Introduction
Bipolar disorders, (BD) I and II, have a global lifetime prevalence of 0.6% and 0.4%, respectively.Reference Merikangas, Jin and He 1 Effective treatment of depressive episodes in BD remains a challenge and bipolar depression contributes to substantial functional impairment.Reference Rosa, González-Ortega and González-Pinto 2 In the World Mental Health survey initiative, severe and very severe role impairment was reported by 75% of BD patients who experienced depression within the 12-months prior.Reference Merikangas, Jin and He 1 A major factor contributing to this impairment is the lack of efficacy of current treatments for bipolar depression, which can be as low as 23.8% for first-line pharmacotherapies.Reference Nierenberg, Ostacher and Calabrese 3 Mounting evidence suggests that a reactive immune system may contribute to the pathophysiology of bipolar disorder and that anti-inflammatory agents may be efficacious bipolar depression.Reference Rosenblat, Kakar and Berk 4 , Reference Jones, Daskalakis and Carvalho 5 More recently, the largest clinical trial to date (n = 266) of anti-inflammatories for bipolar depression found that both minocycline and celecoxib were not superior to placebo in the treatment of bipolar depression.Reference Husain, Chaudhry and Khoso 6 These conflicting results suggest that anti-inflammatories may only be effective for a subset of patients with bipolar depression.
Given the mixed findings from randomized controlled trials (RCTs) of anti-inflammatory agents in mood disorders, anti-inflammatories may not be effective for the syndrome of depression but may target specific symptoms that are more commonly associated with a reactive immune system. The association between a reactive immune system and specific symptoms has been studied in major depressive disorder (MDD) but less so in BD. In MDD, neurovegetative symptoms (eg, low energy, altered appetite) have been associated with inflammation independent of cognitive and mood symptoms, while cognitive (ie, impaired thinking) and mood symptoms (ie, anhedonia, feelings of worthless and guilt) were not associated with inflammation.Reference Majd, Saunders and Engeland 7 Other studies have found that inflammation is associated with fatigue, sleep problems, depressed mood, and anhedonia in depression.Reference Majd, Saunders and Engeland 7 , Reference Milaneschi, Kappelmann and Ye 8 A recent meta-analysis of 56 351 persons with MDD found that five symptoms, that is, little interest in doing things, sleep disturbance, changes in appetite, feeling like everything was an effort, and loss of energy, were associated with higher concentrations of plasma C-reactive protein (CRP).Reference Frank, Jokela and Batty 9 In another sample, specific neurovegetative symptoms of eating, appetite, and tiredness were associated with elevated CRP and not overall depression scores or severity.Reference Franklyn, Stewart and Beaurepaire 10 Symptoms of BD that have been associated with inflammation include disturbances in sleep, suicidality, cognitive dysfunction, and anhedonia.Reference Rosenblat, Brietzke and Mansur 11 , Reference Rosenblat and McIntyre 12 We have previously reported that in a sample from a lower to middle income country (LMIC) lower CRP was associated with higher rates of suicidality in BD; contrary to what has been found in Western populations.Reference Xue, Hodsoll and Khoso 13 This suggests that the associations between inflammation and depressive symptoms in BD may differ in a LMIC setting.
LMICs represent nearly half of the world’s population and over 80% of the disease burden attributed to mental disorders, yet are largely excluded from mental health research. Similarly, studies investigating the relationship between inflammation and depressive symptoms in LMIC populations are scarce. The objective of the current analysis is to: (a) Determine the prevalence of peripheral inflammation in a sample of adults with bipolar depression in a LMIC setting (Pakistan) and (b) To explore associations between plasma CRP, and clinical phenotypes of bipolar depression (demographic variables, clinical variables, and specific symptom scales) in this sample.
Materials and methods
Study design
The original MINDCARE trial was a randomized, double-blinded, multicentre 2 × 2 factorial design trial conducted in outpatient psychiatric clinics in Hyderabad, Karachi, Lahore, and Rawalpindi, Pakistan between May 1, 2016, and March 31, 2019. The study investigated the antidepressant effect of minocycline and celecoxib in adults with bipolar depression with a four-arm (2 × 2) factorial design. The study was approved by the institutional review board of Karachi Medical & Dental College (KMDC). Detailed methodology of the trial has been previously reported.Reference Husain, Chaudhry and Khoso 6
Participants were between the ages of 18 and 65 with a DSM-5 diagnosis of BDI or BDII and concurrent major depressive episode. They were on stable medication for 4 weeks or greater prior to baseline assessment and provided informed consent. Females with child-bearing potential consented to using contraception and monthly pregnancy tests due to the teratogenic risk of minocycline and celecoxib.
