Introduction
Many individuals with Alzheimer’s disease (AD) are cared for at home by family members or friends (Alzheimer’s Association, 2017; Wimo et al., Reference Wimo2017). Such informal caregiving makes a substantial contribution to the overall care of patients with AD dementia (ADD), helping to keep individuals out of long-term institutional care for as long as possible. However, it can have numerous negative financial, social, health, and quality-of-life consequences for the caregiver (“caregiver burden”), which may increase as the disease progresses (Beinart et al., Reference Beinart, Weinman, Wade and Brady2012; Bergvall et al., Reference Bergvall2011; Conde-Sala et al., Reference Conde-Sala, Turró-Garriga, Calvó-Perxas, Vilalta-Franch, Lopez-Pousa and Garre-Olmo2014; Fisher et al., Reference Fisher2011). As a multidimensional construct, the caregiver burden is generally considered to comprise both subjective (e.g. personal, physical, emotional, and social) and more objective (e.g. informal caregiver time and costs) aspects of caring (Park et al., Reference Park, Sung, Kim, Kim and Lee2015).
Costs associated with informal caregiver time form a major component of the societal costs of AD but can be challenging to determine (Darbà et al., Reference Darbà, Kaskens and Lacey2015; Gervès et al., Reference Gervès, Chauvin and Bellanger2014; Shearer et al., Reference Shearer, Green, Ritchie and Zajicek2012; Wimo et al., Reference Wimo2013).
The most widely used measure of perceived caregiver burden, the self-rated Zarit Burden Interview (ZBI; Zarit et al., Reference Zarit, Reever and Bach-Peterson1980), focuses on subjective aspects of this burden. Cross-sectional studies have identified numerous disease-related factors (e.g. the patient’s cognitive status, functional impairment, and behavioral symptoms), as well as various caregiver characteristics (e.g. their relationship to the patient and whether living with the patient or not), as being associated with the subjective caregiver burden (Beinart et al., Reference Beinart, Weinman, Wade and Brady2012; Bergvall et al., Reference Bergvall2011; Brodaty et al., Reference Brodaty, Woodward, Boundy, Ames and Balshaw2014; Conde-Sala et al., Reference Conde-Sala, Turró-Garriga, Calvó-Perxas, Vilalta-Franch, Lopez-Pousa and Garre-Olmo2014; Park et al., Reference Park, Sung, Kim, Kim and Lee2015). More objective but equally important burden indicators, such as caregiver time and costs, and how they change with the progression of ADD severity, have received less attention (Bergvall et al., Reference Bergvall2011; Fisher et al., Reference Fisher2011; Koca et al., Reference Koca, Taşkapilioğlu and Bakar2017; Wolfs et al., Reference Wolfs2012). Identifying drivers of the caregiver burden at different stages of AD could highlight areas in which interventions may benefit both caregivers and patients.
Few longitudinal studies have investigated the impact on informal caregiver burden of AD progression from the early stages of mild cognitive impairment or mild ADD. Existing studies report an increase in subjective caregiver burden (worsening ZBI score) as being associated more with worsening patient functional status and behavioral problems than with cognitive decline (Brodaty et al., Reference Brodaty, Woodward, Boundy, Ames and Balshaw2014; Conde-Sala et al., Reference Conde-Sala, Turró-Garriga, Calvó-Perxas, Vilalta-Franch, Lopez-Pousa and Garre-Olmo2014; Jones et al., Reference Jones2017). Of these studies, only Jones et al. (Reference Jones2017), an 18-month post-hoc analysis of the GERAS study, a prospective longitudinal European observational study designed to assess costs and resource use associated with ADD, investigated the impact of AD progression on objective aspects of the caregiver burden. In this study, progression to a more severe state was associated with increases in caregiver time and total societal costs (including informal care costs).
To increase understanding of the wider impact of caring for a patient with ADD, a post-hoc exploratory analysis of data from the GERAS study was conducted to identify patient and caregiver factors associated with both subjective and objective caregiver outcomes. Outcomes investigated over 36 months included the caregiver burden (assessed using the ZBI) and caregiver informal care time and costs.
Methods
Study design and cohort
GERAS was an 18-month prospective observational study of costs and resource use in community-living patients with ADD and their caregivers conducted in France, Germany, and the U.K.; the study was extended to 36 months in France and Germany. Study design and baseline data for all three countries have been described previously (Wimo et al., Reference Wimo2013). This paper reports a post-hoc analysis of 36-month outcomes in the French and German cohorts.
