Introduction
Bipolar disorder (BD) is a severe mental disorder associated with significant functional disability, elevated suicide risk, increased physical morbidity and premature mortality (Chan et al., Reference Chan, Tong, Wong, Chen and Chang2022a; McIntyre et al., Reference McIntyre, Berk, Brietzke, Goldstein, López-Jaramillo, Kessing, Malhi, Nierenberg, Rosenblat, Majeed, Vieta, Vinberg, Young and Mansur2020; Plans et al., Reference Plans, Barrot, Nieto, Rios, Schulze, Papiol, Mitjans, Vieta and Benabarre2019). Given that it is potentially chronic in nature with relapse-remitting illness course, the majority of patients require maintenance treatment of mood stabilizers. Lithium is the first-line mood-stabilizing medication treatment for BD, and is found to be related to reduced risk of all-cause mortality and suicide (Cipriani et al., Reference Cipriani, Hawton, Stockton and Geddes2013). In recent decades, lithium has shown a declining trend of use due to its narrow therapeutic window and potential physical complications, including hypothyroidism and renal impairment (McKnight et al., Reference McKnight, Adida, Budge, Stockton, Goodwin and Geddes2012; Schoretsanitis et al., Reference Schoretsanitis, de Filippis, Brady, Homan, Suppes and Kane2022), and has been partially replaced by second-generation antipsychotics (SGA) and anticonvulsants (especially valproate) (Bellivier et al., Reference Bellivier, Delavest, Coulomb, Figueira, Langosch, Souery and Vieta2014; Lyall et al., Reference Lyall, Penades and Smith2019; Rhee et al., Reference Rhee, Olfson, Nierenberg and Wilkinson2020). Research assessing mortality risk associated with these alternative mood stabilizers is warranted to inform clinical decision on treatment regimens in BD.
Accumulating research has examined the association of mood stabilizers with mortality risk in BD. An earlier meta-analysis of randomized controlled trials demonstrated superior efficacy of lithium in reducing suicide risk relative to placebo, but no significant difference compared to valproate, olanzapine and quetiapine in patients with unipolar/bipolar depression (Cipriani et al., Reference Cipriani, Hawton, Stockton and Geddes2013). An updated meta-analysis, however, reported that lithium did not differ from placebo or treatment-as-usual in suicide rate reduction (Nabi et al., Reference Nabi, Stansfeld, Plöderl, Wood and Moncrieff2022). Mixed results were observed in recent register-based cohort studies in this respect. Some showed that lithium, but not valproate or antipsychotics, was associated with decreased suicide risk (Song et al., Reference Song, Sjölander, Joas, Bergen, Runeson, Larsson, Landén and Lichtenstein2017; Toffol et al., Reference Toffol, Hätönen, Tanskanen, Lönnqvist, Wahlbeck, Joffe, Tiihonen, Haukka and Partonen2015), while others reported favourable results for both lithium and valproate (Antolín-Concha et al., Reference Antolín-Concha, Lähteenvuo, Vattulainen, Tanskanen, Taipale, Vieta and Tiihonen2020; Chen et al., Reference Chen, Tsai, Chen, Pan, Su, Chen and Kuo2023; Tsai et al., Reference Tsai, Cheng, Chou, Lin, McInnis, Chang and Lan2016), compared to non-treatment. Some (Antolín-Concha et al., Reference Antolín-Concha, Lähteenvuo, Vattulainen, Tanskanen, Taipale, Vieta and Tiihonen2020; Crump et al., Reference Crump, Sundquist, Winkleby and Sundquist2013; Song et al., Reference Song, Sjölander, Joas, Bergen, Runeson, Larsson, Landén and Lichtenstein2017), albeit not all (Hayes et al., Reference Hayes, Pitman, Marston, Walters, Geddes, King and Osborn2016), head-to-head medication comparison studies showed that lithium was associated with reduced suicide rate relative to valproate. Discrepant findings were also reported on all-cause and natural-cause mortality risk in relation to mood stabilizers. A Taiwanese study revealed reduced all-cause and natural-cause mortality associated with lithium and valproate, compared to no mood-stabilizer treatment (Chen et al., Reference Chen, Tsai, Chen, Pan, Su, Chen and Kuo2023). A Finnish study demonstrated no association of elevated all-cause mortality with lithium and valproate (Toffol et al., Reference Toffol, Hätönen, Tanskanen, Lönnqvist, Wahlbeck, Joffe, Tiihonen, Haukka and Partonen2015), while a US study found a lower non-suicide mortality rate in lithium-treated BD patients than in valproate-treated counterparts (Smith et al., Reference Smith, Austin, Kim, Eisen, Kilbourne, Miller, Zivin, Hannemann, Sauer and Valenstein2015).
