Valproate, a salt of valproic acid, is a well-established anti-epileptic medication that is also commonly used in the acute and chronic management of bipolar illness. Its use in women of childbearing age is, however, limited because of its potential to cause foetal abnormalities when taken during pregnancy. The teratogenic potential of valproate was first highlighted in the early 1980s. Reference Robert and Guibaud1 Since then, a variety of congenital malformations, particularly neural tube defects, have been reported in children exposed to valproate in utero. The incidence of neural tube defects is estimated to be around 1-2% of live births, with rates of spina bifida up to 10-20 times those seen in the general population. Reference Ornoy2 The risk of teratogenicity with valproate is estimated to be around three times higher than with other mood stabilising agents such as carbamazepine and lamotrigine Reference Tomson and Battino3 and significantly greater at valproate doses above 1000 mg. Reference Vajda, O'Brien, Hitchcock, Graham, Cook and Lander4 In addition to this, more recent data suggest an increased risk of developmental delays and lower verbal IQs in previously exposed children. Reference Tomson and Battino3,Reference Bromley, Baker and Meador5-Reference Adab, Kini, Vinten, Ayres, Baker and Clayton-Smith7
There are a number of proposed mechanisms for foetal anomalies resulting from maternal valproate exposure. One is that valproate is known to inhibit the effects of folate by affecting folate metabolism and/or absorption. Reference Lagrange8,Reference Ornoy9 It is widely accepted that folate deficiency in pregnancy gives rise to increased rates of neural tube defects. Reference Wolff, Witkop, Miller and Syed10,Reference Daly, Kirke, Molloy, Weir and Scott11 It is also generally agreed that high-dose folate supplementation in the period leading up to conception reduces the rate of neural tube defects in high-risk cases. Reference Lumley, Watson, Watson and Bower12 The protective effect of folate supplementation during maternal valproate exposure remains uncertain, but is assumed to be present.
In July 2006 the National Institute for Health and Clinical Excellence (NICE) issued guidance on the management of bipolar disorder in adults, children and adolescents in primary and secondary care in England and Wales. 13 One of the key recommendations was that valproate should not routinely be prescribed for women of childbearing age and if prescribed, adequate contraception should be ensured and the risks of taking valproate during pregnancy should be explained to the woman. In addition, it was advised that any woman who becomes pregnant while taking valproate should be prescribed at least 4 mg of folate a day. These recommendations are supported by the British Association of Psychopharmacolgy. Reference Goodwin14 The Maudsley Prescribing Guidelines go further, in recommending folate for at least a month before conception. Reference Taylor, Paton and Kapur15
In this paper we report the results of a baseline survey to determine adherence to the NICE guidance for bipolar disorder within the South London and Maudsley NHS Foundation Trust and the impact of the subsequent introduction of a 20-month quality improvement programme.
Method
The audit was performed in five stages: the baseline audit and feedback of results, implementation of a quality improvement programme, re-audit and feedback of results, a second quality improvement programme and final audit.
Definitions
Women of childbearing potential were defined as those aged 15-49 years. Adequate contraception was prescription of an oral, depot, implant or intrauterine device or the use of condoms. Folate cover was the prescription of any preparation containing folic acid 4 mg or above.
Inclusion criteria
All women of childbearing potential prescribed valproate in the trust in-patient and community services receiving medication from the trust pharmacy were included in the audit.
Exclusion criteria
Women aged under 15 years or over 49 years or individuals receiving medication from their general practitioner (GP) were excluded.
Stage 1: baseline audit and feedback of results
Data were collected in February 2008. The prescription charts for all trust in-patients and community patients were reviewed to identify women meeting inclusion criteria. The prescriptions of those identified were scrutinised further for co-prescription of an oral or depot contraceptive preparation as well as any folate preparations. The electronic patient records were searched for evidence of the prescription of an intrauterine device, oestrogen implant, progesterone depot or condom use. Details of any conversations between a healthcare professional and the patient on the risks of becoming pregnant while taking valproate, the importance of the use of a contraceptive or the benefits of folate supplementation were taken from the electronic patient record. In addition, documentation in the notes of an offer to prescribe a contraceptive or folate preparation was noted. The results were disseminated in the trust through the trust clinical governance, medicines management and drug and therapeutics committees.
Stage 2: first quality improvement programme
The quality improvement programme was implemented between January and March 2009. Following the baseline survey, a quality improvement programme was designed by the trust pharmacy department for implementation in all services providing care for women of childbearing potential. The following interventions were put in place.
January 2009
-
• The pharmacy department produced an information sheet containing guidance for prescribers on the use of valproate in women of childbearing potential.
-
• The sheet informed prescribers of the risks of valproate use in pregnancy, the need to ensure women are made aware of this risk and to advise against becoming pregnant during treatment.
-
• In addition, prescribers were advised to prescribe a contraceptive and high-dose folate preparation for these women.
-
• The pharmacy department circulated the information sheet to clinical staff providing services to women of childbearing potential in the trust.
-
• The information was supported by verbal advice from a pharmacist.
