Introduction
Species of Megasporaceae Lumbsch et al. are surprisingly scarce in the Southern Hemisphere. Whereas 97 species are known from North America (Esslinger Reference Esslinger2019), 104 from Russia (Urbanavichus Reference Urbanavichus2010), 40 from Svalbard (Øvstedal et al. Reference Øvstedal, Tønsberg and Elvebakk2009) and 16 from the British Isles (Fletcher et al. Reference Fletcher, Purvis, Coppins, Smith, Aptroot, Coppins, Fletcher, Gilbert, James and Wolseley2009), only six species have been reported from Australia (McCarthy Reference McCarthy2016), seven from New Zealand (Galloway Reference Galloway2007) and only three from each of South Africa (Fryday Reference Fryday2015) and Antarctica (Øvstedal & Lewis Smith Reference Øvstedal and Lewis Smith2001). In southern South America, six taxa have been reported from Argentina (Aspicilia cinerea (L.) Körb., A. mendozae Räsänen, Circinaria caesiocinerea (Nyl. ex Malbr.) A. Nordin et al., C. calcarea (L.) A. Nordin et al., Lobothallia alphoplaca (Wahlenb.) Hafellner and Megaspora verrucosa (Ach.) Arcadia & A. Nordin; Calvelo & Liberatore Reference Calvelo and Liberatore2002) and, because Lecanora masafuerensis Zahlbr. appears not to be a species of Aspicilia as was suggested by Galloway & Quilhot (Reference Galloway and Quilhot1998), only two (C. calcarea and Megaspora verrucosa) from Chile (Galloway & Quilhot Reference Galloway and Quilhot1998). None have previously been reported from the Falkland Islands (Fryday et al. Reference Fryday, Orange, Ahti, Øvstedal and Crabtree2019). Here we describe a new species that is known from three localities on the Falkland Islands, along with a lichenicolous fungus that is present on the holotype collection.
Materials and Methods
Morphological methods
Gross morphology was examined under a dissecting microscope and apothecial characteristics by light microscopy (compound microscope) on hand-cut sections mounted in water, 10% KOH (K), 50% HNO3 (N) or Lugol's reagent (0.15% aqueous IKI). Thallus sections were investigated in water, K and Lugol's reagent. Ascospore measurements of the new species are given as (minimum value–)mean ± standard deviation(–maximum value). Thalline chemistry was investigated by thin-layer chromatography following the methods of Orange et al. (Reference Orange, James and White2001), and nomenclature of apothecial pigments follows Meyer & Printzen (Reference Meyer and Printzen2000).
Additional comparative specimens examined
Lecanora masafuerensis. Chile: Juan Fernandez Islands: Mas Afuera, Quebrada de las Vacas, near two waterfalls of stream in narrow section of canyon, 1965, H. A. Imshaug 36869, 36872; ibid., Quebrada de las Casas, narrow section, 1965, H. A. Imshaug 36697, 36698, 36704 (MSC); ibid., Quebrada de las Casas, 10 iii 1917, C. & I. Scottsberg s. n. (NY—isotype).
Molecular methods
Taxon sampling
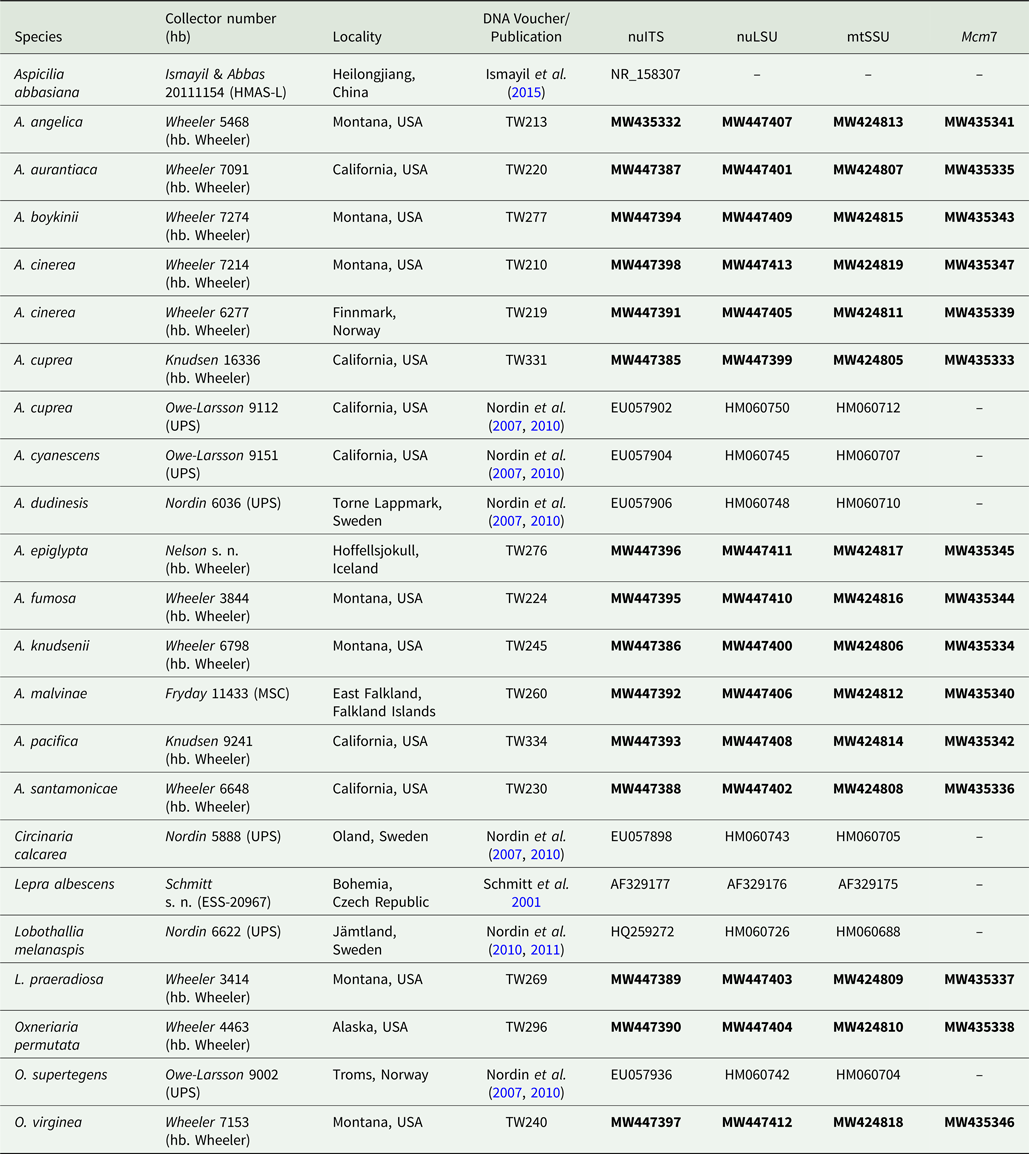
The majority of specimens used in this study were collected between 2007–2017, but further specimens were obtained from herbarium loans and the collecting efforts of other researchers (Table 1). Additional sequences included in the analysis were downloaded from GenBank (Table 1).