Notably, existing population-based cohort studies assessing association of mood stabilizers with mortality risk in BD were hampered by several important methodological limitations. First, most studies focused on suicide outcome only (Antolín-Concha et al., Reference Antolín-Concha, Lähteenvuo, Vattulainen, Tanskanen, Taipale, Vieta and Tiihonen2020; Hayes et al., Reference Hayes, Pitman, Marston, Walters, Geddes, King and Osborn2016; Oquendo et al., Reference Oquendo, Galfalvy, Currier, Grunebaum, Sher, Sullivan, Burke, Harkavy-Friedman, Sublette, Parsey and Mann2011; Song et al., Reference Song, Sjölander, Joas, Bergen, Runeson, Larsson, Landén and Lichtenstein2017; Toffol et al., Reference Toffol, Hätönen, Tanskanen, Lönnqvist, Wahlbeck, Joffe, Tiihonen, Haukka and Partonen2015), and there is limited research evaluating natural-cause mortality which, nonetheless, accounted for the majority of death causes (up to two-thirds) in BD (Chan et al., Reference Chan, Wong, Yung, Chen and Chang2021a; Paljärvi et al., Reference Paljärvi, Herttua, Taipale, Lähteenvuo, Tanskanen, Fazel and Tiihonen2023). Second, past studies did not take into consideration the effect of concomitant or sequential use of multiple mood stabilizers and other psychotropic medications, which might confound the study results (Chen et al., Reference Chen, Tsai, Chen, Pan, Su, Chen and Kuo2023; Lin et al., Reference Lin, Yeh and Pan2023; Toffol et al., Reference Toffol, Hätönen, Tanskanen, Lönnqvist, Wahlbeck, Joffe, Tiihonen, Haukka and Partonen2015). Third, previous studies primarily restricted their analysis to lithium, valproate and broadly categorized antipsychotics (Antolín-Concha et al., Reference Antolín-Concha, Lähteenvuo, Vattulainen, Tanskanen, Taipale, Vieta and Tiihonen2020; Smith et al., Reference Smith, Austin, Kim, Eisen, Kilbourne, Miller, Zivin, Hannemann, Sauer and Valenstein2015; Song et al., Reference Song, Sjölander, Joas, Bergen, Runeson, Larsson, Landén and Lichtenstein2017; Toffol et al., Reference Toffol, Hätönen, Tanskanen, Lönnqvist, Wahlbeck, Joffe, Tiihonen, Haukka and Partonen2015). However, literature conducted in schizophrenia patients indicated differential relationships between all-cause mortality risk and individual antipsychotics (Taipale et al., Reference Taipale, Tanskanen, Mehtala, Vattulainen, Correll and Tiihonen2020). Thus far, no studies have systematically examined the risk of all-cause, natural-cause and unnatural-cause mortality associated with commonly prescribed individual SGAs in BD patients.
We have previously conducted a population-based cohort study to assess premature mortality in BD patients, utilizing a territory-wide medical-record database of public health service in Hong Kong (HK), a metropolitan city located at the south-eastern tip of China, with total population of over 7.5 million. Our results demonstrated that BD patients had 2.6-fold elevated all-cause mortality rate and approximately 7 years of shorter lifespan relative to the general population (Chan et al., Reference Chan, Wong, Yung, Chen and Chang2021a). In the current investigation, we used the same study cohort and aimed to comprehensively evaluate the comparative mortality risk of all-cause, natural-cause and unnatural-cause deaths between mood stabilizers of lithium, valproate and three most commonly prescribed SGAs (i.e., quetiapine, risperidone and olanzapine) in patients with first-diagnosed BD over a 17-year period, taking into account important potential confounders including pre-existing chronic physical diseases, substance/alcohol use disorders and other psychotropic medications. Propensity-score (PS)-weighted models were employed to further optimize covariate adjustment.