In brief, the study enrolled community-dwelling patients aged ≥ 55 years with a diagnosis of probable AD (defined according to criteria in McKhann et al., Reference McKhann, Drachman, Folstein, Katzman, Price and Stadlan1984) and a Mini-Mental State Examination (MMSE) score (Folstein et al., Reference Folstein, Folstein and McHugh1975) of ≤ 26 who presented during the normal course of care. Exclusion criteria included a history, clinical signs, or imaging evidence of stroke or transient ischemic attack; a history of Parkinson’s disease prior to/coincidental with the onset of AD; and probable Lewy-body disease.
All patients had an informal caregiver (a person taking responsibility for most day-to-day decisions and the provision of home care for the patient) who was willing to participate in the study and undertake responsibility for the patient for ≥ 6 months of the year.
Patients were stratified according to baseline disease severity using MMSE criteria (National Institute for Health and Care Excellence, 2011): MMSE 21−26 points—mild AD severity; MMSE 15−20 points—moderate AD severity; MMSE <15 points—moderately severe/severe AD severity. Recruitment was designed to achieve equal numbers of patients in each AD severity group within each country (Wimo et al., Reference Wimo2013). Throughout the study, patients could be prescribed AD treatment as per usual care.
The study was approved by local Ethical Review Boards according to individual country regulations. Written informed consent was obtained from both patient (or legal representative, as determined at study site) and caregiver prior to study enrollment, which occurred between October 2010 and September 2011.
Assessments
Data were collected at baseline and during routine care visits at 6-month intervals up to 36 months in France and Germany. Information collected at baseline for patients and caregivers included sociodemographic data, comorbidities, and medications. Additional data collected and used in our analyses are listed in the Supplementary Material.
Caregiver informal care costs
Caregiver informal care costs (assessed every six months) were calculated based on an opportunity cost approach, taking into account productivity loss for working caregivers and lost leisure time for nonworking caregivers. Caregiver time (calculated as the number of hours spent assisting the patient with basic and instrumental activities of daily living [ADL] [caregiver basic ADL hours and caregiver instrumental ADL hours]) was capped at 24 hours/day. The unit cost of caregiver time for working caregivers was the value of lost production time based on the national average wage per country; for nonworking caregivers, it was the value of lost leisure time, based on 35% of the national average wage per country population (see Wimo et al., Reference Wimo2013). All costs were calculated in € (2010 values). For patients with missing data, costs were imputed based on the reason the data were missing. For patients institutionalized during the study, mean monthly costs from the last visit were used for the period up to institutionalization, followed by zero costs from institutionalization to 36 months. For patients who died, last observation carried forward was used, with costs from the last known visit extrapolated to date of death (no costs were computed thereafter) (Belger et al., Reference Belger2016). For patients who discontinued the study for other reasons, multiple imputation (MI) regression (Rubin, Reference Rubin1987), stratified by MMSE group and using factors selected from those identified by Dodel et al. (Reference Dodel2015), was performed on missing costs.
Statistical analysis
The post-hoc analysis reported was exploratory only. Demographics and baseline characteristics were summarized using descriptive statistics, based on nonmissing observations.
The primary analysis aimed to identify patient and caregiver covariates associated with change from baseline (CFB) over 36 months for the outcome measured ZBI total score, caregiver basic ADL hours, and caregiver instrumental ADL hours. The analysis also identified patient and caregiver covariates associated with informal caregiver costs over 36 months.
The choice of baseline patient and caregiver variables to include in the 36 month models were those covariates significantly associated with the outcomes of interest over 18 months. These were identified by running 100 18-month models using forward and backward selection; 67% of subjects were selected at random for inclusion and variables identified in each model summarized. Inclusion/exclusion of individual variables was based on a significance level of 0.05; variables selected in ≥ 75 iterations were included in the 36-month models.