Table 1. Voucher information and GenBank Accession numbers of sequences used for construction of the phylogenetic tree in Fig. 3. Newly introduced sequences are in bold.
DNA isolation and sequencing
Total DNA was extracted from samples of 10–15 healthy apothecia and surrounding tissue. Two 3 mm steel beads were added to the sample tubes and frozen at −80 °C for 1 h. Samples were then mounted on the TissueLyser II (Qiagen, Germany) and ground in 30 s intervals for 1–2 min at 30/hz. DNA was extracted using the Qiagen DNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer's instructions except for the following modifications: in the first step, samples were incubated in lysis buffer for 1 h and vortexed every 10 mins; in the final step, the samples were eluted in 50 μl AE buffer twice. DNA quantity was tested on an Implen Nanodrop (Implen, München, Germany).
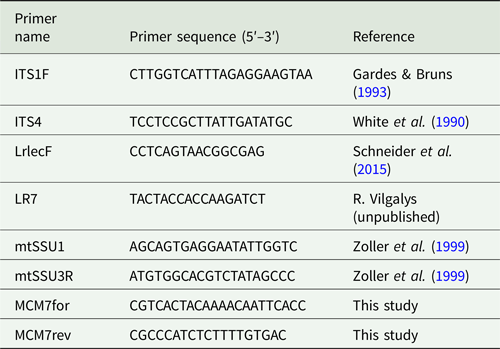
Standard PCR amplifications were conducted in 25 μl reaction volumes using Ready-To-Go PCR Beads (GE Healthcare, UK) following the manufacturer's recommendations. All primers used in this study are listed in Table 2.
Table 2. Primers used in this study.
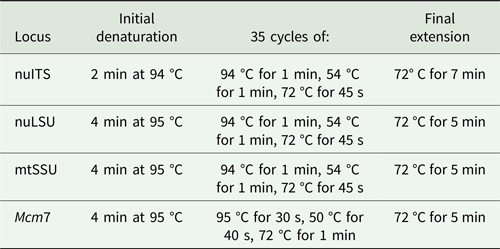
Amplifications were carried out in an Eppendorf Mastercycler Pro thermal cycler (Eppendorf North America, New York, USA) and performed using the protocols in Table 3. PCR products were cleaned using the Qiagen PCR Purification Kit (Qiagen, Germany) or Agencourt AMPure XP beads (Beckman Coulter, Inc., Brea, CA, USA), following the manufacturers’ instructions, and were visualized on 1% agarose gel stained with ethidium bromide. Sequencing reactions were performed by Eurofins Genomics (Louisville, KY, USA).
Table 3. PCR protocols used in this study for given loci.
Sequence alignment
Sequences were quality checked and sequence ends were manually trimmed in AliView (Larsson Reference Larsson2014; http://www.ormbunkar.se/aliview/). Each sequence was checked against the NCBI nucleotide database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to verify that the desired organism was sequenced. Alignments were visually checked in AliView and minor misalignments were manually adjusted.
Phylogenetic analyses
Maximum likelihood (ML) trees for each locus (not shown) were constructed in raxmlGUI 2.0 (Stamatakis Reference Stamatakis2014; Edler et al. Reference Edler, Klein, Antonelli and Silvestro2020), and the bootstrap support values for each clade were compared. Using a 70% bootstrap value threshold, clades were compared and conflict was assumed to be significant when a monophyletic group was supported with bootstrap values ≥ 70% within one locus and the same group of taxa was supported ≥ 70% as non-monophyletic within another locus (Mason-Gamer & Kellogg Reference Mason-Gamer and Kellogg1996). Because no strongly supported conflicts were observed between the four loci, downstream relationships and analyses were performed on the concatenated dataset. Analyses were run using raxmlGUI 2.0 to reconstruct a maximum likelihood concatenated 4-locus tree. We used Lepra albescens as the root and ran 1000 thorough ML bootstraps with the model set to GTRGAMMAI.
The Species
Aspicilia malvinae Fryday & T. B. Wheeler sp. nov.
MycoBank No.: MB 839030
Distinguished from other species of Aspicilia by the thalline chemistry (hypostictic acid) and sequence data.