Methods
Data source
Data of the patient cohort were extracted from the Clinical Data Analysis and Reporting System (CDARS; Hospital Authority Head Office IT Department, 2003), a territory-wide electronic health-record database developed by the Hospital Authority (HA) which is a statutory body delivering government-subsidized, universal health coverage to all HK residents (approximately 92% being Chinese) by managing all public hospitals, specialist and general outpatient clinics in HK. Detailed description of CDARS has been reported elsewhere (Cheung et al., Reference Cheung, Fung, Wong, Tong, Sek, Greyling, Tse and Fung2007). Briefly, CDARS is an integrated, longitudinal patient electronic record system capturing clinical data across all healthcare settings of HA facilities. The database contains patients’ demographics and clinical information including diagnoses, attendances to outpatient clinics and emergency departments, hospital admissions and prescribing/dispensing records of medications. Data on dates and causes of death were retrieved from CDARS via internal linkage to regional death registries from the Immigration Department. Patients’ death status was also directly recorded and verified by CDARS as the vast majority of deaths in HK occur in public hospitals, thereby facilitating accurate ascertainment of death. Clinical data are collected and entered into computerized clinical-management system by treating clinicians and other healthcare professionals, and are then transferred to CDARS for audit and research purposes. CDARS generates unique, anonymized patient identifiers to protect privacy and to link all medical records. This database has been used to conduct high-quality population-based studies on psychiatric disorders including schizophrenia and BD (Chan et al., Reference Chan, Wong, PCF, Chen and Chang2021b, Reference Chan, Wong, Yung, Chen and Chang2021a, Reference Chan, Wong, Yung, Chen and Chang2022b; Chang et al., Reference Chang, Chan, Wong, Hai, Or and Chen2020; Yung et al., Reference Yung, Wong, Chan, Chen and Chang2021, Reference Yung, Wong, Chan, PCF, Chen and Chang2020) and pharmacoepidemiological investigations (Chai et al., Reference Chai, Luo, Man, Lau, Chan, Yip and Wong2022; Hung et al., Reference Hung, Chan, Wong, Fung, Lee and Chang2023; Kan et al., Reference Kan, Chan, Wong, Chen and Chang2022; Law et al., Reference Law, Chan, Wong, Chen and Chang2023).
Study population
We identified all individuals aged ≥15 years who received a first-recorded diagnosis of BD for public psychiatric inpatient or outpatient treatment in HK between 1 January 2002 and 31 December 2018. Diagnosis of BD was recorded and ascertained by the International Classification of Diseases, 10th revision (ICD10 codes: F30 and F31). Diagnostic ascertainment took into consideration the longitudinal illness course (Chang et al., Reference Chang, Pang, Chung and Chan2009) and individuals with their diagnosis changed to schizophrenia or schizoaffective disorder (ICD10 codes: F20 and F25) before the end of follow-up (as their mostly recently assigned principal diagnosis) were excluded. Patients were followed up from the date of their first-recorded BD diagnosis until the date of death or 31 December 2018, whichever came first. The study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. Since individual patient records in this database were completely unidentifiable, no informed consent was required.
Exposure to mood stabilizers
Five mutually exclusive mood-stabilizer exposure groups including lithium, valproate, quetiapine, risperidone and olanzapine were derived based on medication records of included BD patients. Quetiapine, risperidone and olanzapine represented the three most frequently prescribed SGAs in the study cohort (Supplementary Table S1) as the treatment for BD, and were thus selected in the subsequent comparative analyses on mortality risk. BD patients who had been prescribed with one of the five specified mood stabilizers only during study period were classified as users to that mood stabilizer. For patients who had received more than one of the studied mood stabilizers during the study period, their group membership status was determined by the prescribed mood stabilizer with the longest cumulative exposure duration within the study follow-up. BD patients who had not exposed to any of the five studied mood stabilizers were excluded from the analysis.
Outcomes
The main outcome measure was all-cause mortality. Causes of death were also classified according to ICD10 codes, and were divided into natural and unnatural causes. Natural causes comprised infectious and parasitic diseases (A00–B99), neoplasms (C00–D48), metabolic diseases (E00–E90), neurological diseases (G00–G99), cardiovascular diseases (I00–I99), respiratory diseases (J00–J99), digestive diseases (K00–K93) and genitourinary diseases (N00–N99). Unnatural causes included accidents (V01–X59), self-harm (X60–X84) and other external causes (X85–Y98). Patients with unknown death causes were excluded in analyses for mortality of natural and unnatural causes.