A mixed-effects model of repeated measures (MMRM) was used in all analyses; a maximum of six repeated measured data points for each patient were included in the MMRM. The analysis of CFB outcomes used linear regression models; analysis of caregiver costs used generalized linear models with a gamma distribution and a log-link function. The MMRM models included as main effects patient and caregiver baseline covariates found to be significantly associated with the outcomes of interest (CFB in ZBI, caregiver basic ADL hours and caregiver instrumental ADL hours, and caregiver costs) over 18 months; change in score from baseline to 18 months for four time-dependent patient covariates (MMSE, Alzheimer’s Disease Cooperative Study—Activities of Daily Living [ADCS-ADL; Galasko et al., Reference Galasko, Schmitt, Thomas, Jin and Bennett2005] [basic and instrumental] and 12-item Neuropsychiatric Inventory [NPI-12; Cummings, Reference Cummings1997); and baseline scores for these time-dependent covariates.
Mean changes over time are presented as least squares means (± 95% confidence intervals) taken from the MMRM analysis. Sensitivity analyses were performed for ZBI with missing outcomes imputed using MI (Markov chain Monte Carlo method). As the method used to calculate cost data resulted in no missing data, no similar sensitivity analyses were required for informal care costs.
All data were analyzed using SAS software, version 9.2 (SAS Institute, Cary, NC, USA).
Results
A total of 969 patients and their informal caregivers from the French (n = 419) and German (n = 550) GERAS cohorts were included. Caregivers were mostly female (65%), the spouse of the patient (65%), and living with the patient (75%) (Table 1); 64% of caregivers were spouses who lived with the patient. Although caregivers spent over 200 hours/month on care, the overall caregiver burden, as assessed by the ZBI, was relatively low (Table 1). Differences between patients and caregivers in the two countries were apparent. For example, French patients were older, on average, than German patients, and a higher proportion were female. Additionally, caregiver time at baseline was notably higher in Germany than in France.
Table 1. Baseline characteristics of patients and caregivers
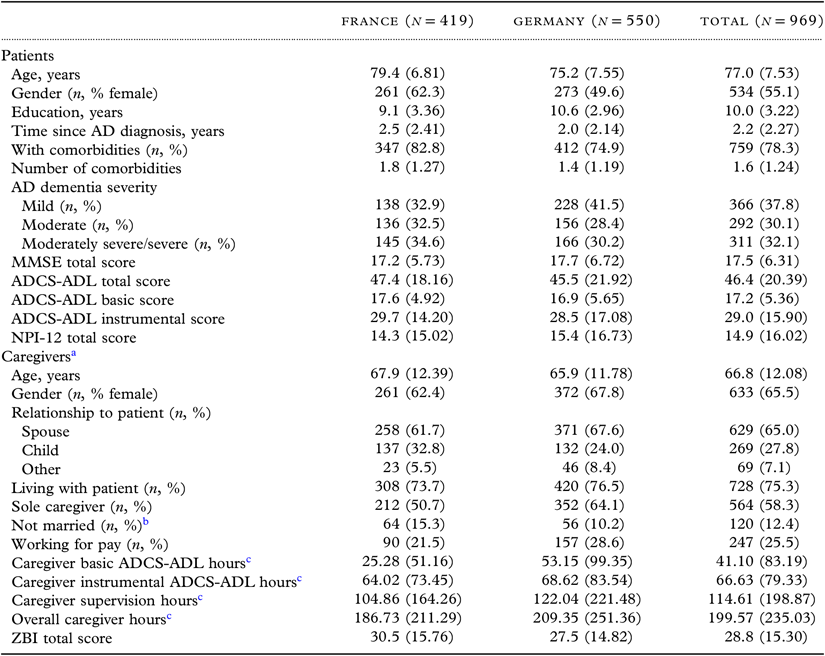
ADCS-ADL = Alzheimer’s Disease Cooperative Study—Activities of Daily Living; MMSE = Mini-Mental State Examination; NPI-12 = 12-item Neuropsychiatric Inventory; ZBI = Zarit Burden Interview.
All variables are reported as mean (SD) for nonmissing observations unless otherwise indicated.
All baseline variables listed were considered in the 18-month models; those found to be significantly associated with the outcome of interest were included in the 36-month models.
a Baseline data missing for two caregivers.
b Divorced/separated, never married, and widowed (includes all caregivers not just spouses/partners).
c Per month.