Exclusion criteria included: serious physical health conditions including chronic infectious diseases; history of allergies to anti-inflammatory drugs; currently using penicillin, anticoagulants, antibiotics, or other anti-inflammatory drugs; history of seizures; DSM-5 diagnosis of substance misuse within 3 months prior to screening; DSM-5 diagnosis of primary psychotic disorder; high suicide risk; experiencing manic or hypomanic symptoms (≥3).Reference Husain, Chaudhry and Khoso 6
Assessments
Measurement of CRP
Blood samples were collected, and CRP was quantified using the Spinreact CRP Latex Agglutination test at baseline and week 12.
Assessment of demographic and clinical characteristics
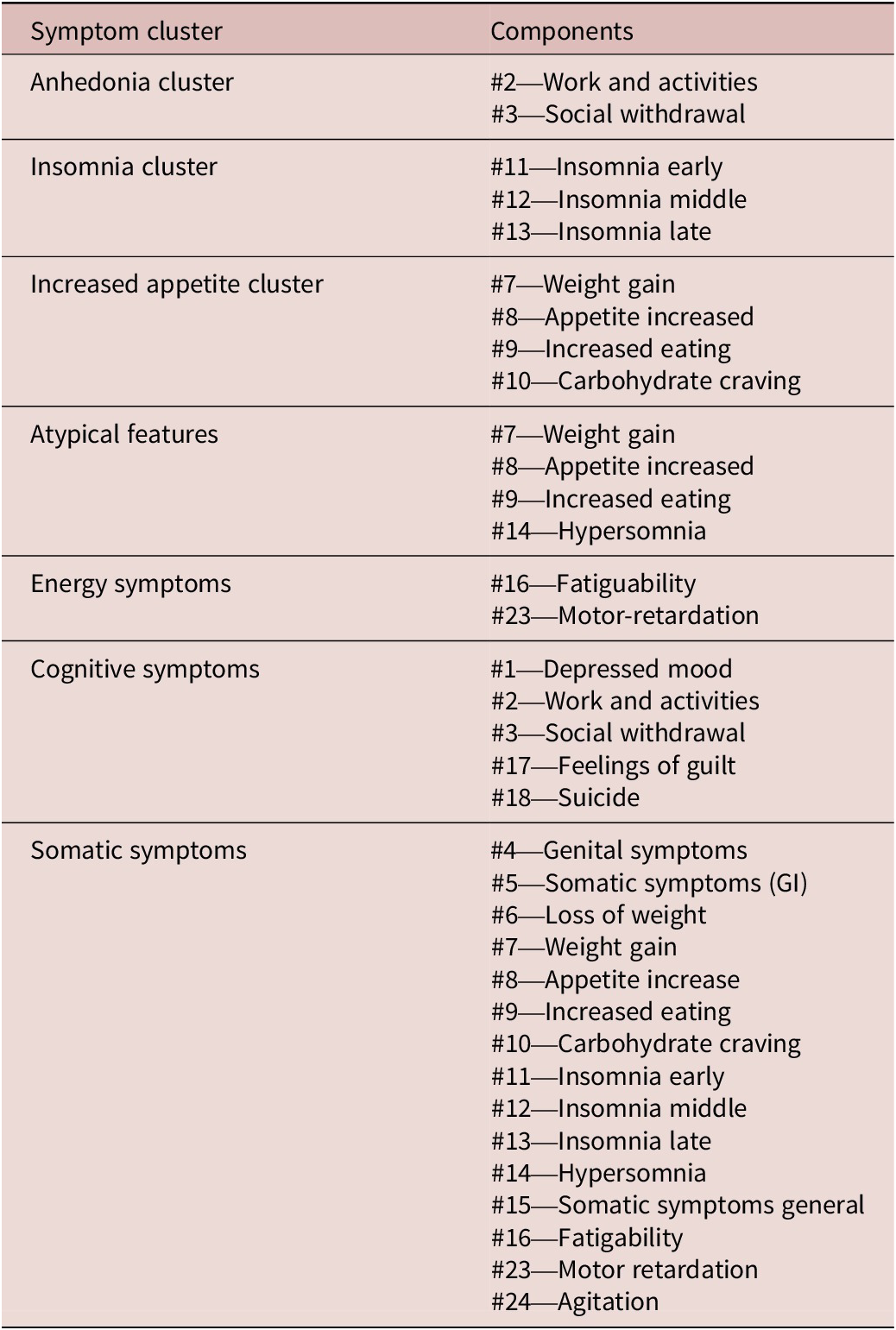
Sociodemographic and patient characteristics such as age, gender, body mass index (BMI), waist circumference (WC), heart rate, blood pressure, socioeconomic status (SES), years of education, number of hospital admissions, and duration of illness (months). SES was defined as either low, low-middle, middle, or upper-class. In our analysis we categorized SES as “low” or “not low” as in the primary report, only 12 participants were in middle- or upper-class. Low SES was defined as a monthly income of less than 17 000 Pakistani rupees (approximately 136 United States Dollars in 2018). Patients were also assessed with the EuroQol-5D (EQ-5D),Reference Rabin and de Charro 14 Generalized Anxiety Disorder scale (GAD-7),Reference Spitzer, Kroenke and Williams 15 Clinical Global Impression-Severity (CGI-S),Reference Busner and Targum 16 Hamilton Depression Rating Scale-17 (HDRS-17),Reference Hamilton 17 and HDRS-24. Each individual item of the HDRS-24 was explored individually as well as grouped into symptom clusters. The symptom clusters explored in our analysis were based on standard clinical clusters, that is, anhedonia (items 2 and 3), insomnia (items 11, 12, and 13), increased appetite (items 7, 8, 9, and 10) atypical features (items 7, 8, 9, and 14), energy symptoms (items 16 and 23), cognitive symptoms (items 1, 2, 3, 17, and 18), and somatic symptoms (Items 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 23, and 24) (Table 1).
