INTRODUCTION
About 25·8 million HIV-seropositive individuals live in sub-Saharan Africa, representing two thirds of the world's total HIV burden [1]. HIV and other sexually transmitted infections (STIs) are the second most common cause of healthy life years lost in women aged 15–44 years in Africa, and account for 17% of the total disease burden in this population [2]. Despite the notable endemicity of HIV in sub-Saharan Africa, HIV seroprevalence in pregnant women differs markedly across this region, ranging from a low of 1% in Senegal and Mauritania to a high of 39% in Swaziland [Reference Asamoah-Odei, Garcia Calleja and Boerma3].
Time trends of HIV seropositivity in pregnant women have been used to monitor the HIV epidemic at the population level [Reference Zaba4]. Although little data are available on HIV seroprevalence in many Central African countries, HIV seroprevalence data in the Democratic Republic of the Congo (DRC) have been documented since 1984 by the National AIDS Control Programme and Project SIDA, the first international HIV/AIDS programme in sub-Saharan Africa. Taken together, HIV prevalence in pregnant women in the DRC has consistently remained at <10% [Reference Batter5–Reference Buvé, Bishikwabo-Nsarhaza and Mutangadura8]. Most recently, HIV prevalence in pregnant women in the capital city Kinshasa has been stable, no more than 5% between 2000–2004 [9–12]. Data in 2002 for 1267 pregnant women from four major cities in the DRC reported a HIV-1 seropositivity of 3·5% [Reference Mulanga11]. Official sentinel survey data for 2003–2004 showed that the HIV-1 seroprevalence in pregnant women in the DRC was 4·5% [12].
In this report, we document the prevalence and associated risk factors of HIV infection and selected STIs in a large representative sample of pregnant women from two major maternity clinics in Kinshasa in 2004.
METHODS
Between April and July 2004, 2138 consecutive antenatal clinic attendees at the Kingasani and Binza maternity clinics in Kinshasa, DRC were invited to participate in a prevalence survey of HIV and selected STIs in order to plan future studies of infection control in pregnant women. Inclusion criteria for participation were: >15 years of age, agreement to participate in HIV testing, and receipt of no previous antenatal care (ANC) during their current pregnancy. A total of 2134 women (99·8%) were eligible and 33 women (1·6%) declined to participate. Informed consent was obtained from each participant, including parental consent from participating minors. All 2101 consenting participants underwent HIV testing, provided a 5 ml blood specimen, and were individually interviewed by trained female nurses on sociodemographic information, previous pregnancy history, and sexual behaviour. In this study, for history of previous birth outcomes, we defined neonatal, infant, and child deaths as deaths <1 month of birth, deaths between 1 month and 1 year of birth, and deaths >1 year of birth, respectively. Limited resources enabled the provision of STI screening for only the first consenting 529 women. Among these women, vaginal swab samples (Starswab II with liquid Stuart's medium, Starplex Scientific, Entobicoke, ON, Canada) were successfully collected by trained female nurses from 524 women at the time of a speculum pelvic examination.
Blood samples were transported on a daily basis to the National AIDS Reference Laboratory in Kinshasa where plasma was separated and then frozen at −20°C. The frozen blood plasma samples were tested for HIV and syphilis within 48 h. HIV-1/-2 serostatus was first tested using two different enzyme-linked immunosorbent assays (ELISAs): Vironostika® HIV Uni-Form II Plus 0 (bioMérieux, Boxtel, The Netherlands) and Enzygnost® Anti-HIV ½ Plus (Dade Behring, Marburg, Germany). If both tests were positive, HIV seropositivity was confirmed with a rapid test assay (Determine HIV-1/-2; Abbott Laboratory, Tokyo, Japan). All women with two positive ELISAs and a positive rapid test result were classified as being HIV positive. Eleven women with indeterminate HIV results (0·5%) and eight with missing samples were excluded from data analyses, leaving a total of 2082 women.
Using residual blood specimens, syphilis seropositivity was determined by a rapid plasma reagin test (RPR: Human GmbH, Wiesbaden, Germany) and a Treponema pallidum haemagglutination assay (TPHA: Serodia®-TP-PA; Fujirebio Inc., Tokyo, Japan) with 525 women. Samples testing positive for RPR or TPHA and 2% of randomly selected negative samples were shipped to the laboratory at the University of North Carolina at Chapel Hill (UNC-CH) for quality control.
Vaginal swab samples were tested for Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) DNA using an amplicor polymerase chain reaction-based assay (PCR, Roche Diagnostics, Indianapolis, IN, USA) at the UNC-CH microbiology laboratory. All positive samples were systematically re-tested for confirmatory purposes. To monitor the adequacy of specimen storage and transport conditions, a vial of Accurun 341 external positive control (BBI Diagnostics, West Bridgewater, MA, USA) containing CT and NG intact organisms was stored and included with each batch of specimens shipped from Kinshasa to UNC-CH. All vials tested positive in the Amplicor CT/NG assay, confirming that specimen storage and transport conditions had been adequate.