MMSE 21-26 points—mild AD severity; MMSE 15−20 points—moderate AD severity; MMSE <15 points—moderately severe/severe AD severity. MMSE: total score 0–30 (higher score = better cognitive function); NPI-12: total score 0–144 (higher score = more severe problems); ADCS-ADL: total score 0–78 (basic ADLs: range 0−22; instrumental ADLs: range 0−56) (higher scores = lower functional impairment [i.e. better functioning]); ZBI: total score 0–88 (higher score = greater burden).
At the 36-month follow-up, 572 (59%) patients had discontinued the study. Main reasons for discontinuation included institutionalization (n = 213 [37%]), death (n = 112 [20%]), and loss to follow-up (n = 247 [43%]).
Caregiver burden (ZBI score)
Mean ZBI total scores increased from baseline (indicative of greater burden) in both France and Germany over the 36 months of the GERAS study (Figure 1).

Figure 1. Least squares (LS) mean change from baseline (±95% confidence interval) over 36 months in caregiver burden (Zarit Burden Index [ZBI] total score).
Primary analysis (Figure 2A) showed that a greater increase in caregiver burden over 36 months was most strongly associated with a worsening in patient functional ability (lower ADCS-ADL instrumental score) and an increase in patient behavioral problems (higher NPI-12 total score) over 18 months. Additionally, a greater increase in caregiver burden was observed with the patient having more years of education and a greater number of comorbidities. A lower increase in caregiver burden over 36 months was seen when the patient had a greater number of caregivers and the participants lived in Germany.

Figure 2. Estimates of change from baseline (CFB) over 36 months (±95% confidence interval) for (A) caregiver burden (Zarit Burden Index [ZBI] score); (B) caregiver basic activities of daily living (ADL) hours; (C) caregiver instrumental ADL hours; and (D) estimates of caregiver informal care costs over 36 months. All mixed-effects models of repeated measures (MMRM) were controlled for country and baseline scores and included patient and caregiver baseline covariates found to be significantly associated with each outcome of interest over 18 months; change in score from baseline to 18 months for four time-dependent patient covariates (Mini-Mental State Examination [MMSE], Alzheimer’s Disease Cooperative Study—Activities of Daily Living [ADCS-ADL] [basic and instrumental], and Neuropsychiatric Inventory-12 [NPI-12]); and baseline scores for these time-dependent covariates.
In sensitivity analyses, the MI model using time-dependent variables replicated the findings of the primary analysis using MMRM (data not shown).
Caregiver basic ADL hours
Mean caregiver basic ADL hours increased from baseline in both France and Germany over the 36 months of the GERAS study but were numerically lower, overall, in France than in Germany (Figure 3A).
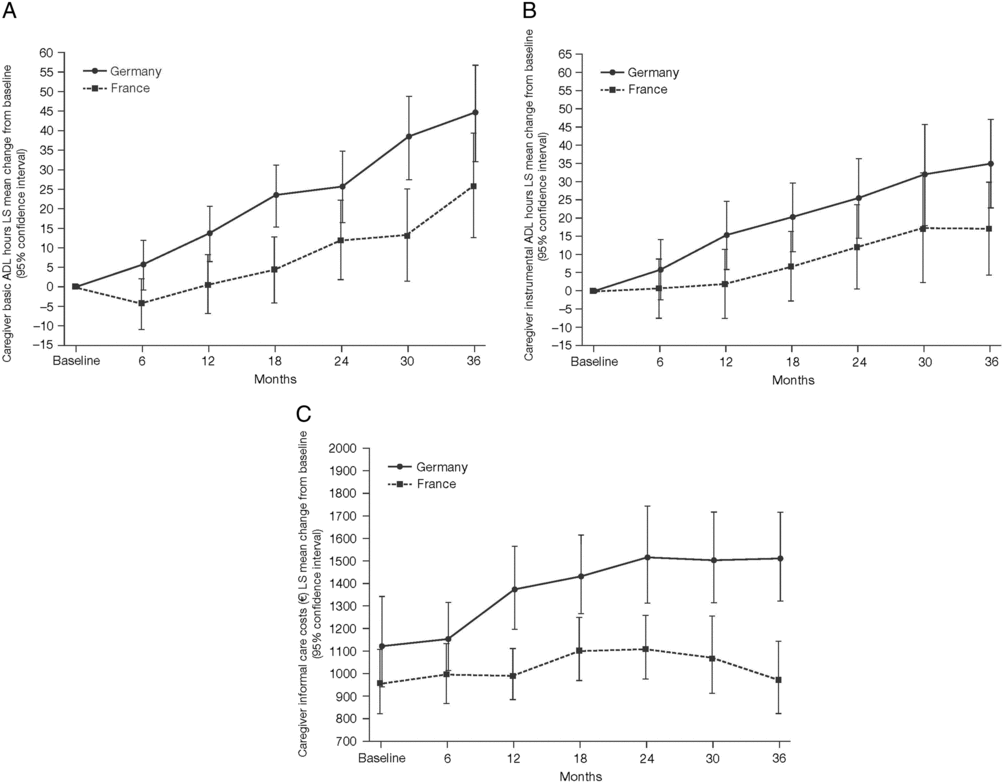
Figure 3. Least squares (LS) mean change from baseline over 36 months (±95% confidence interval) in (A) caregiver basic ADL hours; (B) caregiver instrumental ADL hours; and (C) LS mean caregiver informal care costs over 36 months.