Table 1. Symptom Clusters for HDRS-24
Definition of inflammatory status
We chose CRP >3 mg/L as a definition of low-grade inflammation based upon United States Centers for Diseases Control and Prevention and American Heart Association Guidelines, which considered CRP levels over 3 mg/L to be indicative of an active inflammatory response.Reference Pearson, Mensah and Alexander 18 , Reference Ridker 19 This definition has also been utilized in other studies in BD.Reference Gan, Wu and Liao 20
Statistical analysis
All statistical analysis was conducted with SPSS-28 software. Descriptive statistics were used to explore the distribution of baseline demographic variables, clinical variables, and symptoms. All variables were analyzed grouped by inflammatory status (CRP < 3 mg/L or > 3 mg/L). Kruskal–Wallis test was used to determine any differences in these variables. For categorical variables, we compared difference in inflammatory status using Pearson chi-square tests.
We then completed a series of logistic regression analysis with inflammatory status (CRP < 3 mg/L or > 3 mg/L) as the dependent variable. Each model included one demographic, clinical, or symptom variable of interest as a covariate to assess its association with inflammatory status. SEC, gender, BMI, and age were selected a priori as covariates in each adjusted models given their known association with depressive symptoms and inflammation.
To assess CRP as a continuous variable, we also completed a series of similar linear regression models with log10CRP as the dependent variable. Each model included one demographic or symptom measure as a covariate to assess the association with baseline inflammation. The same covariates as the logistic regression models were included.
Results
Baseline demographic and clinical measures by inflammatory status
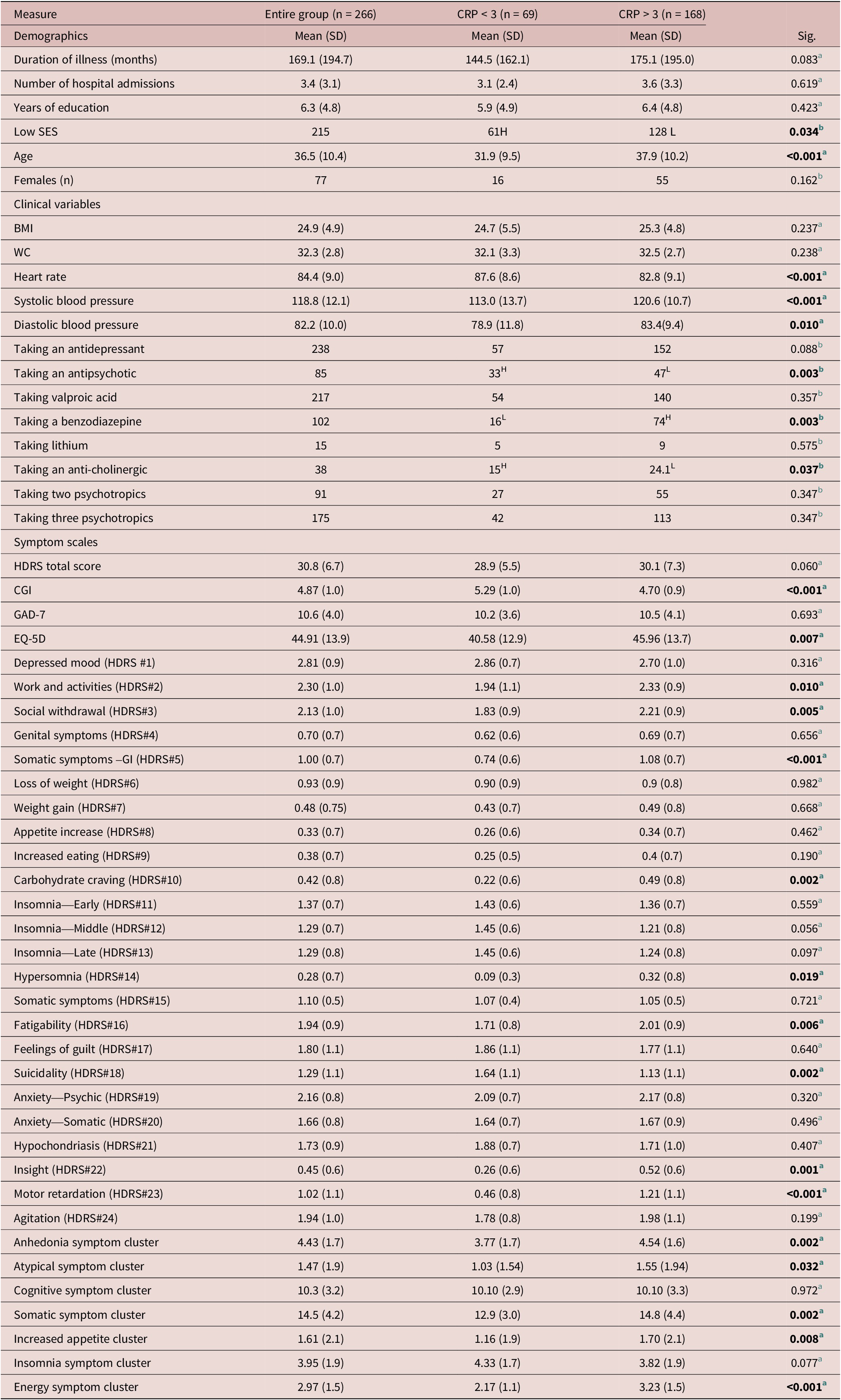
A total of 266 patients were enrolled in the original study, which included 189 (71%) males and 77 (29%) females and mean age was 36.5 (10.4). Table 2 summarizes the baseline demographic, clinical, and symptom variables. Baseline CRP was available from 237 participants with a mean (SD) value of 5.94 (8.54) mg/L. A total of 168 participants (70.9%) had a baseline CRP > 3 mg/L and 69 (29.1%) participants had a baseline CRP < 3 mg/L.
Table 2. Baseline Mean Demographic and Clinical Variables in the Entire Group and Split by Inflammation Status (CRP < 3 or CRP > 3)