PS weighting and covariates
A PS weighting model was performed to limit confounding in the five exposure groups of mood stabilizers. Taken into consideration the availability of clinical information adequately captured in the database, an array of preselected candidate covariates comprising patients’ demographics (sex, age at first-recorded diagnosis of BD, catchment areas of healthcare service), and pre-existing chronic physical diseases (i.e., physical morbidity burden) as quantified by Charlson Comorbidity Index (Charlson et al., Reference Charlson, Pompei, Ales and MacKenzie1987; Deyo et al., Reference Deyo, Cherkin and Ciol1992) as well as the presence of epilepsy, diabetes, hypertension and dyslipidaemia, comorbid substance and alcohol use disorders, history of past psychiatric admission (as a proxy for illness severity) and the use of psychotropics other than the five specified mood stabilizers during the study period including mood-stabilizing anticonvulsants (lamotrigine, carbamazepine), antipsychotics (first-generation antipsychotics and SGAs other than the three specified SGAs) and antidepressants. Details of diagnostic codes for physical and psychiatric morbidities are listed in Supplementary Table S2. Multinomial PS weighting was obtained using generalized boosted models (McCaffrey et al., Reference McCaffrey, Griffin, Almirall, Slaughter, Ramchand and Burgette2013). We sought to estimate the average treatment effect for the PS weighting among the five exposure groups, based on the premise that their membership assignment was an exchangeable option (Desai and Franklin, Reference Desai and Franklin2019). We took the maximum absolute standardized mean difference (ASMD) across exposure groups in each covariate as the diagnostic measure of between-group balance, where ASMD >0.20 denotes notable group differences. Before weighting, a total of 16 (out of 27) comparisons in covariates showed ASMD >0.20 and was reduced to 1 after weighting (Supplementary Figure S1). The imbalanced covariate was additionally adjusted in the PS-weighted regression model to control for remaining imbalances among exposure groups.
Statistical analysis
Demographics, baseline physical comorbidities, substance and alcohol use disorders and prescription profile within follow-up of BD patients among five mood-stabilizer exposure groups were compared. Incidence rates for mortality due to all-, natural- and unnatural-causes among five exposure groups at follow-up were estimated by an exact 95% confidence intervals (CIs) based on a Poisson distribution. PS-weighted Cox proportional-hazards regression models were conducted to evaluate the relative risk of mortality among five exposure groups, with lithium-treated group as the reference category. Kaplan–Meier curves were used to visualize the survival rates of each exposure group. To explore the potential influence of length of exposure to studied mood stabilizers on mortality risk, the analyses were repeated in BD patients of each of the five mood-stabilizer exposure groups with cumulative exposure to the specified mood stabilizer ≥90 days, ≥180 days, ≥365 days and ≥730 days (2 years). We then specifically restricted the analyses to patients with short exposure duration of <90 days, <180 days and <365 days, to the specified mood stabilizers in each exposure group and examined the relationship of short mood-stabilizer exposure duration with mortality risk. Three sets of sensitivity analyses were conducted. First, only patients with the length of cumulative exposure to the specified mood stabilizer ≥90 days (i.e., cumulative exposure to lithium in the lithium-treated group; cumulative exposure to valproate in the valproate-treated group and so forth) and its medication possession ratio (MPR) ≥90% were included to ensure sufficient exposure to the specified mood stabilizer in each exposure group. MPR of the specified mood stabilizer was calculated by dividing the length of its cumulative exposure by the total observation time at follow-up. Prior research has shown that medication prescription data were adequately captured and reliably recorded in CDARS (i.e., electronic medical-record database used in the current study), and were used to generate medication exposure-related parameters including cumulative exposure duration and MPR (Man et al., Reference Man, Coghill, Chan, Lau, Hollis, Liddle, Banaschewski, McCarthy, Neubert, Sayal, Ip, Schuemie, Sturkenboom, Sonuga-Barke, Buitelaar, Carucci, Zuddas, Kovshoff, Garas, Nagy, Inglis, Konrad, Häge, Rosenthal and Wong2017). Second, MPR of the specified mood stabilizer and other studied mood stabilizers in the exposure group was calculated. We only included patients with MPR of the specified mood stabilizer ≥80% and MPR of other studied mood stabilizers <20% in the analyses to reduce confounding effect of the other studied mood stabilizers in each exposure group. Third, monotherapy analysis was conducted by including patients who had been prescribed with only one of the five specified mood stabilizers within the entire follow-up. The proportional-hazards assumptions for all analyses were confirmed using log-minus-log plot (Kleinbaum and Klein, Reference Kleinbaum and Klein2012). Results of all Cox proportional-hazards regression models were presented as hazard ratios (HRs) in 95% CIs. All statistical analyses were performed using R (version 4.0.2). Generalized boosted model was implemented with the twang package (Ridgeway et al., Reference Ridgeway, McCaffrey, Morral, Burgette and Griffin2012). P < 0.05 was considered statistically significant.