Type: Falkland Islands, East Falkland, Lafonia, 3.5 km west of Walker Creek, east side of stream north of road, 51.97705°S, 58.82285°W, 21 m, low dolerite outcrop in Empetrum heath above stream, 12 November 2015, A. M. Fryday 11433 & A. Orange (MSC0057604—holotype).

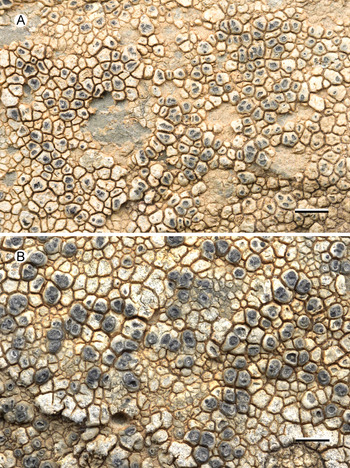
Fig. 1. Aspicilia malvinae (Fryday 11433, holotype). A, thallus with immersed apothecia. B, thallus with ±emergent apothecia. Scales = 2 mm. In colour online.
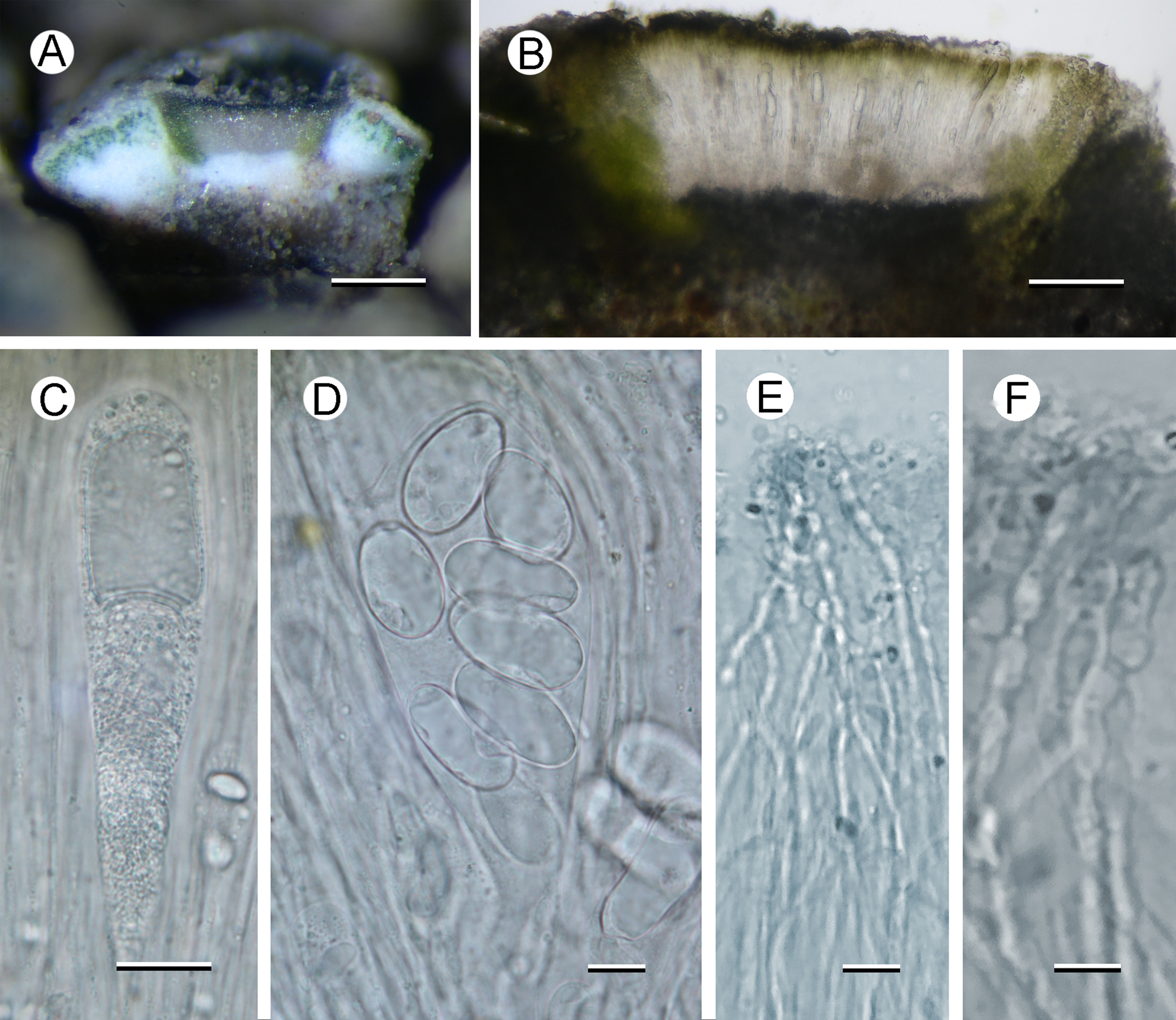
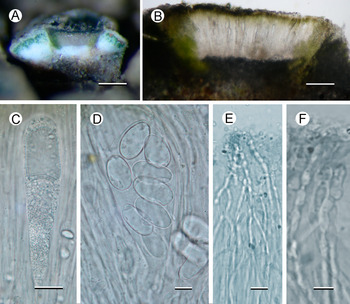
Fig. 2. Aspicilia malvinae (A–C, E & F, Fryday 11433, holotype; D, Fryday 11008). A, section through thallus and apothecium. B, section of apothecium. C, immature ascus. D, mature ascus with ascospores. E & F, paraphyses showing constricted septa in upper part (F). Scales: A = 2 mm; B = 100 μm; C & E = 20 μm; D & F = 10 μm. In colour online.