Abbreviations: BMI, body mass index; CGI, clinical global impression; EQ-5D, EuroQoL; GAD-7, generalized anxiety disorder-7; H, higher than expected; HDRS, Hamilton Depression Rating Scale; L, lower than expected; SES, socioeconomic status; WC, waist circumference.
a Statistical test—Kruskal–Wallis test.
b Pearson chi-square.
Demographic variables
Age and SES were not equally distributed between the low-grade inflammation (CRP > 3 mg/L) group and noninflamed group (CRP < 3 mg/L). The mean age was higher in the low-grade inflammation group (mean: 37.9, SD:10.2) compared to the noninflamed group (mean:31.9, SD:9.5). There was a higher proportion of persons with a low SES in the noninflamed inflammation group (CRP < 3 mg/L) compared to the low-grade inflammation group (CRP > 3 mg/L) (Table 2).
Clinical variables
Clinical variables that were not equally distributed between the two groups were heart rate, blood pressure (systolic and diastolic), and proportion of patients taking an antipsychotic, benzodiazepine, or an anti-cholinergic. The mean heart rate for the low-grade inflammation group (CRP > 3 mg/L) was lower (mean: 82.8, SD: 9.1) compared to the noninflamed group (mean: 87.6, SD: 8.6). The mean blood pressure for the low-grade inflammation group was overall higher (systolic mean: 120.6, SD: 10.7 and diastolic mean: 83.4, SD: 9.4) compared to the noninflamed group (CRP < 3 mg/L) (mean:113.0, SD: 13.7 and mean: 78.9, SD: 11.8 respectively). There was a lower proportion of participants taking an antipsychotic or anti-cholinergic medication in the low-grade inflammation group (Table 2). There was a higher proportion of patients taking a benzodiazepine medication in the low-grade inflammation group (Table 2).
Symptom scale total scores
CGI-S and the EQ-5D scores were not equally distributed between the two groups. The CGI-S (mean: 4.70, SD: 0.9) was lower in the low-grade inflammation group while the EQ-5D was higher in the low-grade inflammation group (mean: 45.96, SD: 13.7) compared to the noninflamed group (CRP < 3 mg/L). A higher CGI-S indicates higher severity and higher EQ-5D indicates a higher perceived health-related quality of life (Table 2).
Individual items of symptom scales
Individual scores that were higher in the low-grade inflammation (CRP > 3 mg/L) group were the HDRS-24 work and activities (HDRS item 2), Social withdrawal (HDRS item 3), Somatic symptoms –GI (HDRS item 5), Carbohydrate craving (HDRS item 10), Hypersomnia (HDRS item 14), Fatigability (HDRS item 16), Insight (HDRS item 22), and Motor retardation (HDRS item 23). The individual suicidality item (HDRS item 18) was higher in the non-inflamed group (CRP < 3 mg/L).
