High, and very high, fasting serum TAG concentrations are associated with a high risk of CVD( Reference Austin1 – Reference Liu, Zeng and Liu3 ) and of pancreatitis, respectively( Reference Valdivielso, Ramirez-Bueno and Ewald4 ). Long-chain n-3 PUFA (LCn3-PUFA), including DHA (22 : 6n-3) and EPA (20 : 5n-3), are known to decrease TAG concentrations( Reference Kris-Etherton, Harris and Appel5 ). However, results from clinical studies have shown that there is a large inter-individual variability in the TAG response to LCn3-PUFA supplementation( Reference Caslake, Miles and Kofler6 – Reference Madden, Williams and Calder10 ). The heterogeneous TAG response to LCn3-PUFA may be attributed to many factors, including measurement errors in the laboratory, day-to-day biological variations, genetic factors and their interaction with other extrinsic factors such as the background diet and concurrent medications( Reference Meisel, Gerloff and Kirchheiner11 ).
A number of studies have examined the variability in TAG response to LCn3-PUFA supplementation( Reference Caslake, Miles and Kofler6 – Reference Madden, Williams and Calder10 ). In the Fish Oil Intervention and Genotype Study, 8-week supplementation of fish oil (1·8 g EPA+DHA/d) reduced mean TAG concentrations by 11·2 % compared with baseline values among healthy subjects( Reference Caslake, Miles and Kofler6 – Reference Madden, Williams and Calder10 ). However, <70 % were defined as responders, that is, with a reduction in TAG >0 mmol/l( Reference Caslake, Miles and Kofler6 , Reference Madden, Williams and Calder10 ). A similar proportion of participants of the Fatty Acid Sensor Study presented a reduction in TAG concentrations after 6-week supplementation with LCn3-PUFA (1·9–2·2 g EPA + 1·1 g DHA/d)( Reference Cormier, Rudkowska and Paradis12 ). One may argue that using a threshold of 0 mmol/l to identify responders (any reduction in TAG concentrations) and non-responders (any increase in TAG concentrations) provides a limited perspective on the true variability of the response to LCn3-PUFA. Indeed, TAG concentrations are known to vary intra-individually by about 20 %( Reference Bookstein, Gidding and Donovan13 ). Thus, any individual change in TAG concentrations that remains within the boundaries of usual intra-individual variation should not be considered as a truly meaningful change. Intra-individual variation, which includes biological variation and analytical variation, may be calculated as the average within-subject standard deviation based on two or more measurements taken on different days when no true change is expected. Any change smaller than this intra-individual variation should be considered within the variability of the measurement, and thus as a non-response. To our knowledge, no study has yet documented the proportion of ‘true’ responders to LCn3-PUFA supplementation.
Furthermore, almost all previous clinical trials on LCn3-PUFA supplements have tested the combined impact of DHA and EPA in various forms and proportions. After years of negative results in studies investigating the association between LCn3-PUFA supplements and cardiovascular risk, the Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT) has recently shown that supplementation with 4 g/d EPA reduced the risk of cardiovascular events by 25 % compared with a mineral oil placebo in individuals with high baseline TAG concentrations( Reference Bhatt, Steg and Miller14 ). These observations highlight the importance of investigating further the individual effects of DHA and EPA on cardiometabolic health. To date, data indicate that DHA may be more potent than EPA in modulating lipids and inflammatory risk factors( Reference Wei and Jacobson15 , Reference Allaire, Couture and Leclerc16 ), including TAG concentrations( Reference Allaire, Couture and Leclerc16 ), but very few studies have undertaken a head-to-head comparison of the differential effect of high-dose DHA and EPA supplementation. The inter- and intra-individual variabilities in TAG response to DHA and EPA, taken separately, remain unknown.
The first objective of the present study was to compare the proportions of individuals whose serum TAG concentrations lowered after high-dose supplementation with either DHA and EPA among men and women with abdominal obesity and a subclinical inflammatory state. The second objective was to identify factors that predict the serum TAG response to DHA and EPA supplementation. We hypothesised that high-dose DHA will meaningfully reduce serum TAG concentrations in more individuals than a similar dose of EPA. We also hypothesised that individuals with the highest serum TAG concentrations at baseline will experience the greatest TAG reductions after both DHA and EPA.
Material and methods
Study design
Details of the study design have been published previously( Reference Allaire, Couture and Leclerc16 ). The present study used a double-blind, randomised, controlled, crossover design with three treatment phases: 3 × 1 g capsule of 90 % purified LCn3-PUFA/d providing (1) 2·7 g/d DHA, (2) 2·7 g/d EPA, (3) 0 g/d DHA+EPA (maize oil control). Each treatment phase had a median duration of 10 weeks and were separated by 9-week washouts. The original and primary objective of the present study was to compare the effect of DHA and EPA on plasma C-reactive protein (CRP) concentrations, and thus, CRP was used as the primary outcome measure for sample size calculation( Reference Allaire, Couture and Leclerc16 ). A priori sample size calculations indicated that n 150 would be needed to detect a minimal difference of 10 % in plasma CRP concentrations when comparing any two of the three treatments with a power of 81 % and P < 0·01 >(two-tailed)( Reference Allaire, Couture and Leclerc16 ). The sample size of n 150 also provided 92 % power to detect a 10 % difference in serum TAG between treatments at P = 0·01 (two-tailed, not shown). This was a secondary analysis of this trial. A total of 154 participants were randomised using an in-house computer program, and all participants signed an informed consent document that was approved by local ethics committees at the beginning of the study. The study protocol was registered on 4 March 2013 at ClinicalTrials.gov (NCT01810003), and the study was conducted according to the Declaration of Helsinki. Allocation to treatments was concealed to participants as well as to study coordinators throughout the study.
Study population
Primary eligibility criteria were to have abdominal obesity (waist circumference ≥80 cm for women, ≥94 cm for men)( Reference Alberti, Zimmet and Shaw17 ) with a subclinical inflammatory state reflected by plasma CRP concentrations >1 but <10 mg/l.