Thallus effuse, several centimetres across, cream to grey, areolate; areoles angular, convex, 0.2–0.5 mm across, usually contiguous but sometimes singular or in small groups on a black hypothallus, areoles separated by deep cracks. Prothallus black, fimbriate at the thallus edge, 0.5 mm wide. Upper cortex c. 50 μm thick with a thin epinecral layer 10–25 μm thick, upper 25 μm of cortex grey in visible light due to medium-sized crystals that only partially dissolve in K, crystals often most frequent between the cortex and the epinecral layer; cortical cells not observed in pale-coloured areoles but grey areas had cortical cells 5–6 μm diam. with a thin, pale grey-brown cap; lateral cortex pale brown due to numerous minute crystals that do not dissolve in K, slightly POL+, cells 5–8 μm diam. Photobiont layer c. 25 μm thick, cells chlorococcoid, 6–16 μm diam.
Apothecia abundant, usually immersed (aspicilioid) but sometimes becoming sessile with a prominent proper margin, black, 0.4–0.5 mm diam. when mature, usually 1 per areole, (occasionally two, rarely three); usually ±round, rarely oblong or linear, angular if > 1 per areole; thalline margin not apparent, formed by the thalline areole; proper margin densely pruinose when immature, becoming epruinose, 0.1 mm wide; disc black, slightly concave, pruinose when immature, becoming epruinose when mature. In section, exciple up to 250 μm wide at the surface, tapering to nothing where it merges with the subhymenium, pale brown but darker at the surface that is N+ green, composed of narrow (1–1.5 μm wide) conglutinated hyphae that become wider (c. 5 μm) and cellular with constricted septa for the final 4–5 cells towards the cortex. Hymenium 140–160 μm, I+ slowly yellow and after c. 5 minutes greenish blue; paraphyses very fine c. 1 μm, the upper 20–25 μm (epihymenium) wider (5 μm) with constricted septa, cells globose to oblong, 5 μm wide by 5–7 μm long; epihymenium olive-brown (K+ brown, N+ bright aeruginose; Caesiocinerea-green); subhymenium hyaline, I+ slowly (after c. 5 minutes) dark blue, composed of ±vertically aligned hyphae with the same kind of medium-sized hyaline crystals as in the cortex, merging with the hypothecium. Hypothecium hyaline, I+ slowly (after c. 5 minutes) bluish mauve, composed of thick (c. 5 μm) randomly organized hyphae. Asci cylindrical when immature, c. 110 × 20 μm, becoming broadly clavate when mature, 80–90 × 40–45 μm; ascospores 8 per ascus, hyaline, broadly ellipsoid, (22–)24.55 ± 1.63(–30) × (11–)14.65 ± 1.53(–18) μm, l/w ratio (1.28–)1.69 ± 0.19(–2.18), n = 20.
Pycnidia not observed.
Chemistry
Thallus C−, K−, KC−, Pd−, but in section slowly K+ yellow. TLC (solvent C): hypostictic acid (red spot at R f 4), faint red spot at R f 1 (probably subhypostictic acid), UV(after charring)++ cream spot at R f 8.
Notes
Of the species of Aspicilia reported from the Southern Hemisphere, only two (viz. Lecanora (Aspicilia) masafuerensis and A. mendozae) are candidates for an earlier name for our species. However, both are reported to have much smaller ascospores than our new species: those of L. masafuerensis are given as 10–18 × 6–8 μm (Zahlbruckner Reference Zahlbruckner and Skottsberg1924) and those of A. mendozae as 9–14 × 8–9 μm (Räsänen Reference Räsänen1941). Examination of specimens in MSC identified as L. masafuerensis and an isotype in NY, revealed slightly larger ascospores than those reported by Zahlbruckner (16.7–22.4 × 8.6–9.5 μm), but the specimens also had a dilute brown epihymenium, apparently Porpidia-type asci and are probably referable to Xenolecia spadicomma (Nyl.) Hertel. In addition, our species contains hypostictic acid as its primary secondary metabolite, which is an uncommon metabolite in lichens and, to the best of our knowledge, found as a major constituent in species of Megasporaceae only in the Chinese species A. abbasiana S. Y. Kondr. et al. (Kondratyuk et al. Reference Kondratyuk, Lőkös, Park, Jang and Jeong2016; syn. A. volcanica Ismayil et al. (Ismayil et al. Reference Ismayil, Abbas and Guo2015)). The ascospores of this species are a similar size ((13–)16–23(–26) × (10–)13–16 μm) to those of A. malvinae and it also occurs on igneous rock. However, it differs in the paraphyses being distinctly moniliform for their whole length, not just the upper cells as in our species, and in the thallus containing stictic and constictic acids in addition to hypostictic acid, which are apparently absent from the thallus of our species. In addition, A. abbasiana is phylogenetically closely related to A. cinerea (Fig. 3) and so is quite distant from our species.