Symptom clusters of symptom scales
Clusters of symptoms that were significantly higher in the low-grade inflammation group include the anhedonia cluster, increased appetite cluster, somatic symptoms cluster, and energy cluster (Table 2).
Associations between low-grade inflammation and clinical variables logistic regression
The results of the logistic regression model controlling for BMI, SES, age, and gender are presented in Table 3 and Figure 1 and are summarized below.
Table 3. Logistic Regression Model with Inflammation Status (CRP < 3 or CRP > 3) as Dependent Variable, Controlling for Age, BMI, Gender, and SES
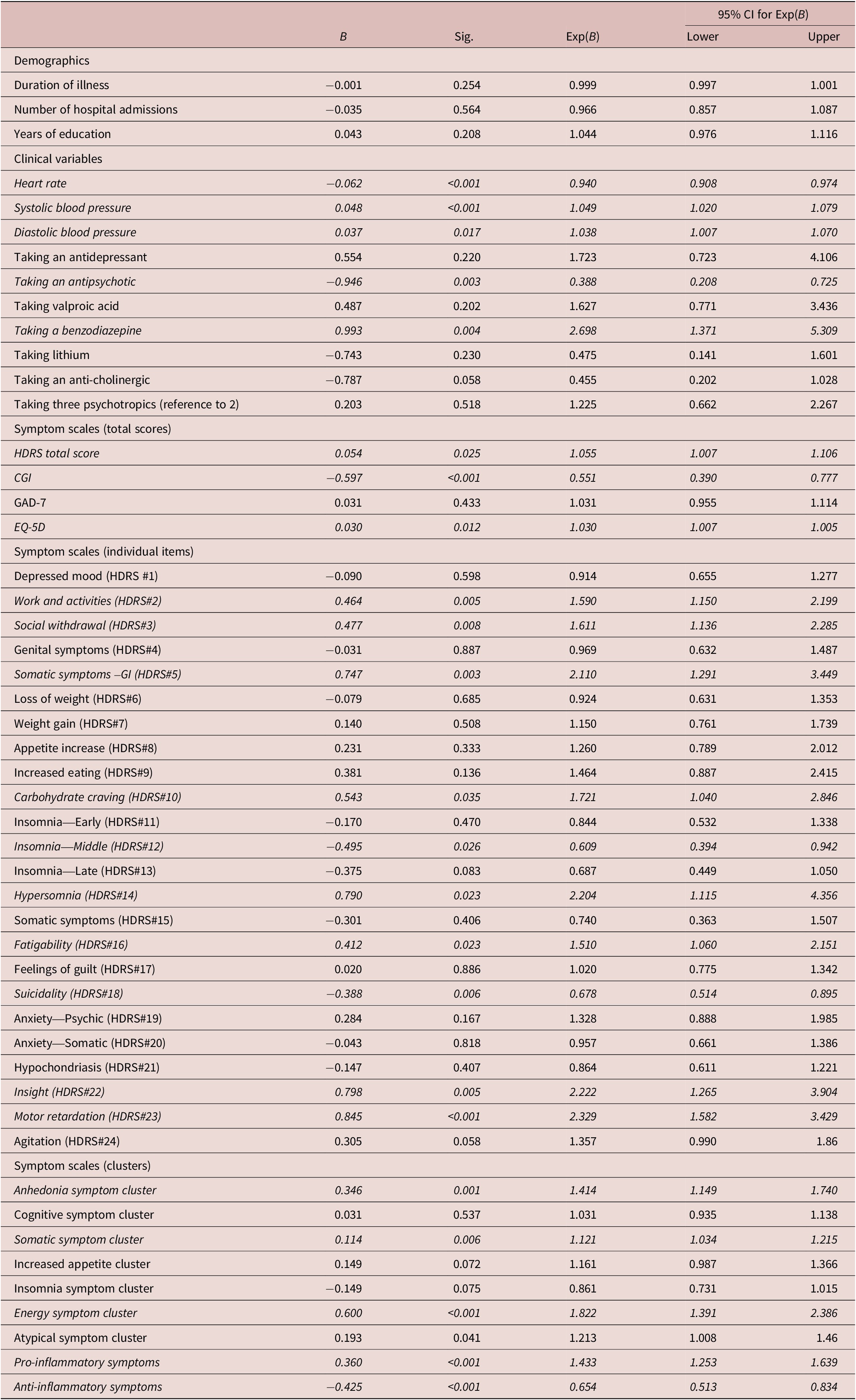
Note: Pro-inflammatory symptoms (2, 3, 5, 10, 14, 16, 22, 23); anti-inflammatory symptoms (12, 18).
Abbreviation: HDRS, Hamilton Depression Rating Scale.