-
• The importance of documenting in the electronic patient record details of any conversations between the clinician and patient was also highlighted in the guidance sheet.
See Appendix for the guidance sheet.
February to April 2009
Women already prescribed valproate
-
• Pharmacy staff identified all women of childbearing age prescribed valproate.
-
• A pharmacist contacted the prescribers of each of the individuals identified to ensure that the women had been informed of the teratogenic risks of valproate and that contraception and folate had been considered.
-
• If a woman had not already received the information, the prescriber was asked to have a discussion with her within 4 weeks of them being contacted by the pharmacy.
-
• A pharmacist arranged to speak to the woman if the prescriber was unable to do so.
Women newly prescribed valproate
-
• In the case of new initiations, women were identified within 2 weeks of the prescription being issued.
-
• The electronic patient record was checked to determine whether the woman had received information on risks in pregnancy.
-
• If a woman had not already received the information the prescriber was asked to have a discussion with her within 4 weeks of them being contacted by the pharmacy.
-
• A pharmacist arranged to speak to the individual if the prescriber was unable to do so.
-
• In addition, a note reminding the clinical team to inform people of the risks of valproate in pregnancy was attached to the individual's medication supply from the pharmacy.
Stage 3: re-audit 1
Data were collected in April 2009 as detailed in Stage 1. Additional information on pregnancy testing was taken from the patients’ notes.
Stage 4: second quality improvement programme
The programme detailed in Stage 2 was repeated between July 2009 and September 2009.
Stage 5: final audit
Data were collected in October 2009 as detailed in Stage 3.
Results
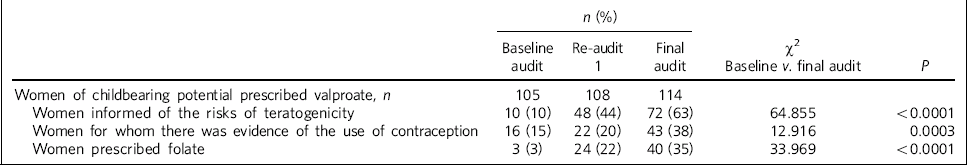
Significant improvement was noted between baseline and final audit in rates of information provision (10% v. 63%, P<0.0001), contraceptive use (15% v. 38%, P = 0.0003) and folate prescription (3% v. 35%, P<0.0001) (Table 1 andFig. 1).
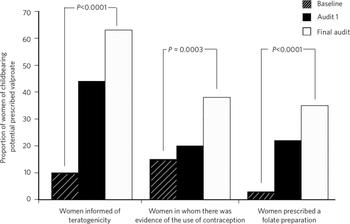
Fig 1 Rates of information provision, contraceptive use and folate prescription at baseline, re-audit 1 and final audit.
Table 1 A summary of results (baseline and re-audit 1 and 2)
| n (%) | |||||
|---|---|---|---|---|---|
| Baseline audit | Re-audit 1 | Final audit | χ2 Baseline v. final audit | P | |
| Women of childbearing potential prescribed valproate, n | 105 | 108 | 114 | ||
| Women informed of the risks of teratogenicity | 10 (10) | 48 (44) | 72 (63) | 64.855 | <0.0001 |
| Women for whom there was evidence of the use of contraception | 16 (15) | 22 (20) | 43 (38) | 12.916 | 0.0003 |
| Women prescribed folate | 3 (3) | 24 (22) | 40 (35) | 33.969 | <0.0001 |
Discussion
Our results show a significant improvement in the proportion of women in the South London and Maudsley NHS Foundation Trust prescribed valproate who were receiving information on its risks in pregnancy, were prescribed folate and were using contraception. This sample was broadly representative of a bipolar cohort for an inner-city facility with an ethnically diverse population. For example, in the final audit 49% of service users were Black, 44% White and 7% were of other ethnicities; 76% had a diagnosis of bipolar affective disorder, 10% schizophrenia, 9% schizoaffective disorder, and 5% had other diagnoses; 90% were using valproate as a mood stabiliser.
Results of a similar survey conducted in a large London mental health trust in 2007 showed that of the women prescribed valproate, fewer than a fifth were informed of its teratogenic potential and under a third were advised about contraception. Reference James, Barnes, Lelliott and Paton16 In the same survey rates of folate prescription were recorded as 4%. These findings are broadly in line with our baseline findings from early 2008.
Adherence to guidelines within mental health services is reported to be variable, with practice often not consistent with clinical recommendations. Reference Wobrock, Weinmann, Falkai and Gaebel17-Reference Ozbilen and Cottrell19 In 2005, we reported non-adherence with NICE guidance for schizophrenia. More recent national surveys examining prescribing of antipsychotics concur with these findings. Reference Paton, Barnes, Cavanagh, Taylor and Lelliott20 Practice in other areas of medicine may be more in line with evidence-based guidance. In 2005, Robertson and colleagues conducted a telephone survey among women who had become pregnant while taking isotretinoin (a known teratogen) to determine whether clinical guidelines relating to its use in women were being followed. Reference Robertson, Polifka, Avner, Chambers, Delevan and Koren21 Around three-quarters of the women surveyed recalled receiving advice on the need for contraception before starting medication, a rate similar to that seen only in our final audit.