Results
Characteristics of the study sample
A total of 12,797 BD patients (mean age = 41.3 years, SD = 15.4) were identified within the study period by the medical-record database. In this whole BD cohort, 3701 patients were excluded as their BD diagnosis was not incident in nature within the study period. Among the incident BD cohort (n = 9094), 957 patients who were not prescribed with any of the five studied mood stabilizers within the study period were excluded from the subsequent analyses, resulting in a total of 8137 patients as the final study cohort (mean age = 39.2 years, SD = 15.4). The mean duration of follow-up for BD patients was 7.2 years (SD = 4.7). A total of 488 deaths was recorded in the overall sample, of which 358 (73.4%) had known causes. There were 1027, 3580, 797, 1975 and 757 patients assigned to lithium, valproate, olanzapine, quetiapine and risperidone exposure groups, respectively. Lengths of cumulative exposure per mood stabilizer in each of the five studied mood-stabilizer exposure groups are shown in Supplementary Table S3. Patients in the lithium-treated group tended to be younger and had lower prevalence of hypertension, dyslipidaemia, diabetes and substance use disorder than those in the other exposure groups (Table 1).
Table 1. Characteristics of mood-stabilizer exposure groups

Note: FGA, first-generation antipsychotics; SD, standard deviation; SGA, second-generation antipsychotics.
a Chi-square and one-way ANOVA tests were conducted for analysis of categorical and continuous variables, respectively.
b At least one medical comorbidity was measured at baseline.
c Charlson Comorbidity Index (CCI) score is a widely used and well-validated measure for physical morbidity burden (Charlson et al., Reference Charlson, Pompei, Ales and MacKenzie1987; Deyo et al., Reference Deyo, Cherkin and Ciol1992), which assesses the presence of 17 chronic physical diseases including cerebrovascular disease, hemiplegia or paraplegia, myocardial infarction, congestive heart failure, peripheral vascular disease, respiratory diseases, peptic ulcer disease, mild liver disease, moderate or severe liver disease, renal diseases, rheumatological diseases, malignancy without metastasis, metastatic solid tumour, diabetes metabolic disturbances, diabetes with chronic complications, AIDS or HIV and dementia. In the current study, age-adjusted adapted CCI was computed. As diabetes was a disease of interest, it was evaluated separately and excluded from CCI score calculation. The higher the CCI score, the greater the physical comorbidity burden (in terms of the number and severity of physical multi-comorbidity).
d Medications could be prescribed concomitantly or sequentially during study follow-up.
e Other anticonvulsants included prescriptions of carbamazepine or lamotrigine.
f Other second-generation antipsychotics (SGA) included any prescription of clozapine, amisulpride, paliperidone, aripiprazole, lurasidone, ziprasidone, sertindole.
All-cause, natural-cause and unnatural-cause mortality risk and mood stabilizers
As shown in Table 2, the incidence rate per 1000 person-years were 5.9 (95% CI: 4.5–7.6), 8.4 (7.4–9.5), 11.1 (8.3–14.9), 7.4 (6.0–9.2) and 12.0 (9.3–15.6) for all-cause mortality in lithium, valproate, olanzapine, quetiapine and risperidone exposure groups, respectively. PS-weighted Cox regression models indicated significantly elevated risk of all-cause mortality associated with olanzapine (HR: 2.07 [95% CI: 1.33–3.22]) and risperidone (1.66 [1.08–2.55]), relative to lithium.