In the primary analysis (Figure 2B), a greater increase in caregiver basic ADL hours over 36 months was associated with a worsening in patient functioning (lower ADCS-ADL basic score) over 18 months, the caregiver living with the patient, and the participants living in Germany. Lower patient functioning at baseline (lower ADCS-ADL basic score) was associated with the greatest increase in caregiver basic ADL hours over 36 months.
Caregiver instrumental ADL hours
Mean caregiver instrumental ADL hours increased from baseline in both France and Germany over the 36 months of the GERAS study but were again numerically lower, overall, in France than in Germany (Figure 3B).
In the primary analysis, including time-dependent factors (Figure 2C), a greater increase in caregiver instrumental ADL hours over 36 months was associated with a worsening in patient functioning (lower ADCS-ADL instrumental score) over 18 months. Additionally, a greater increase in caregiver instrumental ADL hours was observed when caregivers (including adult-child and spousal/partner caregivers) were not married or were living with the patient and when the participants lived in Germany. Lower baseline functioning (lower ADCS-ADL basic and instrumental scores) was associated with a greater increase in caregiver instrumental ADL hours over 36 months.
Caregiver informal care costs
Mean caregiver informal care costs increased from baseline in Germany over the 36 months of the GERAS study. In contrast, in France, costs rose slowly over the first 24 months of the study, returning to original levels during the final 12 months. Overall, costs were lower in France than in Germany at all timepoints (Figure 3C).
Primary analysis (Figure 2D) showed that higher informal care costs over 36 months were associated with a worsening in patient function (lower ADCS-ADL instrumental score) over 18 months, the caregiver (adult-child or spousal/partner) being unmarried, the caregiver living with the patient, the caregiver working for pay, and the participants living in Germany. Lower baseline functioning (lower ADCS-ADL instrumental score) was associated with higher 36-month informal care costs.
Discussion
This study provides evidence that a decline in function, but not cognition, over a period of 18 months in community-dwelling patients with ADD impacts key caregiver outcomes over 36 months. In the context of a correlation between decline in function and decline in cognition, these analyses assessed which factors have the largest influence on caregiver outcomes, and with both function and cognition in the analyses, our results indicate that after accounting for function then decline in cognition does not add a statistically significant change in the caregivers’ outcomes.
Findings of an association between a decline in functional ability and an increase in subjective caregiver burden (assessed via the ZBI) have been reported in previous longitudinal studies (Brodaty et al., Reference Brodaty, Woodward, Boundy, Ames and Balshaw2014, in a general dementia population; Conde-Sala et al., Reference Conde-Sala, Turró-Garriga, Calvó-Perxas, Vilalta-Franch, Lopez-Pousa and Garre-Olmo2014; Jones et al., Reference Jones2017), but the current study is the first to provide confirmation that wider, more objective aspects of the caregiver burden—caregiver hours and informal care costs—are also impacted by increases in patient functional impairments over time. Hence, the early introduction of interventions aimed at slowing functional decline in patients with ADD may help reduce all aspects of the caregiver burden (subjective and objective). Various pharmacological and nonpharmacological (e.g. exercise) interventions have demonstrated efficacy in delaying functional decline in individuals with dementia, including those with AD (Laver et al., Reference Laver, Dyer, Whitehead, Clemson and Crotty2016).