Compliance
Compliance to supplementation was assessed by counting supplements that were returned by participants. As previously reported, subjects with a compliance <80 % were considered non-compliant and therefore excluded from analyses (n 2)( Reference Allaire, Couture and Leclerc16 ).
Assessment of anthropometric and cardiometabolic markers
Methods of the present study have been published previously( Reference Pirro, Bergeron and Dagenais18 , Reference Allaire, Vors and Tremblay19 ). Briefly, anthropometric measures, including weight and waist circumference, were obtained according to standardised procedures( Reference Lohman and Roche20 ) at screening, beginning and end of each treatment phase. Blood samples were collected after a 12-h overnight fasting at screening, beginning of each treatment phase and at the end of each treatment phases. Total cholesterol, LDL-cholesterol (LDL-C), HDL-cholesterol (HDL-C), TAG, total apo B100 (apo B), CRP, adiponectin, IL-6, LDL peak particle size, mean LDL particles size, proportion of LDL in the various size categories (small and large), insulin and glucose were measured before and after each study phase. TNF-α, proprotein convertase subtilisin/kexin type 9 (PCSK9) and IL-18 were measured after each treatment phase. Serum non-HDL-C concentration was calculated as the difference between total serum cholesterol concentrations and HDL-C concentrations.
Fatty acid composition of erythrocyte membranes was analysed by OmegaQuant Analytics, LLC according to the Omega-3 Index methodology( Reference Allaire, Harris and Vors21 ). Each fatty acid was expressed as a weight percentage of total identified fatty acids after a response factor correction (based on calibration curves) was applied to each fatty acid.
Gene expression of lipid metabolism
Fasting fresh blood was collected in PAXgene Blood RNA tubes (Becton Dickinson) after each treatment in a subsample of 44 randomly selected participants for gene expression analyses. RNA was isolated using a PAXgene RNA-kit according to the manufacturer’s instructions (Qiagen). The quantity of total RNA was measured using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies), and total RNA quality was assayed on an Agilent BioAnalyzer 2100 (Agilent Technologies). Reverse transcription was performed on 1·5–2 μg total RNA. cDNA corresponding to 20 ng of total RNA was used to perform fluorescent-based real-time PCR quantification using LightCycler 480 (Roche Diagnostics). The genes targeted were 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase), LDL-receptor (LDL-R) and sterol regulatory element-binding protein 1c and 2 (SREBP1c and SREBP2). Values were normalised to the expression of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
All personnel involved in the intervention and measurements of study outcomes were blinded to treatments.
Statistical analyses
As per our a priori defined statistical plan( Reference Allaire, Couture and Leclerc16 ), and since the maize oil control treatment had no significant impact on serum TAG concentrations compared with baseline value (–0·04±0·04 mmol/l; P = 0·31; data not shown), the change in serum TAG concentrations after DHA and EPA supplementation was calculated as the difference between post-treatment serum TAG concentrations (EPA or DHA) minus serum TAG concentrations after the control phase.
Mean intra-individual variation in serum TAG concentrations, reflecting the combination of usual biological as well as analytical variation, was calculated as the standard deviation of mean TAG values from four off-treatment samples, that is, one sample taken at screening and samples taken before each of the three treatment phases, which were preceded by a median washout period of 9 weeks from the previous treatment. The mean intra-individual variation in TAG concentrations in our sample was ±0·25 mmol/l. The median intra-individual variation was ±0·20 mmol/l (min. ±0·04 mmol/l; max. ±0·91 mmol/l). Responders to either DHA or EPA were defined as those in whom serum TAG reduced by >0·25 mmol/l compared with the control phase. Non-responders were those in whom TAG variation was within the ±0·25 mmol/l variation. Concordant responders were those in whom both DHA and EPA supplementation reduced serum TAG concentrations by >0·25 mmol/l compared with the control treatment.
Pearson correlation analysis was used to assess the association between TAG response to DHA and TAG response to EPA. Changes in anthropometric measurements and cardiometabolic marker values among the subgroups of responders were compared using generalised linear models (proc MIXED with a variance components (VC) or a compound symmetry (CS) covariance matrix for unrepeated and repeated measurements, respectively) for continuous variables and Fisher’s exact tests for categorical variables. Models with the VC covariance matrix were adjusted for age, sex and waist circumference at screening, and models with the CS covariance matrix were adjusted for age, sex, waist circumference at screening and value of the variable of interest after the control phase. EPA- and DHA-induced changes in cardiometabolic outcomes compared with values after the control phase were determined with the LSMEANS (least squares means) statement and were tested against the null hypothesis in the mixed models. Differences in cardiometabolic outcomes between EPA and DHA were determined by the main treatment effect (no post hoc test needed)( Reference Allaire, Couture and Leclerc16 ). The skewness of the distribution of the variables and model residuals was assessed and data were transformed when required. All analyses were computed using SAS v9.3 (SAS Institute Inc.).
Results
Subjects’ characteristics
As shown previously, of the 154 individuals randomised to the treatment sequences, a total of 121 subjects completed all three study phases and were included in the analyses( Reference Allaire, Couture and Leclerc16 ). Screening characteristics of these subjects are presented in Table 1. All subjects had a relatively high waist circumference and serum CRP concentrations as per the main entry criteria. The mean age was 58±10 years for men (n 36) and 52±16 years for women (n 85). The mean screening TAG concentration was within the normal range and similar among men and women (1·4±0·8 υ. 1·4±0·7 mmol/l, respectively).
Table 1. Characteristics at screening of the 121 subjects included in the analyses
(Mean values and standard deviations; numbers and percentages)
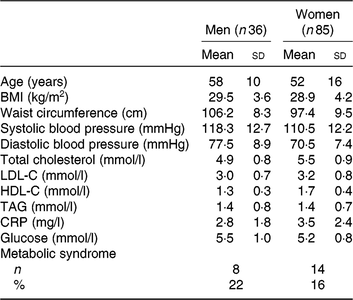
LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; CRP, C-reactive protein.