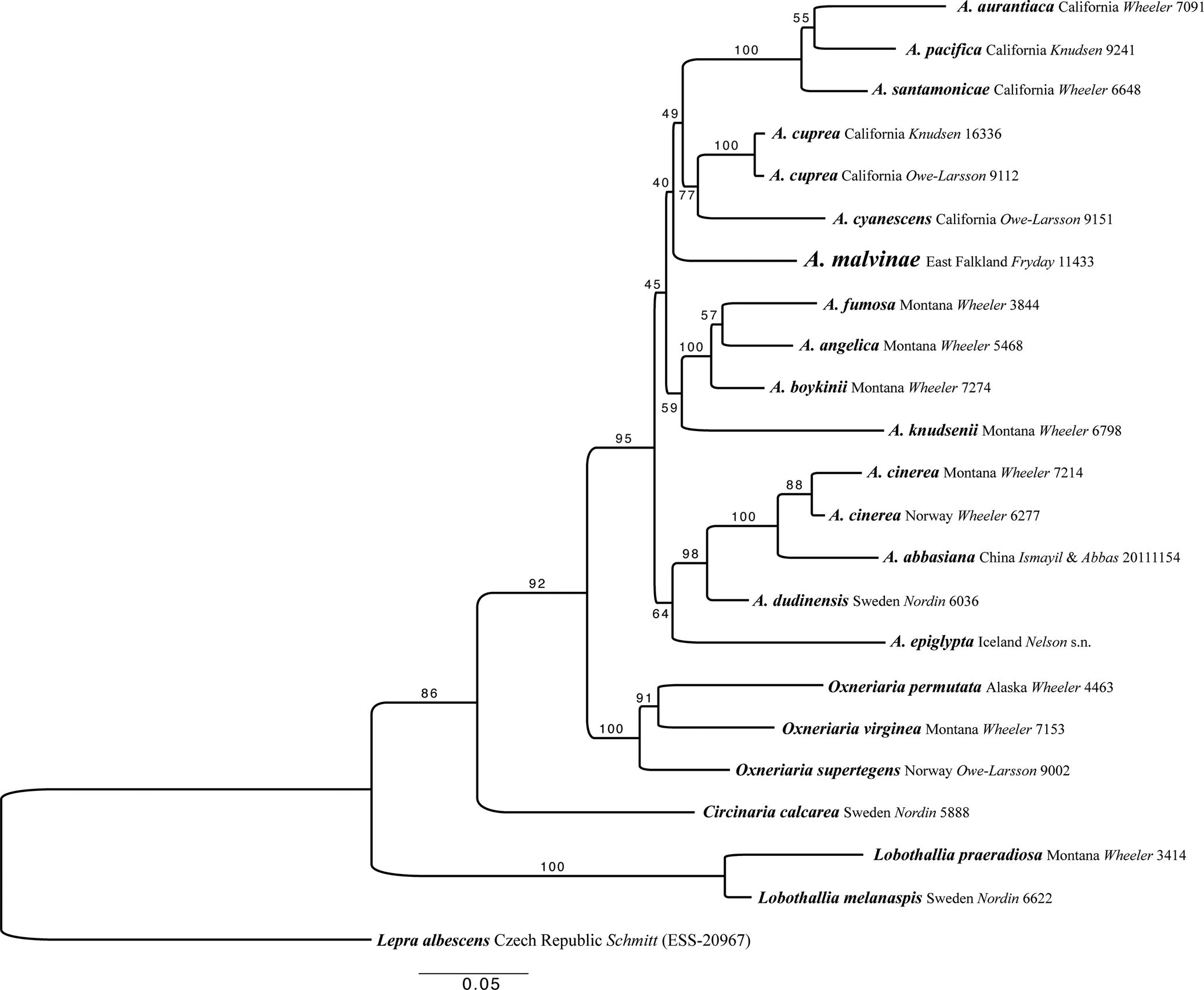
Fig. 3. Maximum likelihood (ML) tree of the concatenated (ITS, LSU, mtSSU and Mcm7) dataset for members of Aspicilia and related species. Analyses were performed using raxmlGUI 2.0 (Stamatakis Reference Stamatakis2014; Edler et al. Reference Edler, Klein, Antonelli and Silvestro2020). Maximum likelihood bootstrap values are shown above each branch. The newly introduced species is in larger font and voucher information for all specimens is provided (see Table 1 for further details). Abbreviation: A. = Aspicilia.
The four collections of this species were all collected from rocks close to streams, the holotype and the two collections from West Falkland (Fryday 11008, Orange 23271) from near the coast, and the other collection from East Falkland (Fryday 11431) from a somewhat higher altitude (67 m) inland. The two collections from East Falkland were on dolerite and although the site from which both the West Falkland collections were made, the Patricia Luxton NNR, is also primarily dolerite, at least one of the collections (Fryday 11008) was from sandstone. Unfortunately, the other collection from East Falkland (Orange 23171) was not accessible for this study.
The holotype and one of the West Falkland collections (Orange 23271) had a nearly identical ITS sequence and the three coastal collections were also morphologically similar. The inland collection (Fryday 11431) was also morphologically similar although the paraphyses appeared to be slightly more moniliform (the area with constricted septa extending through the upper 5–6 cells, whereas for the other collections only the upper 3–4 cells had constricted septa) but this appears to be a variable character. However, the presence of hypostictic acid, an uncommon substance in Aspicilia, as a major substance in all three available collections (Fryday 11008, 11431 & 11433) strongly suggests that they represent a single taxon. Mature asci and ascospores were infrequent in the holotype collection and the other collection from East Falkland (Fryday 11431), although these were frequent in a collection from West Falkland (Fryday 11008).
Aspicilia malvinae is in a highly supported group outside of the A. cinerea clade and was recovered as sister to the ‘cyanescens’ clade and the ‘americana’ clade, but with no support (Fig. 3). This ambiguity arises as the single gene trees of ITS and LSU place A. malvinae either within the unsupported ‘americana’ group or within the unsupported ‘cyanescens’ group, respectively. The relationships within Aspicilia s. str. are unresolved, not due to gene tree discordance, because none of the single gene trees are supported at any of these conflicting nodes, but due to a lack of data from within the group. More sampling is needed to resolve the relationships between the clades within Aspicilia s. str. However, the separation of A. malvinae from the A. cinerea clade, which contains A. abbasiana, the only other Aspicilia species containing hypostictic acid as a major substance, is highly supported. The relationships between the genera Lobothallia, Circinaria, Oxneriaria and Aspicilia are also highly supported here (Fig. 3).