Table 2. Mortality risk of mood-stabilizer exposure groups
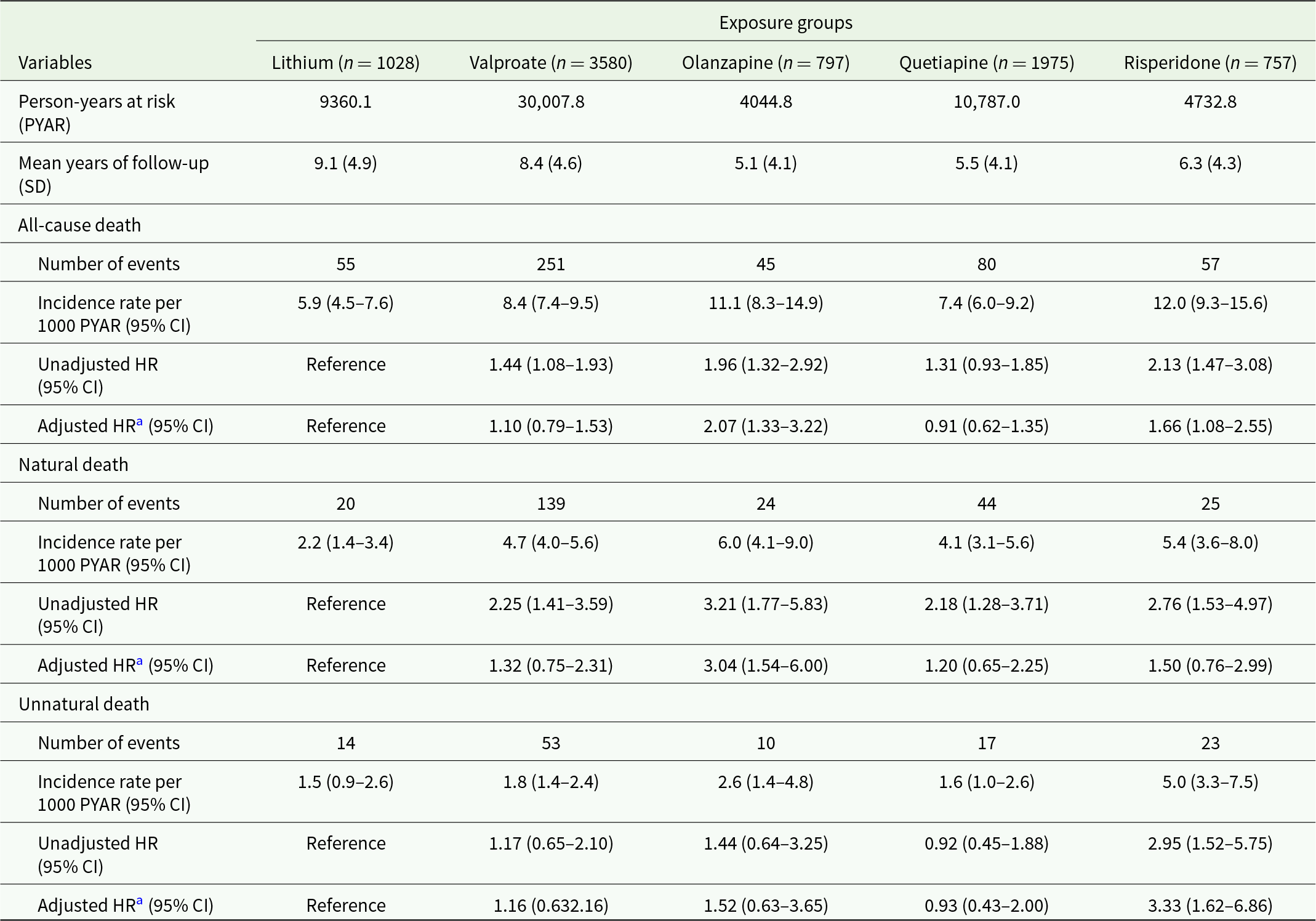
Note: CI, confidence interval; HR, hazard ratio; SD, standard deviation.
a The model was propensity-score weighted with additional adjustment for concomitant/ sequential prescription of studied mood stabilizers other than the specified one.