TAG response to DHA and EPA
As previously shown, the overall mean reduction in serum TAG concentrations compared with control was greater with DHA than with EPA (–13·3 υ. –11·9 %; P < 0·01)(Reference Allaire, Couture and Leclerc16). The individual TAG response to DHA and EPA supplementation is presented in Fig. 1. The proportion of responders (those in whom serum TAG concentrations reduced by >0·25 mmol/l) was greater with DHA than with EPA (45 and 32 %, respectively; P < 0·001). However, the TAG reduction was similar in magnitude between the two groups (–0·59 mmol/l for DHA and –0·57 mmol/l for EPA). Inversely, the variation in TAG concentrations remained within the ±0·25 mmol/l intra-individual variation range in fewer participants after DHA than after EPA (47 and 57 %, respectively; P < 0·01). The proportion of participants in whom serum TAG concentrations increased by >0·25 mmol/l was similar after DHA and EPA (8 and 11 %, respectively; P = 0·29).
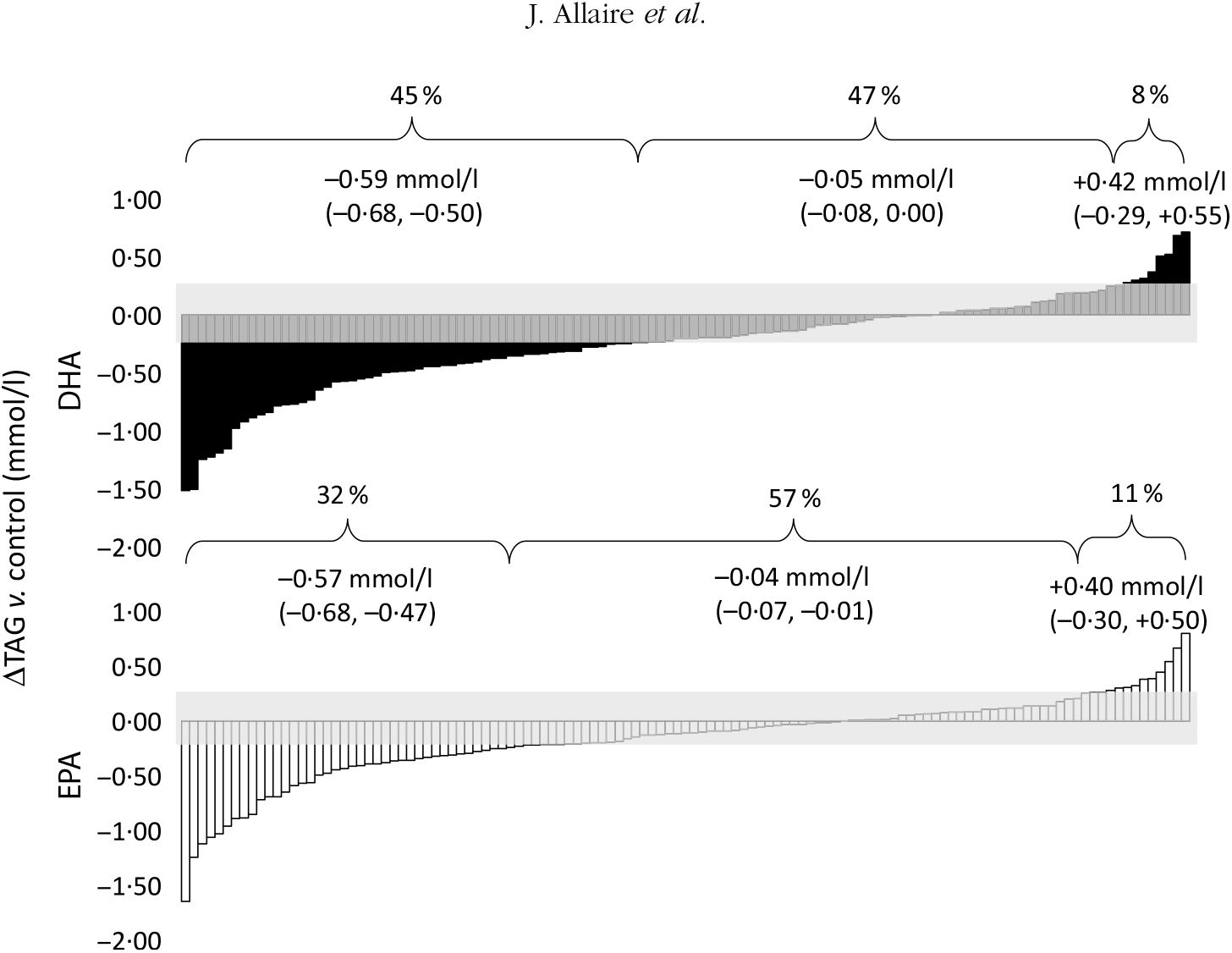
Fig. 1. Individual changes in TAG (v. control) after DHA and EPA supplementation. One column represents one subject. Data were sorted to show the range of variations in TAG response after both DHA (▪) and EPA (□). The grey zone represents the intra-individual variation range of ±0·25 mmol/l. Values in brackets are 95 % confidence intervals. For DHA: 45 % (n 54) were responders, 47 % (n 57) were non-responders and 8 % (n 10) showed an increase in TAG >0·25 mmol/l. For EPA: 32 % (n 39) were responders, 57 % (n 69) were non-responders and 11 % (n 13) showed an increase in TAG >0·25 mmol/l.
The percentage of shared variance in TAG response to DHA and EPA was 39·0 % (r 0·62; P < 0·001; data not shown). A total of 26 % participants were concordant responders to both DHA and EPA; 34 % presented no change in TAG after both DHA and EPA supplementation; and 2 % presented an increase in TAG after both supplements (data not shown). A total of 2 % participants presented a fully discordant TAG response to DHA and EPA (reduction >0·25 mmol/l in TAG with DHA and increase >0·25 mmol/l with EPA, or vice versa; data not shown).