Additional collections examined
Falkland Islands: West Falkland: Chartres, Patricia Luxton NNR, 51.72560°S, 59.98474°W, 15 m, sloping rock with Bucklandiella sp., 2015, Fryday 11008 (MSC); ibid., 51.72824°S, 59.98484°W, seasonally irrigated bedrock sloping at 40°, level with ground, unshaded, aspect 60°, with Pertusaria alterimosa, Pertusaria cerebrinula/spegazzinii, Parmelia saxatilis, Massalongia patagonica, Bucklandiella sp., 2015, A. Orange 23271 (NMW). (The amount of moss suggests that this is a moist microhabitat by Falkland Islands standards). East Falkland: Mt Usborne, Camilla Creek, 51.716467°S, 58.898183°W, 67 m, bare, stony area in grass/Empetrum heath, 2015, A. M. Fryday 11431 (MSC).
Lichenicolous fungi
The collections of Aspicilia malvinae support two lichenicolous fungi: an Endococcus that is best accommodated in Endococcus propinquus s. lat. (Körb.) Trevis., reported here for the first time from the Falkland Islands, and a species of Sagediopsis that is frequent on the holotype and is described here as new to science.
Endococcus propinquus s. lat. (Körb.) Trevis.
Conspect. Verruc., 17 (1860).
Ascomata 0.15–0.20 mm diam. with a depressed ostiole; ascospores brown, 1-septate, broadly ellipsoid (8–)9.45 ± 0.76(–10) × (5.5–)6.6 ± 0.77(–8) μm; l/w ratio (1.125–)1.44 ± 0.14(–1.67); n = 10 (Fryday 11431—MSC).
Two species of Endococcus were reported by Diederich et al. (Reference Diederich, Lawrey and Ertz2018) as occurring only on Aspicilia: E. calcaricola (Mudd) Nyl. (as Microthelia calcaricola Mudd) and E. verrucosus Hafellner. The application of the name Endococcus calcaricola is uncertain; it has been included as a synonym of E. rugulosus Nyl. (Index Fungorum Partnership 2021), which has ascospores 13–17 × 5–8 μm (Ihlen & Wedin Reference Ihlen and Wedin2008). Hafellner (Reference Hafellner1994) gives the ascospore dimensions of E. verrucosus as (13–)14–17(–18) × 7–9 μm, whereas Zhurbenko & Notov (Reference Zhurbenko and Notov2015) report them as (7.5–)10–15 (–21) × (5–)6–8(–10) μm. Therefore, the ascospores of both these species are notably larger than those of the species reported here. Two other species were mentioned by Diederich et al. (Reference Diederich, Lawrey and Ertz2018) as occurring on several lichen genera including Aspicilia, and by Ihlen & Wedin (Reference Ihlen and Wedin2008) as occurring on Aspicilia: E. perpusillus Nyl., with ascospores (12–)15–25(–30) × 5–9 μm, and E. propinquus s. lat., with ascospores 7–11 μm long (Ihlen & Wedin Reference Ihlen and Wedin2008). Although E. propinquus s. str. occurs only on the thalli of Porpidia spp., E. propinquus s. lat. is widespread and has been recorded on a wide variety of crustose lichens, including Aspicilia (Ihlen & Wedin Reference Ihlen and Wedin2008), and this would appear to be the best accommodation for our fungus.
Specimen examined
Falkland Islands: East Falkland: Mt Usborne, Camilla Creek, 51.716467°S, 58.898183°W, 67 m, bare, stony area in grass/Empetrum heath, 2015, A. M. Fryday 11431 (MSC).
Sagediopsis epimalvinae Etayo, T. B. Wheeler & Fryday sp. nov.
MycoBank No.: MB 839031
Lichenicolous fungus growing on Aspicilia malvinae. Similar to Sagediopsis fissurisedens that grows on Aspilidea (syn. Aspicilia) myrinii, but differing in the much smaller perithecia, 0.15–0.20 mm diam., smaller asci, 52–60 × 11–15 μm, and longer and narrower ascospores (16–)18–20(–22) × (3.5–)4–5(–6) μm.
Type: Falkland Islands, East Falkland, Lafonia, 3.5 km west of Walker Creek, east side of stream north of road, 51.97705°S, 58.82285°W, 21 m, lichenicolous on Aspicilia malvinae on a low dolerite outcrop in Empetrum heath above stream, 12 November 2015, A. M. Fryday 11433a & A. Orange (MSC0057605—holotype).