We also found that behavioral decline impacted subjective caregiver burden, as reported in previous longitudinal and cross-sectional studies (Bergvall et al., Reference Bergvall2011; Brodaty et al., Reference Brodaty, Woodward, Boundy, Ames and Balshaw2014; Conde-Sala et al., Reference Conde-Sala, Turró-Garriga, Calvó-Perxas, Vilalta-Franch, Lopez-Pousa and Garre-Olmo2014). A prospective, observational study reported an improvement in behavioral disturbances and a reduction in subjective caregiver burden (assessed via the ZBI) in patients with ADD at 12 months (Agüera-Ortiz et al., Reference Agüera-Ortiz, Frank-Garcìa, Gil and Moreno2010). However, differences between the current study and this earlier trial hinder comparisons. The earlier study was conducted in patients with moderate-to-severe ADD (MMSE score <20), a substantial proportion of whom were in residential care. Additionally, the study used the Blessed Dementia Scale, not the NPI-12, to assess behavioral disturbances and found that the use of pharmacotherapy and, in contrast to the current study, patients having a higher level of education, were associated with a lower caregiver burden.
In a systematic review examining associations between specific items on the NPI-12 and caregiver burden in dementia (mainly AD), Terum et al. (Reference Terum, Andersen, Rongve, Aarsland, Svendsboe and Testad2017) found that irritability, followed by agitation, sleep disturbances, anxiety, apathy, and delusion seemed to have the greatest impact on caregiver burden. While we did not explore which NPI-12 items were most relevant to the change in caregiver burden over time, our study found that an increase in NPI-12 total score was associated with greater caregiver burden in ADD but not increased caregiver time or informal care costs.
We found no evidence that a decline in cognition over 18 months, having accounted for all other variables in the models, including the decline in function, was associated with changes in subjective or objective measures of caregiver burden over 36 months. A recent report that a decline in cognitive function (together with increasing functional impairment and level of neuropsychiatric symptoms) was associated with higher caregiver burden in a longitudinal study in patients with subjective cognitive decline or progressive neurocognitive disorder followed for approximately 12 months suggests that reductions in patient cognition may have more of an impact on caregiver burden in the predementia phase (Dauphinot et al., Reference Dauphinot, Ravier, Novais, Delphin-Combe, Mouchoux and Krolak-Salmon2016).
Although there has been an assumption that increasing caregiver hours will impact informal care costs (Wimo et al., Reference Wimo2013; Wolfs et al., Reference Wolfs2012), the current analysis in patients with ADD demonstrated a direct association between functional decline over 18 months (but not cognitive or behavioral decline) and an increase in both informal caregiver time and informal care costs over 36 months. The longitudinal Predictors Study, in community-dwelling patients with mild AD, found that increasing impairments in function were associated with increased caregiver time and costs over a four-year period but also reported no such association with cognitive or behavioral problems (Zhu et al., Reference Zhu2006; Reference Zhu2008).
Another key factor that the current study identified as associated with objective aspects of the long-term caregiver burden was the caregiver living with the patient at baseline. Unlike previous longitudinal studies examining the factors associated with caregiver burden over time (Conde-Sala et al., Reference Conde-Sala, Turró-Garriga, Calvó-Perxas, Vilalta-Franch, Lopez-Pousa and Garre-Olmo2014; Viñas-Diez et al., Reference Viñas-Diez2017), we found no association between the caregiver cohabiting with the patient at baseline and greater subjective caregiver burden, as assessed using the ZBI, at 36 months. However, living with the patient at baseline was associated with increases in objective measures, such as caregiver basic and instrumental ADL hours and informal care costs. These findings suggest that the proximity of the caregiver to the patient, allowing him/her to be readily available to assist with the patient’s care needs, is a significant risk factor for a higher objective caregiver burden. Living with a patient with ADD has been identified as a factor associated with a greater caregiver burden (Kim et al., Reference Kim, Chang, Rose and Kim2012; Raccichini et al., Reference Raccichini, Spazzafumo, Castellani, Civerchia, Pelliccioni and Scarpino2015; Reed et al., Reference Reed2014). Greater increases in caregiver instrumental ADL hours and informal care costs over 36 months were also observed in caregivers (adult-child or spousal/partner) who were not married at baseline.