For natural-cause mortality, the incidence rates per 1000 person-years were 2.2 (1.4–3.4), 4.7 (4.0–5.6), 6.0 (4.1–9.0), 4.1 (3.1–5.6) and 5.4 (3.6–8.0) in lithium, valproate, olanzapine, quetiapine and risperidone exposure groups, respectively. In particular, exposure to olanzapine was associated with a significantly higher rate of natural-cause mortality than lithium, with an adjusted HR of 3.04 (1.54–6.00). Regarding unnatural-cause mortality, the incidence rate in lithium, valproate, olanzapine, quetiapine and risperidone exposure groups were 1.5 (0.9–2.6), 1.8 (1.4–2.4), 2.6 (1.4–4.8), 1.6 (1.0–2.6) and 5.0 (3.3–7.5), respectively. The risperidone exposure group demonstrated a significantly increased risk of unnatural-cause mortality risk compared to the lithium group (HR: 3.33 [1.62–6.86]). Kaplan–Meier curves showing survival rates of five exposure groups are shown in Fig. 1. Findings of the additional analyses of cumulative exposure to studied mood stabilizers ≥90 days, ≥180 days, ≥365 days and ≥730 days (Supplementary Table S4) were consistent with those of the primary analyses. For the analyses restricting to patients with comparatively short duration of exposure to studied mood stabilizers, olanzapine remained significantly associated with increased risk of natural-cause mortality in exposure <180 days and <365 days (but not <90 days) relative to lithium. Association between risperidone and elevated unnatural-cause mortality risk was not observed compared to lithium in all of the three short exposure durations.
Sensitivity analyses restricting to patients with length of cumulative exposure to the specified mood stabilizer ≥90 days and its MPR ≥90% yielded a similar pattern of mortality risk across the five exposure groups (Supplementary Table S6). In the other two sets of sensitivity analyses which included (i) patients with MPR of the specified mood stabilizer ≥80% and the other mood stabilizers <20%, and (ii) patients with mood-stabilizer monotherapy, we observed that olanzapine and risperidone were both associated with increased risk of all-cause and natural-cause mortality (relative to lithium group), and the association between risperidone and unnatural-cause mortality risk became non-significant (Supplementary Tables S7 and S8).

Figure 1. Survival curves for mood-stabilizer exposure groups: (a) all-cause deaths; (b) natural-cause deaths and (c) unnatural-cause deaths.
Discussion
To our knowledge, this investigation is one of the few population-based cohort studies comprehensively examining risk of all-, natural- and unnatural-cause mortality associated with mood stabilizers, and the first study evaluating risk of natural-cause mortality in relation to individual SGA agents relative to lithium in BD patients. We had employed PS weighting models to optimize control for confounding factors including chronic physical comorbidities, substance and alcohol use disorders and the use of other psychotropic medications to delineate the independent mortality risk of BD patients in mood-stabilizer exposure groups. Our data showed that olanzapine and risperidone users both had a significantly higher rate of all-cause mortality than patients in lithium-treated group. We also observed that olanzapine was associated with elevated risk of natural-cause mortality, while risperidone was related to increased risk of unnatural-cause mortality. There were no significant differences between lithium-, valproate- and quetiapine-treated groups in risk of all-, natural- and unnatural-cause mortality.