(Fig. 4)
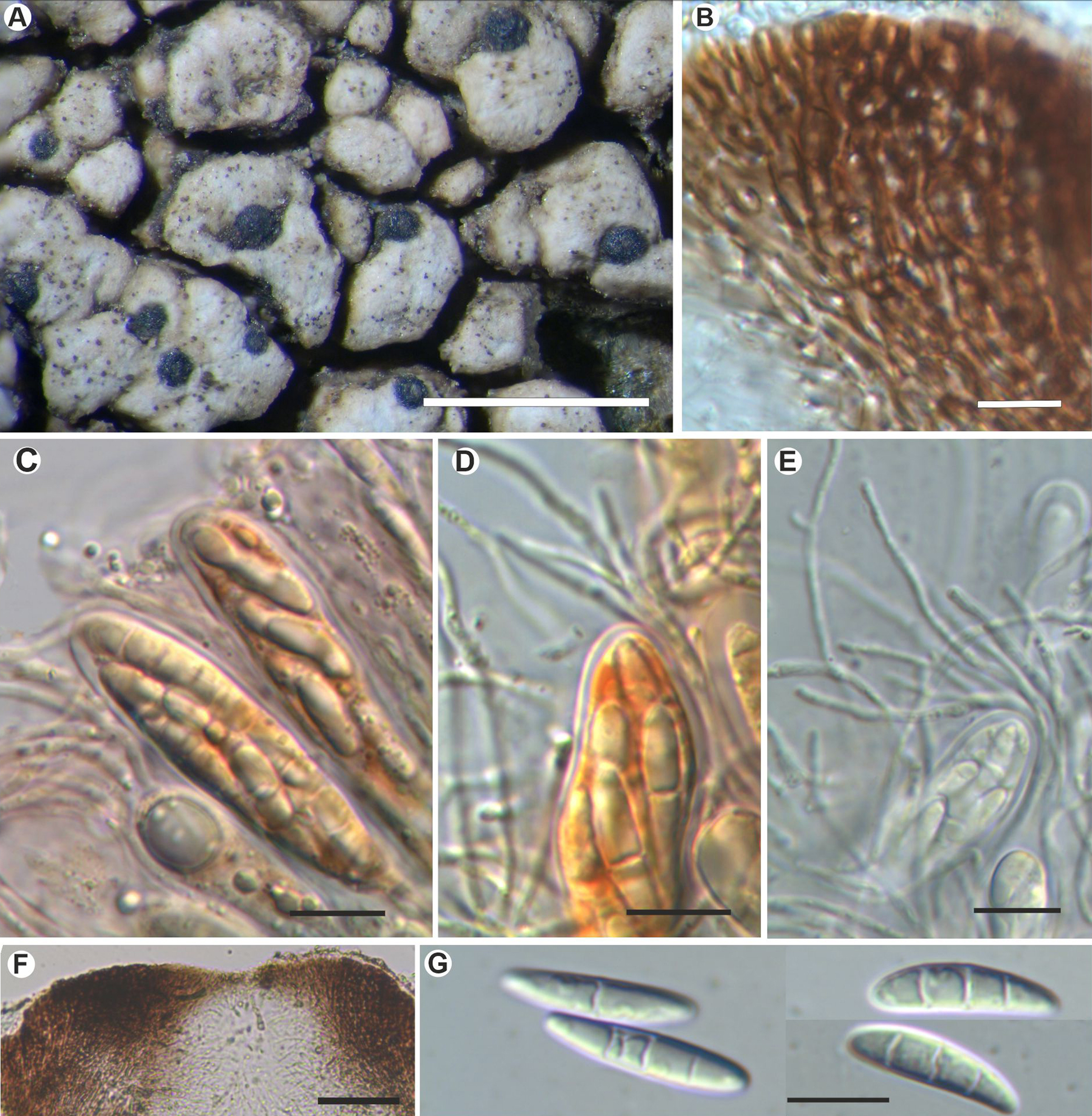
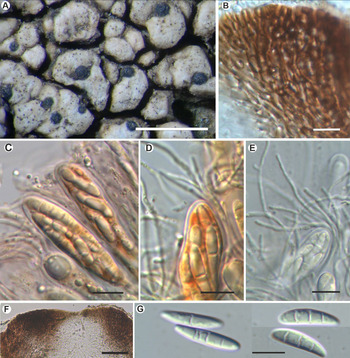
Fig. 4. Sagediopsis epimalvinae (Fryday 11433a, holotype). A, habitus of several Sagediopsis perithecia on areolae of Aspicilia malvinae. B, upper part of exciple showing elongated hyphae disposition and periphysoids in ostiolar channel. C & D, upper part of asci and paraphyses (in I). E, ascus with simple and branched paraphyses (in water). F, upper part of a perithecium showing the thickened upper exciple and periphysoids. G, ascospores. Scales: A = 1 mm; B–E & G = 10 μm; F = 25 μm. In colour online.
Independent thallus not formed but apothecia production in the host is apparently suppressed, hyphae below ascomata hyaline.
Ascomata perithecioid, not clypeate but with the wall markedly thickened above, 150–200 μm diam., obovate but flattened above, not radially split, with central ostiole sometimes not visible, black, matt, immersed, finally slightly protruding from the thallus of Aspicilia malvinae. Exciple entire, black, greatly thickened apically giving a flat surface up to 100 μm thick, in section surrounded by a hyaline, non-cellular coat 4–5 μm thick, dark brown above to somewhat lighter brown below, K−, hyphae around ostiole elongated; base and lateral parts of exciple 15–20(–35) μm thick, composed of several layers of more or less elongated cells with a thick cell wall and small lumina, 4–7 × 1.5–2 μm. Periphysoids growing down from around ostiole, abundant, immersed in a colourless gel, simple to branched, even anastomosed, especially at the base, 10–30 × 1 μm. Hymenial gel I+ reddish, KI+ blue; subhymenium with large colourless oil droplets, 1–6 μm diam. Hamathecium of persistent paraphysoids, flexuose, not thickened at the apex, septate, simple or sparingly branched, not constricted at the septa, 1–1.5 μm thick, with many small colourless oil droplets. Asci clavate to cylindrical, with a short ‘foot’, thin-walled laterally (1 μm) but thicker apically, 2–3 μm, 8-spored, 52–60 × 11–15 μm (n = 6), wall I+ reddish, turning bluish in part and KI+ pale blue, tholus I−, ascoplast I+ orange. Ascospores biseriate in the asci, hyaline only finally slightly brownish, ellipsoid to fusiform to slightly soleiform, with upper part wider than lower, (0–)3-septate, not or hardly constricted at the septa, with a large oil droplet in each cell occupying most of the cell except the very ends, thin and smooth-walled, without perispore, straight, rarely curved, (16–)18–20(–22) × (3.5–)4–5(–6) μm (n = 40).