Concordant responders to DHA and EPA
Table 2 presents the characteristics of the participants with a concordant reduction (n 32) in TAG concentration after DHA and EPA supplementation compared with all other participants, after the control phase. Responders to both DHA and EPA had higher serum non-HDL-C (4·01 v. 3·50 mmol/l; P = 0·02), TAG (1·90 v. 1·21 mmol/l; P < 0·001), PCSK9 (241·35 v. 203·00 ng/ml; P = 0·02) and insulin (110·34 v. 98·40 pmol/l; P = 0·03) concentrations and a greater proportion of large LDL particles (11·50 v. 8·35 %; P = 0·03) after the control phase compared with the other participants (i.e. non-responders and those with a discordant response to EPA and DHA). The reduction in TAG concentration among concordant responders was similar after DHA and EPA supplementation (–0·67 v. –0·61 mmol/l, respectively; P = 0·22; online Supplementary Table S1). However, the increase in HDL-C concentration among concordant responders was greater after DHA than after EPA supplementation (+0·14 v. –0·01 mmol/l, respectively; P < 0·001) and the reduction in IL-18 concentrations was greater after DHA than after EPA supplementation (–36·79 v. –10·60 pg/ml, respectively; P = 0·02; online Supplementary Table S1).
Table 2. Characteristics after the control phase of participants with a concordant reduction in TAG concentration after both DHA and EPA supplementation and of participants among other categories of responders to DHA and EPA
(Unadjusted mean values and standard deviations; numbers and percentages)
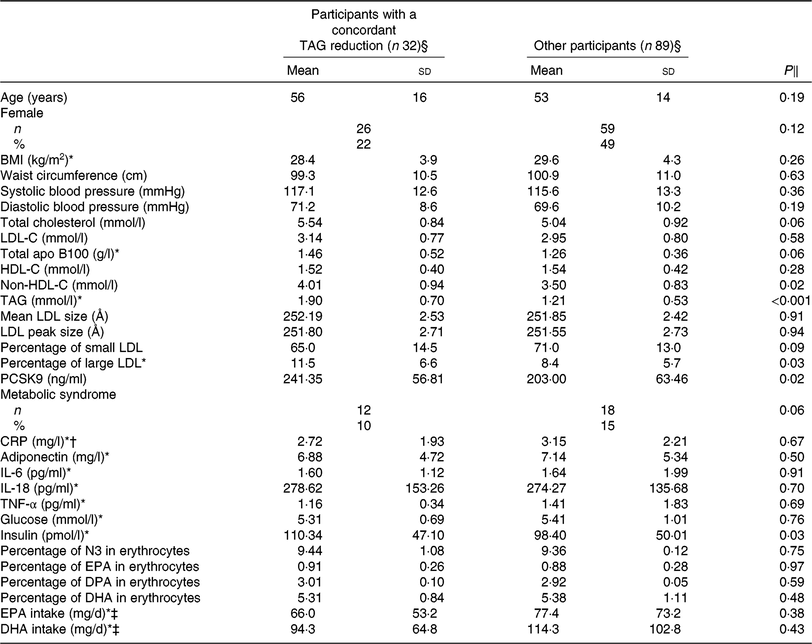
LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; CRP, C-reactive protein; N3, long-chain n-3 fatty acids; DPA, docosapentaenoic acid.
* Analyses were performed on log-transformed values due to the skewness of the distribution.
† n 29 for the concordant reduction and 84 for other responders due to the exclusion of CRP >10 mg/l.
‡ n 27 for concordant reduction and 77 for other responders.
§ Concordant reduction group comprised individuals with a reduction in TAG concentration >0·25 mmol/l after both DHA and EPA. Other responders group included all other individuals.
‖ P values were obtained with generalised linear models for continuous values or Fisher’s exact tests for proportions. Models were adjusted for age, sex and waist circumference at screening.
Correlates of TAG response to DHA
Compared with non-responders to DHA, responders to DHA had a greater concentration of TAG (P < 0·001), PCSK9 (P < 0·01) and insulin (P = 0·02) after the control phase (Table 3). As shown in Table 4, a reduction in TAG concentration among responders to DHA supplementation was observed along with a concomitant reduction in BMI (–0·3 kg/m2; P = 0·03), diastolic blood pressure (–2·4 mmHg; P = 0·04), PCSK9 (–40·1 ng/ml; P < 0·001), CRP (–0·33 mg/l; P = 0·02) and IL-18 (–35·1 pg/ml; P = 0·003) concentrations, as well as an increase in HDL-C (+0·13 mmol/l; P = 0·001). Changes in BMI, total cholesterol and non-HDL-C concentrations with DHA were significantly different among TAG responders and non-responders to DHA (all P < 0·05; Table 4).
Table 3. Anthropometric measures and cardiometabolic risk factors of different groups of responders to DHA supplementation after the control phase
(Unadjusted mean values and standard deviations; numbers and percentages)

BP, blood pressure; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; CRP, C-reactive protein; N3, long-chain n-3 fatty acids; DPA, docosapentaenoic acid.
a,b Mean values with unlike superscript letters were significantly different (P < 0·05).
* Analyses were performed on log-transformed values due to the skewness of distribution.
† n 48 for responders (reduction) and 55 for non-responders due to the exclusion of CRP >10 mg/l.
‡ The responders group included individuals with a reduction in TAG concentration >0·25 mmol/l after EPA (v. control). The non-responders group included individuals with a change in TAG concentration between –0·25 and +0·25 mmol/l after EPA (v. control).
§ Main treatment P values for comparison between groups were determined by the main effect. P values were obtained with generalised linear models (multiple comparisons between groups adjusted with Tukey–Kramer) and are adjusted for age, sex and waist circumference after the control phase for continuous values and using Fisher’s exact tests for proportions.