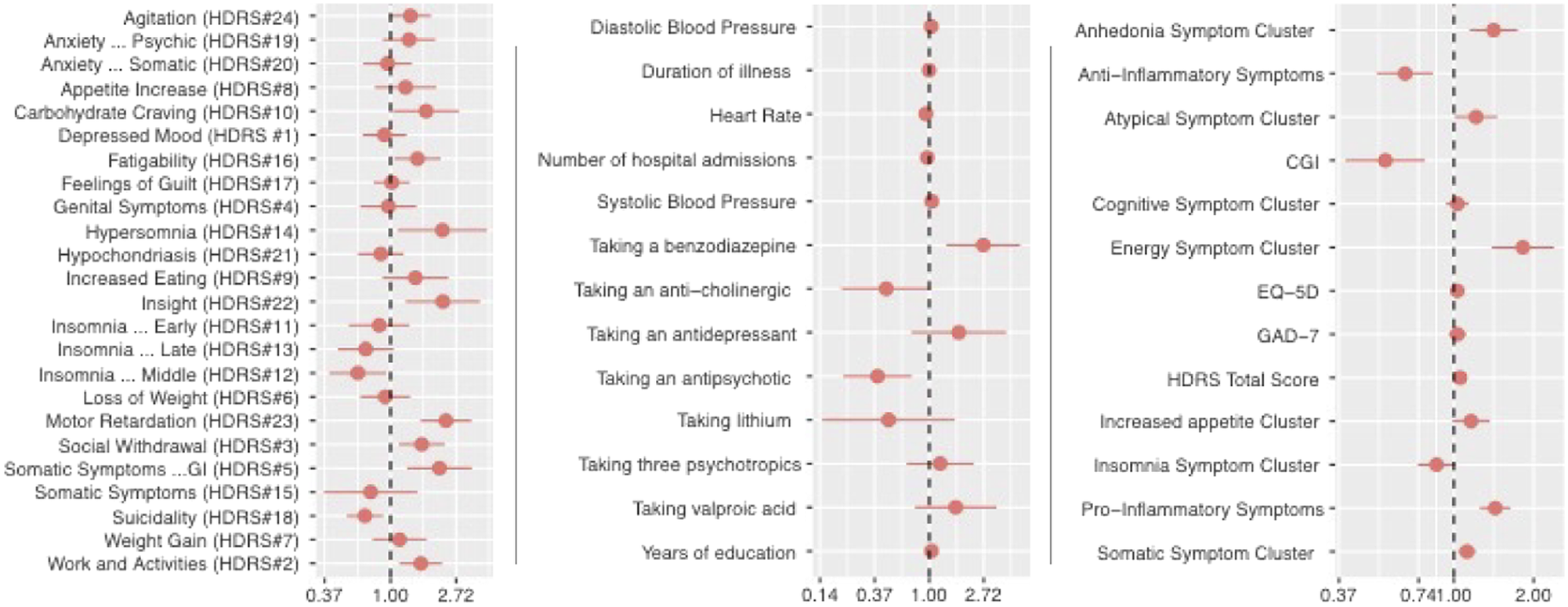
Figure 1. Odds ratio and 95% confidence interval of low-grade inflammation (CRP > 3) controlling for age, BMI, gender, and SES.
Demographic and clinical variables
Higher systolic blood pressure (OR:1.049; CI: 1.020-1.079), diastolic blood pressure (OR:1.038; CI:1.007-1.070), and taking a benzodiazepine (OR:2.698; CI: 1.371-5.309) was associated with higher likelihood of low-grade inflammation (Table 3). Heart rate (OR: 0.940; CI: 0.908-0.074) and taking an antipsychotic (OR: 0.388; CI: 0.208-0.725) was negatively associated with low-grade inflammation.
Symptom scale total scores
A higher total score of the HDRS (OR: 1.055; CI: 1.007-1.106) and the EQ-5D (OR: 1.030; CI: 1.007-1.005) were associated with a higher likelihood of having low-grade inflammation (Table 3). The CGI was negatively associated with low-grade inflammation (OR: 0.551; CI: 0.390-0.777).
Individual item scores
Work and Activities (HDRS item 2), Social Withdrawal (HDRS item 3), Somatic Symptoms –GI (HDRS item 5), Carbohydrate Craving (HDRS item 10), Hypersomnia (HDRS item 14), Fatigability (HDRS item 16), Insight (HDRS item 22), and Motor Retardation (HDRS item 23) were associated with a higher likelihood of low-grade inflammation. Motor Retardation (HDRS item 23) had the greatest association (OR: 2.329; CI: 1.582-3.429). Insomnia—middle and suicidality were negatively associated with low-grade inflammation.
Symptom clusters
There were four symptoms clusters that were associated with a higher likelihood of low-grade inflammation: anhedonia symptom clusters, somatic symptom clusters, energy symptom clusters, and pro-inflammatory symptom clusters (Table 1). The anti-inflammatory cluster was negatively associated low-grade inflammation. The pro-inflammatory and anti-inflammatory symptom clusters are the individual items that were respectively associated with low-grade inflammation.