Quality improvement programmes in mental health also appear to vary in their success at delivering change. The Prescribing Observatory for Mental Health recently introduced two national programmes to improve medicines’ management practices in mental health organisations across the UK. Reference Paton, Barnes, Cavanagh, Taylor and Lelliott20,Reference Barnes, Paton, Hancock, Cavanagh, Taylor and Lelliott22 The first, designed to reduce rates of prescribing of high-dose and combinations of antipsychotics failed to bring about a change in practice, despite identifying factors associated with dubious prescribing practices and targeting a number of change interventions. In the second programme, rates of physical health monitoring doubled after the introduction of a 12-month intervention programme. The scale of improvement, although noteworthy, must be viewed in the context of absolute patient numbers: the proportion of individuals screened for metabolic syndrome at re-audit was still only 35%. Interestingly, the antipsychotic high-dose and polypharmacy quality improvement programme has yielded a greater improvement in the longer term. 23
Our results show that it is possible to change practice through the introduction of a persistent and sustained intervention programme. This was evident from the incremental improvement in all our audit standards over the course of the quality improvement programme, which reached statistical and clinical significance between baseline and final audit. The improvement in the rate of information provision was particularly striking because of the scale of improvement noted. This may have been a result of the multidisciplinary approach to information provision in the trust, with both doctors and pharmacists counselling women on the risks of medication in pregnancy. Overall, the ongoing identification by the pharmacy of women prescribed valproate and subsequent reminders to prescribers of the need to counsel these women on the risks in pregnancy appears to have been an effective measure.
Folate prescription and contraceptive use in our survey, although improved, remained at an overall low level compared with the proportion of women given information on teratogenicity. It is widely accepted that high-dose folate supplementation may have a protective effect in women who are deemed to be at a high risk of having a baby with neural tube defects. It is unclear, however, whether prophylactic use of high-dose folate in women prescribed valproate confers the same benefits. Reference Duncan, Mercho, Lopes-Cendes, Seni, Benjamin and Dubeau24 There is also a suggestion that preconceptual high-dose folate supplementation can lead to twin births. Reference Duncan, Mercho, Lopes-Cendes, Seni, Benjamin and Dubeau24-Reference Vollset, Gjessing, Tandberg, Ronning, Irgens and Baste26 It is possible that either or both of these factors contributed to the low rates of folate prescription in the women in our survey.
Discussions between patients and the clinical team about medication can be difficult and adherence with medication is often poor in the acute phase. A more probable explanation for the low recorded rates of folate and contraceptive use may be that the pharmacological management of acute hypomania is given priority over the long-term prescription of medication for the prevention of, or safety in, pregnancy.
It is clearly crucial that folate supplementation is offered to, and options for contraception are discussed with, individuals prescribed valproate. Nearly a half of the women included in our last survey were tested for pregnancy and nine pregnancies were confirmed. The first of these figures points to the high prevalence of unprotected sexual activity in this patient group. The second demonstrates that a number of pregnancies do occur in women taking valproate.
There are two noteworthy limitations in our study. The first, as with all audits, is that data were collected at a point in time. In the case of new prescriptions the time to audit was 2 weeks after initiation of valproate. It is very possible that in many cases individuals were too unwell to receive information about teratogenicity or consent to contraception at this stage. Second, information on risk counselling in pregnancy was taken from the electronic patient record, by employing a keyword search of the record. It is possible that documentation of information may have been made using different keywords from the ones we used. Bearing both of these factors in mind it is reasonable to suggest that rates of information provision, contraceptive use and folate prescription may in fact have been higher than those recorded in our audit.
In summary, our results show that adherence to the NICE guidance for bipolar illness in respect to valproate was poor at baseline. The subsequent introduction of a quality improvement programme was successful in bringing about a position change in practice. However, despite a significant improvement in all areas surveyed, the quality improvement programme was not successful in ensuring full adherence to NICE guidelines.
Appendix
Guidance on prescribing valproate for women of childbearing age
In July 2006 the National Institute for Health and Clinical Excellence issued guidance on the management of bipolar disorder. One of the key recommendations is that when valproate is prescribed for women of childbearing age, adequate contraception should be used and the risks of taking valproate during pregnancy should be explained to the patient.
When prescribing valproate to a woman of childbearing age the prescriber must:
-
• evaluate the woman's intention to and likelihood of becoming pregnant
-
• inform the patient that the use of valproate in pregnancy is associated with foetal malformations, particularly neural tube defects
-
• inform the patient of the importance of not becoming pregnant while taking valproate
-
• advise the patient that prescription of a contraceptive is advised
-
• offer the patient a contraceptive of their choice
-
• advise the patient that prescription of folic acid 5 mg is advised
-
• prescribe a folate preparation (5 mg)
-
• document in the patient's notes:
-
• that the above has been done
-
• patient's refusal of contraception and/or folate
-
• a summary of the discussion with the patient.
-





eLetters
No eLetters have been published for this article.