Table 4. Change in anthropometric variables and in cardiometabolic risk factors among different groups of responders to DHA supplementation (v. control)
(Unadjusted mean values with their standard errors)
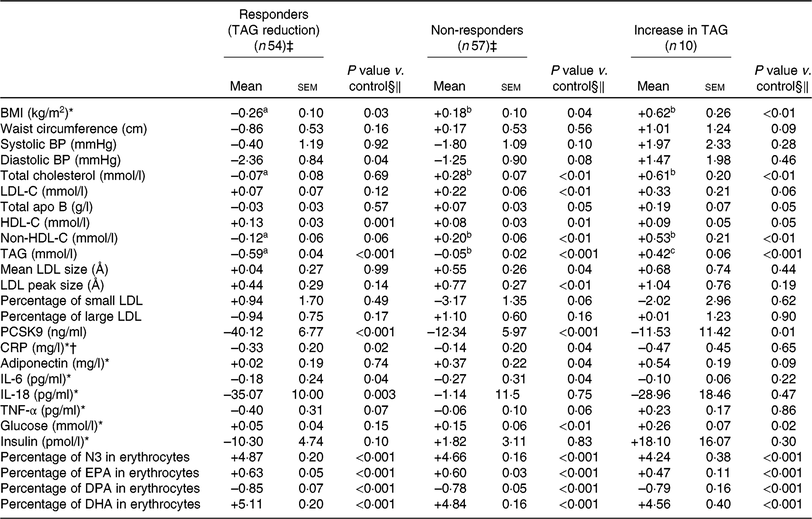
BP, blood pressure; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; CRP, C-reactive protein; N3, long-chain n-3 fatty acids; DPA, docosapentaenoic acid.
a,b,c Mean values with unlike superscript letters were significantly different (P < 0·05)
* Analyses were performed on log-transformed values due to the skewness of distribution.
† n 46 for responders (reduction) and 54 for non-responders due to the exclusion of CRP >10 mg/l.
‡ The responders group included individuals with a reduction in TAG concentration >0·25 mmol/l after DHA (v. control). The non-responders group included individuals with a change in TAG concentration between –0·25 and +0·25 mmol/l after DHA (v. control).
§ Adjusted for age, sex, waist circumference at screening and value of the variable of interest after the control phase.
‖ P values for DHA and EPA changes compared with control values in the outcome were determined with the LSMEANS (least squares means) statement and were tested against the null hypothesis. P values were obtained with generalised linear models (multiple comparisons between groups adjusted with Tukey–Kramer).
Correlates of TAG response to EPA
Similarly, responders to EPA had greater TAG (P < 0·001) and PCSK9 (P = 0·03) concentrations after the control phase than non-responders (Table 5). A reduction in TAG concentration among responders to EPA was observed along with a reduction in PCSK9 (–39·9 ng/ml; P < 0·001) and IL-6 (–0·69 pg/ml; P = 0·03) concentrations after EPA supplementation (Table 6). None of these changes among responders to EPA was different from those seen in non-responders (Table 6).
Table 5. Anthropometric variables and cardiometabolic risk factors among different groups of responders to EPA supplementation after the control phase
(Unadjusted mean values and standard deviations; numbers and percentages)
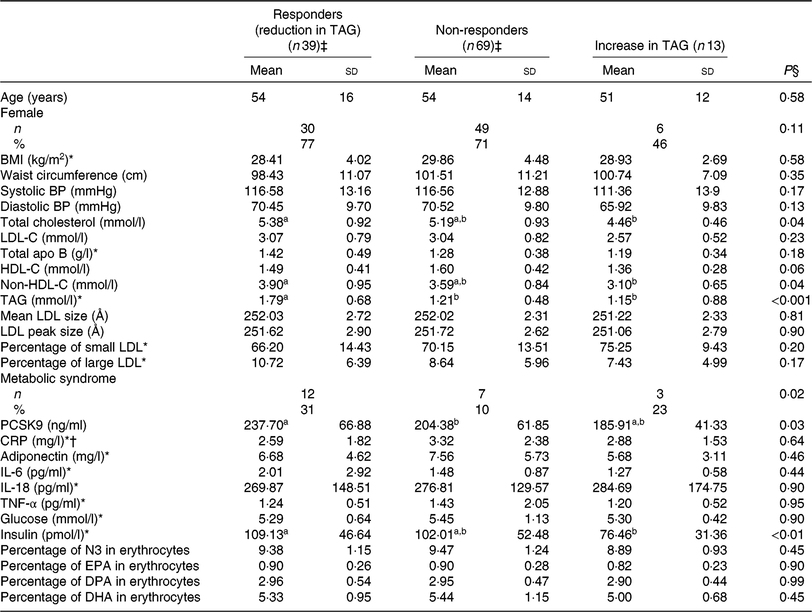
BP, blood pressure; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; CRP, C-reactive protein; N3, long-chain n-3 fatty acids; DPA, docosapentaenoic acid.
a,b Mean values with unlike superscript letters were significantly different (P < 0·05).
* Analyses were performed on log-transformed values due to the skewness of distribution.
† n 34 for responders (reduction) and 65 for non-responders due to the exclusion of CRP >10 mg/l.
‡ The responders group included individuals with a reduction in TAG concentration >0·25 mmol/l after EPA (v. control). The non-responders group included individuals with a change in TAG concentration between –0·25 and +0·25 mmol/l after EPA (v. control).
§ Main treatment P values for comparison between groups were determined by the main effect. P values were obtained with generalised linear models (multiple comparisons between groups adjusted with Tukey–Kramer) and were adjusted for age, sex and waist circumference after the control phase for continuous values and using Fisher’s exact tests for proportions.
Table 6. Changes in anthropometric variables and cardiometabolic risk factors among different groups of responders to EPA supplementation (v. control)
(Unadjusted mean values with their standard errors)

BP, blood pressure; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol; PCSK9, proprotein convertase subtilisin/kexin type 9; CRP, C-reactive protein; N3, long-chain n-3 fatty acids; DPA, docosapentaenoic acid.
a,b Mean values with unlike superscript letters were significantly different (P < 0·05).