This study was conducted in a large, well-characterized, selected patient cohort from which longitudinal data were collected prospectively over a 36-month period. However, as the study enrolled approximately equal numbers of patients in each AD severity group (resulting in the inclusion of a larger proportion of patients with moderately severe/severe AD than would be typical of community-dwelling AD patients), its findings may not be representative of the full caregiver burden spectrum. The study assessed the effect of cognitive, functional, and behavioral decline on caregiver outcomes, all important facets of AD progression and recommended efficacy endpoints for clinical trials in AD (European Medicines Agency, 2016). The use of a standardized measure of resource use, including informal care, facilitated the pooling of data from two countries; however, the potential difficulties in interpreting pooled data from different countries, with differing health and social care systems, and varying approaches to the management of AD, should be noted. This is reflected in the higher baseline levels of caregiver time, the greater increases in caregiver basic and instrumental ADL hours, the higher informal care costs, and lower increases in subjective caregiver burden (ZBI score) over 36 months seen in caregivers living in Germany than in those living in France. These findings are consistent with those of a previous GERAS report analyzing the factors driving between-country differences in the costs of AD, which found societal costs (driven largely by informal care costs) over 18 months to be higher in Germany than in France (Reed et al., Reference Reed2017).
Only community-dwelling patients retained in the study were followed-up at 36 months, leading to possible bias from missing data, a common occurrence in longitudinal studies in older adults where patients may be lost to follow-up for reasons including institutionalization and death (Coley et al., Reference Coley, Gardette, Cantet, Gillette-Guyonnet, Nourhashemi, Vellas and Andrieu2011; Hardy et al., Reference Hardy, Allore and Studenski2009). However, sensitivity analyses in which missing ZBI scores were imputed using MI found substantively similar results to the main analyses, suggesting that the pattern of missing outcome values had little impact on the results.
Although function was assessed during the study less regularly than cognition or behavioral symptoms, the associations between functional assessments and all caregiver outcomes were strong, despite the level of patients discontinuing from the study.
The analysis does not take into account the influence of any health and social care resources being provided to support patients and caregivers. Additionally, other measures of caregiver impact may be relevant but were not explored in this study.
Conclusion
The study provides evidence that long-term (36-month) informal caregiver burden is driven by worsening functional abilities and behavioral symptoms, but not cognitive decline, over 18 months in community-dwelling patients with ADD. Caregivers of patients with ADD therefore offer a unique perspective on the impact of the disease, and their inclusion is warranted during evaluations of programs and interventions aimed at reducing caregiver burden. Prompt care planning for people with early-stage ADD could help trigger targeted support for caregivers, especially those living with an affected person. Such initiatives, and effective treatment options aimed at reducing patient functional impairments and behavioral problems, could help mitigate the impact of long-term informal caring for a patient with ADD and need to be evaluated.
Conflict of interest
Catherine Reed, Mark Belger, J. Scott Andrews and Antje Tockhorn-Heidenreich are employees of Eli Lilly and Company. Roy W. Jones, Anders Wimo, Richard Dodel and Josep Maria Haro have received financial compensation from Eli Lilly for participation in the GERAS Advisory Board. RWJ has received honoraria for speaking at/chairing symposia and has been a member of advisory boards for Eli Lilly. AW has received financial compensation from Lundbeck, Novartis, Biogen, and Roche for participation in advisory boards. RD has received research funding, honoraria, and financial compensation for consultancy or advisory board membership from a number of pharmaceutical companies, including Abbott/AbbVie, Baxter, Eli Lilly, Novartis, Octapharma, and Pfizer, and from some medical societies. JMH has received financial compensation from Otsuka, Roche, and Lundbeck for participation in advisory boards.
Description of authors’ roles
CR, MB, JSA and ATH made substantial contributions to study conception and design and the acquisition and analysis of data. MB was involved in statistical analysis and interpretation of data. RWJ, AW, RD and JMH were involved in the conduct of the study as members of the study advisory board. All authors were involved in drafting the manuscript or revising it critically for important intellectual content and have given final approval of the version to be published. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content and has agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
The authors would like to acknowledge Gill Gummer and Deirdre Elmhirst (Rx Communications, Mold, U.K.) for medical writing assistance with the preparation of this manuscript, funded by Eli Lilly and Company.
This study is sponsored and funded by Eli Lilly and Company Limited.
Supplementary material
To view supplementary material for this paper, please visit https://doi.org/10.1017/S1041610219000425