To identify risk factors for HIV seropositivity, odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated using multivariate logistic regression adjusting for maternal age [Reference Sagay13, Reference Buvé14] and site of ANC. For sparse data (cell counts with sample size ⩽5), exact logistic regression was performed using LogXact 4 for Windows (Cytel Software Corporation, Cambridge, MA, USA).
The protocol was approved by the Institutional Review Boards at UNC-CH, RTI International, and the Kinshasa School of Public Health.
RESULTS
We detected a HIV prevalence rate of 1·9% (95% CI 1·3–2·5) in the 2082 participating pregnant women whose mean age was 27·3 years (range 15–45 years) (Table 1). HIV seropositivity was low in women from both antenatal clinics (1·6% in Kingasani and 2·2% in Binza, P=0·33), and in the first 529 women who consented to have STI testing (1·5%) (data not shown). No active syphilis case was detected in 525 women. Three samples (0·6%) were RPR positive but TPHA negative, suggesting false-positive results. External quality control of the syphilis test performed at the UNC-CH laboratory confirmed these results. CT and NG infection prevalence rates were 1·7% and 0·4%, respectively in 521 women. None of the patients were found to have more than one STI.
Table 1. Prevalence of HIV and selected sexually transmitted infections in female antenatal clinic attendees at two maternity clinics in Kinshasa, Democratic Republic of the Congo
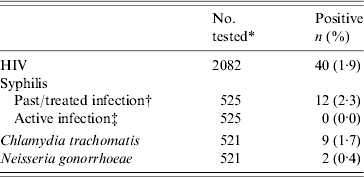
* Numbers may differ due to missing samples and refusals.
† Defined as Treponema pallidum haemagglutination assay (TPHA) positive and rapid plasma reagin test (RPR) negative.
‡ Defined as TPHA and RPR positive.
No significant association was found between women's age and HIV prevalence (Table 2). Women aged between 25 and 29 years had a slightly higher HIV risk, with the lowest prevalence in the youngest and the oldest women, although these differences were not statistically significant. A woman's HIV risk was significantly associated with her reporting a male partner as having had other female sexual partners (OR 2·7, 95% CI 1·2–6·2), although only a small proportion of women (10·4%) reported this behaviour. A pregnant woman's reported history of previous neonatal deaths was not related to her risk of HIV infection; however, a history of previous infant (OR 2·5, 95% CI 1·1–5·6) and child (OR 2·7, 95% CI 1·2–5·9) deaths were positively associated with HIV infection. When the statistically significant factors were modelled simultaneously, a woman's report of a male partner having ever had other female sexual partner(s), infant deaths, and child deaths remained significant.
Table 2. Risk factors for HIV infection in 2082 female antenatal clinic attendees at two maternity clinics in Kinshasa, Democratic Republic of the Congo

CI, Confidence interval; OR, odds ratio.
* Adjusted for age and site of antenatal clinic.
† Reference category.
‡ P<0·05.
§ Data analysed by exact logistic regression.
ǁ Standard logistic regression result.
DISCUSSION
This study confirms the continuing paradoxically low HIV seroprevalence in Kinshasa, DRC, a location where many of the factors found to enable HIV infection in other regions of sub-Saharan Africa are highly prevalent [Reference Mulanga-Kabeya7, Reference Buvé, Bishikwabo-Nsarhaza and Mutangadura8]. These enablers include political and social instability, widespread poverty, long-lasting conflicts in the Eastern parts of the country, commercial sex and lack of access to effective testing and treatment of STIs. Despite these highly prevalent enabling conditions, the persistently stable and low HIV prevalence rates remain a striking and unexplained phenomenon.
The HIV prevalence rate in our study participants (1·9%) was consistent with the results from the Global AIDS Programme in Kinshasa that documented a 1·9% HIV-1 seroprevalence in 38 603 pregnant women surveyed at 21 maternity clinics (excluding Kingasani and Binza clinics) from 2004 to July 2005 (F. Behets, personal communication). The cumulative HIV prevalence at the Kingasani and Binza maternity clinics from 2003 to 2005 was 2·4% among 37 988 pregnant women (R. Matendo, personal communication). Similarly low HIV seroprevalence rates were reported in pregnant women in neighbouring countries in 2001–2002 including Angola (2%) and the Republic of the Congo (4%) [Reference Asamoah-Odei, Garcia Calleja and Boerma3], although high HIV prevalence rates have been reported in nearby Zambia (23·5%) [10] and the Central African Republic (CAR; 15%) [Reference Asamoah-Odei, Garcia Calleja and Boerma3].
Our study provides new data on the burden of selected STIs (CT and NG) in pregnant women from the DRC using sensitive PCR-based laboratory testing. Antenatal attendees from the two largest maternity clinics in Kinshasa had a low prevalence of both infections (1·7% and 0·4%, respectively). A previous study in Kinshasa [Reference Vuylsteke15] found a higher prevalence of CT (5·2%) and NG (1·6%) infections in 1160 pregnant women using an enzyme immunoassay and cultured endocervical smears, respectively. The low prevalence of cervical infections found in our present study appears to be real, given the higher sensitivity of the PCR-based laboratory assay used. In our study, no active syphilis was found and only 0·6% of women screened tested RPR positive, TPHA negative. These later results are consistent with the low syphilis prevalence rate of 0·43% by RPR only found in 1619 female antenatal clinic attendees from three maternity clinics in Kinshasa in 2003 [16].