* Analyses were performed on log-transformed values due to the skewness of distribution.
† n 36 for responders (reduction) and 64 for non-responders due to the exclusion of CRP >10 mg/l.
‡ The responders group included individuals with a reduction in TAG concentration >0·25 mmol/l after EPA (v. control). The non-responders group included individuals with a change in TAG concentration between –0·25 and +0·25 mmol/l after EPA (v. control).
§ Adjusted for age, sex, waist circumference at screening and value of the variable of interest after the control phase.
‖ P values for DHA and EPA changes compared with control values in the outcome were determined with the LSMEANS (least squares means) statement and were tested against the null hypothesis. P values were obtained with generalised linear models (multiple comparisons between groups adjusted with Tukey–Kramer).
Participants with a low intra-individual TAG variability (i.e. variability <0·20 mmol/l based on four off-treatment measurements, which is the median variability of the sample) were more likely to be responders to both DHA and EPA (OR 3·35; 95 % CI 1·12, 10·08) than participants with an individual TAG variability >0·20 mmol/l (data not shown).
Gene expression of lipid metabolism
Neither DHA nor EPA supplementation modified the expression of HMG-CoA reductase, LDL-R, SREBP1c and SREBP2 in whole blood cells compared with the control treatment among the 44 participants (online Supplementary Table S2). Moreover, neither DHA nor EPA modified the gene expression of HMG-CoA reductase, LDL-R, SREBP1c and SREBP2 among TAG responders or non-responders to DHA and EPA (data not shown).
Discussion
To the best of our knowledge, this is the first study to identify and characterise TAG responders to DHA and EPA supplementation among individuals at an increased risk of CVD( Reference Jellinger, Handelsman and Rosenblit22 ). This randomised, controlled, crossover study revealed important inter- and intra-variability in serum TAG response to similar doses of DHA and EPA. First, the greater reduction in mean TAG concentration with high-dose DHA compared with high-dose EPA( Reference Allaire, Couture and Leclerc16 ) was largely due to the fact that there were more ‘true’ responders to DHA than to EPA supplementation, that is, in whom TAG reduction was greater than the intra-individual variation of 0·25 mmol/l. Indeed, the magnitude of reduction in TAG concentration after DHA and EPA was similar among responders. Second, only a small proportion of participants (26 %) were ‘true’ responders to both DHA and EPA. Finally, individuals with elevated non-HDL-C, TAG and insulin concentrations and with a lower TAG variability were more likely to present a reduction in TAG concentration after high-dose DHA and EPA.
Variability in responses to pharmacological treatments has been widely reported in the literature( Reference Nicolas, Espie and Molimard23 ), and thus variability in response to LCn3-PUFA supplementation was not surprising. Clinical studies have consistently shown a mean TAG reduction after LCn3-PUFA supplementation, but a substantial inter-individual variability in TAG response to LCn3-PUFA supplementation has also been observed( Reference Caslake, Miles and Kofler6 , Reference Minihane, Khan and Leigh-Firbank8 , Reference Minihane9 , Reference Cormier, Rudkowska and Paradis12 ). Previous studies have assessed the changes in TAG concentration using a reference of 0 mmol/l to identify responders and non-responders to LCn3-PUFA. We argue that a change in serum TAG concentration that remains within the boundaries of intra-individual variability, that is, accounting for biological as well measurement variability, should not be considered ‘meaningful’. Hence, the proportion of individuals with a ‘meaningful’ change in TAG (positive or negative) may be smaller than what previous studies have suggested. Indeed, the present study suggests that the proportion of individuals with a ‘meaningful’ TAG reduction was approximately 30 % for EPA and 45 % for DHA rather than the 70 % reported previously( Reference Caslake, Miles and Kofler6 , Reference Minihane, Khan and Leigh-Firbank8 , Reference Minihane9 , Reference Cormier, Rudkowska and Paradis12 ). This suggests that <50 % of individuals (at least those with baseline TAG within the normal range) may benefit from LCn3-PUFA supplementation in terms of TAG lowering.
The present study showed that a greater reduction in mean TAG concentration observed after DHA compared with EPA( Reference Allaire, Couture and Leclerc16 ) seemed largely due to this greater proportion of responders to DHA compared with EPA because the magnitude of TAG lowering was similar between responders to DHA and EPA supplementation. LCn3-PUFA are known to reduce TAG concentration primarily by reducing the production of VLDL-apo B100 and by increasing the conversion of VLDL- to LDL-apo B100( Reference Lamarche and Couture24 ). However, we previously showed that DHA and EPA exert similar effects on the rate of VLDL-apo B100 production and catabolism and, hence, on VLDL-apo B pool size( Reference Allaire, Vors and Tremblay19 ). These observations support the hypothesis that a larger reduction in TAG concentration after DHA compared with EPA was rather due to a greater proportion of responders to DHA compared with EPA than to a greater hypotriacylglycerolaemic effect of DHA compared with EPA. Sex, BMI, baseline TAG concentration and a number of gene polymorphisms have been identified as potential contributors of TAG response to LCn3-PUFA supplementation( Reference Madden, Williams and Calder10 , Reference Skulas-Ray, Kris-Etherton and Harris25 , Reference Sirtori, Tremoli and Sirtori26 ). These covariates may explain, at least partly, why DHA exerts an hypotriacylglycerolaemic effect in a greater proportion of individuals compared with EPA.