Log10 CRP linear regression models
The results of the linear regression with log10CRP as the dependent variable are presented in Supplementary Table 1 and Supplementary Figure 1 and summarized below.
There were no demographic variables that were associated with the log10CRP in the linear regression models. A higher heart rate was associated with a lower log10CRP (B: −0.012, CI: −0.018 to −0.006, P < .001). Taking an antidepressant was associated with a higher log10CRP (B: 0.187, CI: 0.019 to 0.355, P: .03). Taking an anti-cholinergic medication was associated with a lower log10CRP (B: −0.163, CI: −0.321 to −0.006, P: .042).
A higher score in the CGI-S was associated with a lower log10CRP (B: −0.097, CI: −0.154 to −0.040, P = .001). The EQ-5D and HDRS total score were not associated with the continuous variable log10CRP.
There were 3 individual items that were associated with the log10CRP at baseline. These included hypersomnia (B: 0.082, CI: 0.001 to 0.163, P: .048), fatiguability (B: 0.077, CI: 0.014 to 0.139, P: .017), and motor retardation (B: 0.068, CI: 0.014 to 0.12, P: .014). The “pro-inflammatory symptoms” cluster was significantly associated with log10CRP (B: 0.029, CI: 0.12 to 0.045, P: <.001), in addition to the energy cluster (B: 0.061, CI: 0.024 to 0.098, P: .001). Suicidality (B: −0.090, CI: −0.139 to −0.041, P < .001) and the “anti-inflammatory symptom cluster” (B: −0.068, CI: −0.109 to −0.027, P: .001) were negatively associated with baseline log10CRP.
Discussion
The current secondary analysis of the MINDCARE trial found a high prevalence (70.9%) of low-grade inflammation in a sample of Pakistani adults with bipolar depression. When controlling for SES, age, BMI, and gender, we found that elevated blood pressure, taking an antipsychotic or benzodiazepine, HDRS total, and EQ-5D scores were associated with low-grade inflammation. Symptoms that were associated with low-grade inflammation were related to anhedonia, gastrointestinal somatic complaints, carbohydrate craving, hypersomnia, decreased energy, and motor retardation.
The prevalence of low-grade inflammation in our sample is inconsistent with evidence from other settings. A recent study in a Han-Chinese BD sample (n = 430), found that rates of low-grade inflammation were 10.1%.Reference Gan, Wu and Liao 20 This sample was similar in that they were treatment-seeking participants; however, less than half of the sample was currently depressed with only a moderate HDRS-17 (18.4) score. Other samples of BD, mostly from Western countries, have reported lower rates of low-grade inflammation ranging from 20% to 40% depending on phase of illness.Reference Osimo, Cardinal and Jones 21 , Reference Wysokiński, Margulska and Strzelecki 22 An explanation for the higher rates of low-grade inflammation in our sample could be related to current medications. Previous studies have included a significant proportion of patients that were treatment naïve while all participants in the MINDCARE trial were taking at least two psychotropic medications at baseline. Previous research has shown that psychotropic medications have an influence on inflammatory markers.Reference Osimo, Cardinal and Jones 21 In the current sample antipsychotic medication was associated with lower rates of inflammation. This association has been previously reported in patients with schizophrenia, with antipsychotics leading to reduced expression of immune genes and down-regulation of cytokine levels. Given the cytokine role in anorexigenic responses, reduction in inflammation could contribute to change in eating behaviors and changes in metabolic parameters associated with antipsychotics.Reference Fonseka, Müller and Kennedy 23
Another explanation may be related to relative SES. A recent meta-analysis in nonpatient populations in North America, found that higher rates of inflammation are found in persons with lower SES.Reference Muscatell, Brosso and Humphreys 24 Our sample, from a LMIC, represents a population with a relative lower SES compared to those in high-income settings, which may explain the high prevalence of low-grade inflammation. Though in our population, high SES was associated with low-grade inflammation, our sample was heavily biased toward low to medium SES. Within this spectrum of SES there may be complex factors that are unaccounted for and contributing to this association. For example, mounting evidence has shown that relative inequality, perceived SES, and neighborhood SES have a significant impact on overall health.Reference Operario, Adler and Williams 25 , Reference Sampson, Morenoff and Gannon-Rowley 26 Future work should explore the relationship between SES and inflammation within LMIC countries and compare this to findings from high-income countries.
