Czerniak, Holick, Paolella, Agarwal and Combelles2011). Antioxidant properties of FF differently affected oxidative stress in oocytes during IVM. In cows, bovine FF (bFF) from follicles of 9–13 mm diameter enhanced GSH levels in oocytes compared with bFF from follicles of 3–8 mm diameter (Park et al., Reference Park, Cho and Yu2014). Basini et al. (Reference Basini, Simona, Santini and Grasselli2008) reported that the concentrations of hydrogen peroxide and hydroperoxides were reduced in swine FFs during follicle development, however the activity of enzymatic and non-enzymatic ROS scavengers decreased. These studies demonstrated that pFF contains various antioxidants, such as superoxide dismutase, catalase, glutathione peroxidase and other non-enzymatic antioxidants.
In the present study, we found that LFF treatment during IVM-I of porcine oocytes enhanced cumulus expansion and GSH levels. Regardless of IVM stage, nuclear maturation, GSH levels and blastocyst formation were also increased by LFF treatment, whereas intracellular ROS was reduced. These results showed that supplementation of pFF from large-sized follicles could improve oocyte maturation and developmental competence in pigs through reducing oxidative stress and enhancing responsiveness to gonadotropins.
Financial support
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Education) (2019R1A2C1004307).
Conflict of interest
None
Ethical standards
All animal experimentation in this study was approved by the Kangwon National University Institutional Animal Care and Use Committee (KIACUC-09–0139).