EPA may be converted into docosapentaenoic acid (DPA; 22 : 5n-3) and DHA through a metabolic pathway involving in vivo elongation, desaturation and peroxisomal β-oxidation in humans( Reference Cottin, Sanders and Hall27 ). We observed that changes of LCn3-PUFA content in erythrocytes after DHA and EPA supplementation were similar between responders and non-responders compared with control (Tables 4 and 6), suggesting that TAG response appeared to be independent of the rate of incorporation of DHA and EPA into erythrocytes. Polymorphisms in genes involved in endogenous LCn3-PUFA biosynthesis (e.g. fatty acid desaturase 1 and 2 (FADS1 and FADS2)), metabolism (e.g. peroxisome proliferator-activated receptors family (PPAR), ATP citrate lyase (ACYL) and acetyl-CoA carboxylase (ACACA)) and transport (e.g. apo E (apo E)) have been identified as modulators of TAG response to LCn3-PUFA supplementation( Reference Cormier, Rudkowska and Paradis12 , Reference Bouchard-Mercier, Rudkowska and Lemieux28 – Reference Lindi, Schwab and Louheranta30 ). To our knowledge, only one randomised controlled trial has assessed how the apo E genotype modulates the TAG response to DHA and EPA-rich oils( Reference Olano-Martin, Anil and Caslake31 ). The study found no effect of the apo E genotype on TAG response to a 4-week supplementation with either 3·3 g/d EPA or 3·7 g/d DHA in a sample of thirty-eight healthy normolipidaemic males( Reference Olano-Martin, Anil and Caslake31 ). Importantly, the TAG response was modelled as a continuous variable, and thus, the intra-individual variability in TAG response was not considered. Moreover, the association between the apo E genotype and the consistency in TAG response to DHA and EPA has not been documented. The present study revealed that supplementation with either DHA or EPA exerted no effect on the gene expression of HMG-CoA reductase, LDL-R, SREBP1c and SREBP2 (online Supplementary Table S2) measured in whole blood cells. We previously observed that supplementation with either DHA or EPA had similar effects on FADS1, FADS2, ELOVL fatty acid elongase 2 (ELOVL2) and ELOVL5 ( Reference Allaire, Harris and Vors21 ). Of note, a change in the expression of these genes after DHA and EPA supplementation was similar among the subgroups of TAG responders to DHA and EPA (data not shown). These observations remain to be validated in future studies.
Our data showed that TAG responses to DHA and EPA were inconsistent, with only 26 % of participants showing meaningful TAG reductions after both DHA and EPA supplementation. However, responders to a given LCn3-PUFA (i.e. DHA or EPA) were more likely to be responders to the other fatty acid, and so were those with a smaller intra-individual variability (<0·20 mmol/l) in their TAG concentrations. Participants with a concordant reduction in TAG concentrations after DHA and EPA supplementation also had higher non-HDL-C, TAG and insulin concentrations at baseline compared with non-responders. Consistent with these observations, participants with higher TAG concentrations at baseline usually achieved the greatest TAG reduction after LCn3-PUFA supplementation(Reference Skulas-Ray, Kris-Etherton and Harris25). In a small clinical study conducted in hypertriacylglycerolaemic individuals, men with a lower BMI were more likely to present a reduction in TAG concentration >30 % in response to a LCn3-PUFA-and-metformin-combined supplementation(Reference Sirtori, Tremoli and Sirtori26). Also consistent with our observations, the results of the Fatty Acids Sensor Study conducted among 254 healthy subjects showed that neither age, sex nor BMI explained the variability in TAG response to LCn3-PUFA supplementation in a multivariate model(Reference Thifault, Cormier and Bouchard-Mercier7). However, responders to LCn3-PUFA supplementation were those with the highest TAG and insulin concentrations, and the lowest HDL-C concentrations at baseline(Reference Rudkowska, Guenard and Julien32). Thus, a small number of studies have shown that individuals with high TAG, low HDL-C concentrations and alterations in glucose/insulin homeostasis at baseline are those most likely to be responders to LCn3-PUFA supplementation, at least in terms of TAG lowering. Because DHA and EPA are nearly always consumed and studied in combination, further research is needed to determine the impact of sex, body weight and alterations of the glucose, insulin and lipid metabolism on the consistency in TAG response to individual fatty acids.
Interestingly, we observed that responders to DHA or to EPA had greater serum PCSK9 concentration at ‘baseline’ (i.e. after the control phase) than non-responders. PCSK9 regulates cholesterol metabolism by degrading cellular LDL-receptors, hence blunting the clearance of LDL from the circulation, and then leading to an increase in LDL concentration(Reference Tavori, Rashid and Fazio33). We previously showed that DHA and EPA reduce PCSK9 concentrations in a similar manner(Reference Allaire, Vors and Tremblay19). Accordingly, responders presented a reduction in PCSK9 concentration after either DHA or EPA, which did not differ, however, from changes seen in groups of non-responders. While the role of PCSK9 in regulating cholesterol homeostasis is well understood, its impact on TAG metabolism is less well known. The relationship between LCn3-PUFA, PCSK9 and TAG metabolism remains to be investigated in the future.
It has been consistently reported that a reduction in LDL-C concentrations lowered the incidence of major cardiovascular events(Reference Mihaylova and Emberson34). However, LCn3-PUFA supplementation tended to slightly increase LDL-C concentrations, and DHA may do more so than EPA(Reference Allaire, Couture and Leclerc16). The use of LCn3-PUFA in order to prevent cardiovascular events may appear counterintuitive in this context. In the present study, supplementation with DHA and EPA had neutral effect on LDL-C concentration among TAG responders. Moreover, a reduction in TAG concentration after DHA among responders was also associated with favourable changes in diastolic blood pressure, HDL-C, CRP and IL-18 concentrations. A reduction in TAG after EPA among responders was associated with reduced IL-6 concentrations. These observations support the previous assumption that DHA may be more potent than EPA in modulating cardiometabolic risk factors( Reference Allaire, Couture and Leclerc16 ), especially among those with a meaningful reduction in TAG concentration after LCn3-PUFA supplementation. Because a high proportion of individuals are non-responders to LCn3-PUFA and that TAG concentration may even increase in a small proportion of them, it is not surprising that most previous clinical trials failed to observe a reduction in CVD risk after LCn3-PUFA supplementation( Reference Aung, Halsey and Kromhout35 – Reference Manson, Cook and Lee37 ). This does not exclude the possibility that LCn3-PUFA supplementation may exert beneficial effects on cardiovascular risk, through the modulation of TAG concentration as well as of other cardiovascular risk factors, in some individuals. More recently, data from the REDUCE-IT study showed that supplementation with EPA alone (4 g/d) reduced the risk of cardiovascular events by 25 % in individuals with a high baseline TAG concentration( Reference Bhatt, Steg and Miller14 ). Considering that DHA may be a more potent modulator of cardiometabolic health than EPA( Reference Wei and Jacobson15 , Reference Allaire, Couture and Leclerc16 ), it will be more interesting to further investigate the potential cardiovascular benefits of DHA alone in future hard end-point studies.