Conidiomata not observed.
Notes
Our new species fits well with the genus Sagediopsis by having immersed perithecioid ascomata with a flattened upper zone; a thickened upper exciple formed by elongated hyphae around the ostiole; periphysoids immersed in gel growing down from around the ostiole; abundant paraphysoids intermixed with the asci; a hymenial gel reacting reddish to blue with Lugol's reagent or IKI; Verrucaria-type asci that are thickened apically and 8-spored; ellipsoid to fusiform, transversally septate ascospores.
No other species of Sagediopsis are known from Aspicilia, although two were reported from Aspilidea myrinii (Fr.) Hafellner before it was transferred from Aspicilia to Aspilidea: S. fissurisedens Hafellner (Hafellner Reference Hafellner1993) and S. aspiciliae Nik-Hoffm. & Hafellner (Hoffmann & Hafellner Reference Hoffmann and Hafellner2000). Sagediopsis fissurisedens has several features in common with our new species: ascomata with the exciple markedly thickened above, a similar hamathecium, 8-spored asci and 3-septate ascospores. However, it differs in the much larger perithecia (0.4–0.7 mm diam.), the larger asci (60–80 × 13–17 μm) that are I−, and the slightly shorter but notably wider ascospores, 12–17 × 5–8 μm. Sagediopsis aspiciliae is very different, also with larger ascomata (120–360(–400) μm diam.), larger asci (60–100 × 9–13.5 μm) and ascospores that are simple to rarely 1-septate and ellipsoidal, (10–)10.5–14.1(–15) × (5–)5.2–6.9(–8) μm, but it has a hemiamyloid hymenium gel similar to S. epimalvinae.
Sagediopsis species have also been reported from other genera of Pertusariales. Sagediopsis pertusariicola Zhurb. was described growing on Pertusaria (Zhurbenko Reference Zhurbenko2009). This species has larger, glossy perithecia, (200–)250–400(–500) μm diam., and ascospores with upper and lower parts mostly equal in width, sometimes with pointed ends. Sagediopsis campsteriana (Lindsay) D. Hawksw. & R. Sant. is very similar to our new species but seems to be an exclusive parasite of Ochrolechia sp. Hawksworth (Reference Hawksworth1975) and Triebel (Reference Triebel1993) described it with ellipsoid ascospores, (1–)3(–4)-septate, (12–)15–20(–25) × 4–6 μm. According to the most recent description by Zhurbenko (Reference Zhurbenko2009), its ascomata are larger, 150–250(–400) μm diam., and usually immersed to sometimes erumpent to almost superficial (a sessile, obpyriform perithecium is drawn in Hawksworth (Reference Hawksworth1975)) and it has slightly larger asci, 60–73 × 10–13 μm. In all of these species growing on Pertusariales, a subhymenium with many colourless oil droplets has not been recorded.
Other species of Sagediopsis are known from species of Lecideaceae. Sagediopsis aquatica (Stein) Triebel (Rambold et al. Reference Rambold, Hertel and Triebel1990), which occurs on Koerberiella wimmeriana (Körb.) Stein and is known only from Europe, has ascospores (22–)27–36(–45) × (2.5–)3–3.5(–4) μm that are narrowly fusiform to acicular and acuminate at the basal end. Sagediopsis barbara (Th. Fr.) R. Sant. & Triebel, which is restricted to Porpidia spp. (Triebel Reference Triebel1989), has larger ascomata (to 450 μm diam.) and larger ascospores (20–)27–39.5(–46) × (3–)3.5–4.5(–5) μm. Sagediopsis dissimilis Triebel was described growing on Paraporpidia leptocarpa (Nyl.) Rambold & Hertel in Australasia (Triebel Reference Triebel1993) and has 0–1-septate ascospores, (7.5–)8–10.5(–12) × (4–)4.5–6(–6.5) μm.
Other species of the genus have been reported from a range of unrelated host genera. Sagediopsis bayozturkii Halıcı et al. was described on Acarospora macrocyclos (Halıcı et al. Reference Halıcı, Güllü and Parnikoza2017). It differs from S. epimalvinae by its smaller perithecia (90–150 μm diam.) and smaller ascospores ((10–)11–14(–15) × 4–5 μm), as well as in several other features. Sagediopsis lomnitzensis (Stein) Orange on Ionaspis lacustris (With.) Lutzoni and I. odora (Ach.) Th. Fr. (Orange Reference Orange2002) has a perithecial wall that is I+ sometimes violet to blue in part, and smaller, 1-septate, halonate ascospores, (9.5–)11–18 × 5–7(–8) μm. Sagediopsis vasilyevae Zhurb. on Rhizocarpon has much larger ascospores, (37.5–)41.7–50.3(–53.0) × (2.5–)2.9–3.5(–3.8) μm (Zhurbenko & Yakovchenko Reference Zhurbenko and Yakovchenko2014).
Acknowledgements
Fieldwork on the Falkland Islands by the first author was funded by the UK Government through DEFRA and the Darwin Initiative as part of the project Lower Plants Inventory and Conservation in the Falkland Islands (Reference number DPLUS017). Support for fieldwork and advice regarding landowners was provided by Falklands Conservation. We are grateful to Alan Orange (Cardiff) for sharing his unpublished sequences of Aspicilia malvinae with us and to Kerry Knudsen (Prague) for additional specimens included in this study. We also thank the curators of NY for the loan of their isotype of Lecanora masafuerensis, and the curators of BM, GB, UPS & W for details of the type specimens of this species housed in their herbaria.
Author ORCIDs
Alan Fryday, 0000-0002-5310-9232; Timothy Wheeler, 0000-0003-0668-8662; Javier Etayo, 0000-0003-0392-0710.