Our study found several specific symptoms that are associated with both low-grade inflammation. Anhedonia has been repeatedly associated with a pro-inflammatory state in both BD and MDD as evidenced by cross-sectional studies and responsiveness of anhedonia to anti-inflammatory drugs.Reference Rosenblat and McIntyre 12 , Reference Lee, Mansur and Brietzke 27 In our sample, we found that higher rates of anhedonia were seen in those with low-grade inflammation. In addition, low-grade inflammation was also associated with lower energy, hypersomnia, somatic symptoms, and psychomotor retardation. In the Han-Chinese population, low-grade inflammation was not associated with hypersomnia but was associated with leaden paralysis.Reference Gan, Wu and Liao 20 The same study reported that low-grade inflammation was associated with a recent suicide attempt, while in our sample low-grade inflammation was associated with less suicidality as previously reported.Reference Xue, Hodsoll and Khoso 13 As discussed above, one explanation may be the lower rates of depression in the Han Chinese sample vs our sample. Furthermore, both samples represent relatively unique groups with distinct psychosocial circumstances, lifestyle factors, and cultural practices. Sleep disturbances are a key feature of BD and have been linked to a pro-inflammatory state, with a bidirectional relationship.Reference Rosenblat and McIntyre 12 In the context of MDD, a large meta-analysis has shown increased CRP to be associated with little interest in doing things, sleep disturbance, changes in appetite, feeling like everything was an effort, and loss of energy.Reference Frank, Jokela and Batty 9 In a sample of MDD and BD, the effect of CRP on connectivity mediated significant relationships between CRP and anhedonia and motor slowing.Reference Felger, Li and Haroon 28 Overall, our results are consistent with these studies, which reported that anhedonia, somatic, sleep, and fatigue-related symptoms are associated with higher CRP.
Limitations
This present study is an exploratory study with limitations. First, the study cannot conclude a causal relationship between inflammation and depressive symptoms in BD. We limited the current study to a cross-sectional analysis given the primary study did not report any anti-inflammatory effects or antidepressant treatment response of the study drugs over placebo. The current study only used CRP as the sole marker for inflammation. Other direct markers of inflammation, such as cytokines, may have a different correlation with the symptoms and provide insight about which subset of the BD population is responsive to anti-inflammatory agents. Most of the sample recruited to the MINDCARE trial were males hence the findings may not be generalizable to females. This is likely due to the under-representation of females in the treatment-seeking population in Pakistan. Studies suggest that there may be a gender difference regarding the association between CRP and symptom severity.Reference Köhler-Forsberg, Buttenschøn and Tansey 29 Additionally, due to factors, including socioeconomic and lifestyle differences, the findings from Pakistan, a LMIC, are not necessarily generalizable to high-income countries or other LMICs. Future studies should explore psychosocial, lifestyle, and cultural factors that contribute to this relationship.
Conclusions
Our findings suggest a high prevalence of low-grade inflammation in Pakistani patients with bipolar depression. In this sample, there are several symptoms that may be more associated with inflammation such as anhedonia, hypersomnia, fatigability, and motor retardation. Further research validating these findings in larger samples is needed. If replicated, clinical trials of anti-inflammatory agents stratifying patients based upon this “inflammatory” clinical phenotype may improve treatment outcomes in a subset of patients with bipolar depression in LMICs.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1092852923002316.
Author contribution
B.D.M.J. and U.M. contributed equally to this work. All authors participated in the conceptualization and article preparation. All authors have approved the final manuscript.
Financial support
This study was funded by the Stanley Medical Research Institute, grant ID-15T 004, and was supported in part by an Academic Scholars Award to M.I.H. from the Department of Psychiatry, University of Toronto, Canada. This report represents independent research part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London, UK, the UK Maudsley National Health Service (NHS) Foundation Trust, and King’s College London, London, UK. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, or UK Department of Health.
Disclosure
I.B.C. and N.H. have given lectures and advice to Eli Lilly, Bristol Myers Squibb, Lundbeck, Astra Zeneca, and Janssen pharmaceuticals for which they or their employing institution have been reimbursed. M.I.H., I.B.C., and N.H. were previously Trustees of the Pakistan Institute of Learning and Living. A.H.Y. has been commissioned to provide lectures and advice to all major pharmaceutical companies with drugs used in affective and related disorders. A.H.Y. has undertaken investigator-initiated studies funded by Astra Zeneca, Eli Lilly, Lundbeck, and Wyeth. B.H.M. currently receives research support from Brain Canada, the Canadian Institutes of Health Research, the CAMH Foundation, the Patient-Centered Outcomes Research Institute (PCORI), the US National Institute of Health (NIH), Capital Solution Design LLC (software used in a study funded by CAMH Foundation), and HAPPYneuron (software used in a study funded by Brain Canada). Within the past 5 years, he has also received research support (medications for NIH-funded clinical trials) from Bristol-Myers, Eli Lilly, and Pfizer. He directly owns stocks of General Electric (less than $5000). M.I.H. is a PI for a trial sponsored by COMPASS Pathways Limited. B.D.M.J. has received fellowship support from the Canadian Institute of Health Research. The remaining authors have nothing to disclose.