The present study has several strengths but also some limitations. To our knowledge, this is the first crossover trial to assess the variability in TAG response to DHA and EPA supplementation taken individually and using the intra-individual TAG variation to assess the response. The results of the present study are specific to a population with high waist circumference and elevated CRP concentrations only. Also, the sample size was calculated a priori to compare the effect of DHA and EPA on CRP concentrations and not to characterise the responders to LCn3-PUFA supplementation. Despite being sufficient to characterise the TAG response to DHA and EPA supplementation, the number of subjects in the study (n 121) may have been insufficient to identify the predictors of TAG response. Participants were asked to maintain their usual dietary habits and were also counselled on how to exclude fatty fish meals, fish oil supplements, flax products, walnuts and LCn3-PUFA-enriched products during the study. Since the diet was not assessed before the study, we cannot exclude the fact that the background diet may have influenced TAG variability as well as its response to DHA and/or EPA supplementation. The variability in response during clinical trials may be attributed to non-compliance to interventions. However, since the mean compliance to supplementation based on returned capsules was >95 % for all three phases of the present study and the changes of DHA and EPA content in erythrocytes were consistent with the given treatment (i.e. DHA and EPA, respectively)( Reference Allaire, Couture and Leclerc16 ), the intra- and inter-individual variability in TAG response observed in the present study is more likely attributable to other factors rather than as a consequence of non-compliance.
In sum, data from this randomised, controlled, crossover trial showed that a small proportion of individuals presented a meaningful reduction in TAG concentration after supplementation with either high-dose DHA or EPA. A greater reduction in TAG concentration after DHA compared with EPA is mainly explained by a greater proportion of responders to DHA than to EPA. In this population at risk of CVD, participants with high TAG, non-HDL-C and insulin concentrations at baseline and those with TAG levels that appeared to be less variable were more likely to present a concordant reduction in TAG concentration after both DHA and EPA supplementation. In light of the present data and of data from the REDUCE-IT study, further studies must be conducted to better identify individuals who may benefit from DHA and EPA supplementation and to compare the effect of DHA and EPA on cardiovascular outcomes.
Acknowledgements
We are grateful to the subjects for their excellent collaboration and the staff of the Institute of Nutrition and Functional Foods and the CHU de Québec.
Financial support for this randomised, controlled trial was provided via a grant from the Canadian Institutes for Health Research (CIHR, MOP-123494) (B. L., P. C., A. T.). Douglas Laboratories provided the EPA, DHA and control capsules used in this study. Neither CIHR nor Douglas Laboratories were involved in designing the study; conducting the study; collection, management, analysis or interpretation of the data; preparation and review of the manuscript prior to submission. J. A. is a recipient of a PhD scholarship from the CIHR and the Fonds de recherche du Québec – Santé. C. V. is a postdoctoral fellow supported by the European Marie Skłodowska-Curie Actions.
B. L., P. C. and A. T. designed and obtained funding for this study. P. C. was responsible for the medical supervision of the study. W. S. H. and K. H. J. conducted the laboratory analyses. C. V. provided significant help with the analysis of the results. J. A. performed statistical analyses and wrote the manuscript, which was reviewed critically by all authors. B. L. had primary responsibility over the final content. All authors critically revised the manuscript and contributed intellectually to its development, provided final approval of the submitted manuscript, had full access to all of the data in the study, take responsibility for the integrity and accuracy of the data in the analyses, affirm that the article is an honest, accurate and transparent account of the study being reported and that no important aspects of the study have been omitted.
B. L. has received funding in the last 5 years from the Canadian Institutes for Health Research, the Natural Sciences and Engineering Research Council of Canada, Agriculture and Agri-Food Canada (Growing Forward programme supported by the Dairy Farmers of Canada (2013–2017), Canola Council of Canada, Flax Council of Canada, Dow Agrosciences (2013–2018)), Dairy Research Institute (2013–2017), Dairy Australia (2013–2017), Merck Frosst (2016–2018) and Atrium Innovations (2012–2019).
P. C. has received funding in the last 5 years from the Canadian Institutes for Health Research, Agriculture and Agri-Food Canada (Growing Forward programme supported by the Dairy Farmers of Canada, Canola Council of Canada, Flax Council of Canada, Dow Agrosciences), Dairy Research Institute, Dairy Australia, Merck Frosst, Pfizer and Atrium Innovations.
A. T.’s funding of the past 5 years as principal investigator came from the Canadian Institutes for Health Research, the Natural Sciences and Engineering Research Council of Canada, the Fonds de recherche du Québec – Santé, the Fondation de l’Institut universitaire de cardiologie et de pneumologie de Québec, as well as investigator-initiated funding from Johnson & Johnson Medical Companies and Pfizer for studies unrelated to the present report in addition to financial support from Medtronic for a Research Chair on bariatric and metabolic surgery.
W. S. H. is the President and Founder of OmegaQuant, LLC, and K. H. J. is the Director of Research. OmegaQuant is a commercial research laboratory specialising in fatty acid analysis.
Other authors have no disclosure.
The authors have no conflict of interest in relation to this work.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114519000552