A woman's risk of HIV infection was significantly associated with her reporting a male partner as having had other female sexual partners. These data are consistent with population-based studies from Nigeria showing a higher risk of STIs in women reporting a partner with higher risk sexual behaviour [Reference Sagay13, Reference Thomas17]. Given the low HIV seroprevalence reported here, sexual network analyses documenting the extent of partner change and of concurrent sexual partnerships in this population may provide further insights [Reference Lagarde18].
Civil war, political instability, and widespread poverty in the DRC could have been expected to increase the vulnerability of the population to HIV and other STI infections, yet the prevalences of HIV and selected STIs reported here were low. HIV acquisition and transmission can be facilitated by other STI infections through multiple immunological and biological pathways [Reference Cohen19–Reference Cohen21]. The low prevalence of selected STIs found here may provide one potential explanation for the stable, low HIV seroprevalence in the DRC relative to other sub-Saharan African countries. Behavioural factors including a high prevalence of male circumcision have been shown to significantly reduce HIV risk [Reference Weiss, Quigley and Hayes22, 23]. The circumcision rate is estimated to be 80–90% in Congolese males in Kinshasa (B. Lapika, personal communication). The low and stable HIV prevalence in the DRC might also be attributable to high HIV-related mortality that could have maintained the balance between the number of new infections cases and HIV deaths [Reference Mulanga-Kabeya7]. A better access to quality health care could contribute to prolongation of life in people living with HIV/AIDS, and hence a longer duration of HIV infection in HIV positives and high levels of HIV prevalence in a population [Reference Boerma24]. However, in the DRC this is not the case, due to the rapidly deteriorating health service system and lack of access to health care as a consequence of decades of mismanagement and corruption, and extreme poverty [Reference Mulanga11].
Finally, the extremely high diversity of HIV-1 subtypes found in the DRC may be associated with the relatively slow HIV transmission patterns compared to other sub-Saharan African countries with high prevalence where only one HIV-1 subtype (subtype C) is predominant [Reference Vidal25, Reference Janssens, Buvé and Nkengasong26]. As observed in the DRC, the largest variety of HIV-1 subtypes is found in Central Africa where the HIV prevalence has been low except in CAR (15%) and Cameroon (8%) (data for ANC attendees) [Reference Asamoah-Odei, Garcia Calleja and Boerma3]. Interestingly, subtype E which is primarily responsible for the explosion of the HIV epidemic in the heterosexual population in Thailand, is commonly found in CAR but not in other African countries [Reference Janssens, Buvé and Nkengasong26]. Further studies are required to identify biological differences in infectivity, transmissibility, and immunogenicity among these HIV-1 subtypes that may explain trends in HIV seroprevalence in countries such as DRC [Reference Geretti27, Reference Vidal28].
Our study results are based on a large sample size and sensitive laboratory PCR techniques for the ascertainment of selected STIs. Furthermore, HIV seroprevalence was determined at two of the largest maternity clinics where 14·2% of the 114 144 deliveries occur in Kinshasa (Office of District Medical Inspection, Kinshasa, DRC). An estimated 85% of pregnant women attend at least one ANC visit during pregnancy in Kinshasa [29].
Nevertheless, our study has certain limitations. Despite the large number of women enrolled in the study, the sample size was too small to examine the association between HIV and other STIs [Reference Cohen19–Reference Cohen21], due to the low prevalence of both HIV and selected STIs. Selecting only two maternity clinics may not represent the pregnant population from different sociodemographic and geographical areas in Kinshasa. Data on other important behavioural risk factors for HIV and STI infections such as condom use, number of current or lifetime male sexual partners, and the prevalence of sex work in the target population were not available as this was beyond the scope of the study.
The historically stable and low seroprevalence of HIV in pregnant women was confirmed at two of the largest maternity clinics in Kinshasa, DRC. The prevalence rates of CT, NG, and syphilis were also lower than expected in this population. Understanding the factors contributing to the low and stable HIV prevalence in the DRC, including further analyses of the balance between HIV/AIDS deaths and newly infected cases, patterns of sexual behaviours, and the prevalence of other STIs such as herpes simplex virus 2 (HSV-2) are important to prevent the possible future expansion of an HIV epidemic in the low HIV transmission areas in Africa.
ACKNOWLEDGEMENTS
The authors acknowledge K. Rich (UNC-CH Microbiology Laboratory) and S. Edidi (DRC National AIDS Reference Laboratory) for laboratory technical support. G. Mpanya, M. Onyamboko, and K. Mwandagalirwa worked in the planning and implementation phases of the study. This work was financed by the Global Network for Women's and Children's Health Research (NICHD U01 HD043475-01) and the Bill & Melinda Gates Foundation. J. Smith was supported through a Center for AIDS Research grant (5 P30 AI050410-07 NIAID). Logistical support for STI testing was provided by the North Carolina STI and Topical Microbicide Cooperative Research Center grant (5 U19 AI031496-14).
DECLARATION OF INTEREST
None.