Specifically, findings of the primary analyses revealed that olanzapine and risperidone were related to significantly higher risk of natural- and unnatural-cause mortality than lithium, respectively. The significant association between olanzapine and increased natural-cause mortality was consistently affirmed in additional analyses on various lengths of cumulative exposure (except short exposure of <90 days) as well as three sets of sensitivity analyses. The relationship between risperidone and a higher rate of mortality due to unnatural causes (relative to lithium) did not reach statistical significance in additional analyses on short exposure duration (<365 days, albeit remained significant for longer exposure of ≥1 and ≥2 years) and in the sensitivity analyses restricting to low MPR of other mood stabilizers and monotherapy (i.e., risperidone-only), precluding definitive conclusion in this respect. The markedly higher risk estimate for unnatural-cause mortality in the risperidone-treated group than other mood-stabilizer exposure groups in three sets of sensitivity analyses, however, constituted a signal that warranted further investigation. A Swedish study comparing mortality risk of BD patients treated with individual SGA agents to those treated with lithium also revealed that olanzapine and risperidone were both associated with modestly increased mortality risk (Crump et al., Reference Crump, Sundquist, Winkleby and Sundquist2013). The excess mortality related to olanzapine and risperidone compared to lithium may have several possible explanations. First, a large body of evidence demonstrated increased prevalence of physical diseases such as metabolic syndrome, diabetes and ischemic heart diseases in individuals treated with antipsychotics, especially SGAs (Pillinger et al., Reference Pillinger, McCutcheon, Vano, Mizuno, Arumuham, Hindley, Beck, Natesan, Efthimiou, Cipriani and Howes2020). In particular, among SGAs, olanzapine has been shown to exhibit the worst metabolic profiles (Citrome et al., Reference Citrome, Collins, Nordstrom, Rosen, Baker, Nadkarni and Kalsekar2013; Galling et al., Reference Galling, Roldán, Nielsen, Nielsen, Gerhard, Carbon, Stubbs, Vancampfort, De Hert, Olfson, Kahl, Martin, Guo, Lane, Sung, Liao, Arango and Correll2016; Li et al., Reference Li, Peng, Li, Liu, Lv, Yang, Yu, Deng, Zhang, Fang, Huo, Chen, Sun and Li2019; Pillinger et al., Reference Pillinger, McCutcheon, Vano, Mizuno, Arumuham, Hindley, Beck, Natesan, Efthimiou, Cipriani and Howes2020). Conversely, lithium may confer beneficial effect on physiological functions such as maintaining vascular elasticity by facilitating elastin biosynthesis (Xu et al., Reference Xu, Wang, Li, Li, Qu, Tong, Zhang, Bai and Fan2021). Our findings of lack of significant difference between quetiapine and lithium in terms of the association with natural-cause (and all-cause) mortality risk, however, were at odds with the results of olanzapine and risperidone. Further investigation is required to verify our findings in this respect and to delineate factors attributable to potential differential mortality risk between individual SGAs in BD. Second, owing to the narrow therapeutic index, BD patients treated with lithium receive close medical surveillance via specialist psychiatric services which offer regular clinical monitoring and blood tests on lithium serum level as well as other bodily functions for potential physical complications (e.g., thyroid and renal function tests). Comparatively, monitoring (with blood tests) for patients receiving valproate and antipsychotics is generally less frequent than those prescribed with lithium in our local practice. Then more intensive monitoring of lithium-treated patients may therefore increase the likelihood of earlier detection of physical conditions with subsequent interventions. Third, individuals prescribed with olanzapine or risperidone (or other antipsychotics) might represent a subgroup of BD patients with greater illness severity. For instance, olanzapine/risperidone could be commenced for treating psychotic symptoms in BD, or after a failure of initial treatment of conventional mood stabilizers (including lithium and valproate). In this context, treatment with olanzapine/risperidone would indicate the greater illness severity of the underlying BD, which in turn might be associated with a higher risk of excess mortality, i.e., illness severity as potential confounder. Notably, SGA has replaced lithium as the most-frequently prescribed mood stabilizer in the recent decades (Lyall et al., Reference Lyall, Penades and Smith2019; Rhee et al., Reference Rhee, Olfson, Nierenberg and Wilkinson2020). The excess mortality related to olanzapine and risperidone relative to lithium observed in our data indicates the need for research to further clarify the factors contributing to such association, thereby facilitating the risk–benefit evaluation of SGA use as an alternative mood-stabilizing treatment in BD. Importantly, the result that lithium was related to a lower mortality risk than olanzapine and risperidone, and comparable mortality risk with quetiapine provides further evidence supporting the need to adhere to the clinical guidelines which consistently recommend lithium (and often quetiapine) as the first-line maintenance treatment for BD (Goodwin et al., Reference Goodwin, Haddad, Ferrier, Aronson, Barnes, Cipriani, Coghill, Fazel, Geddes, Grunze, Holmes, Howes, Hudson, Hunt, Jones, Macmillan, McAllister-Williams, Miklowitz, Morriss, Munafò, Paton, Saharkian, Saunders, Sinclair, Taylor, Vieta and Young2016; Grunze et al., Reference Grunze, Vieta, Goodwin, Bowden, Licht, Möller and Kasper2013; NICE, 2014; Yatham et al., Reference Yatham, Kennedy, Parikh, Schaffer, Bond, Frey, Sharma, Goldstein, Rej, Beaulieu, Alda, MacQueen, Milev, Ravindran, O’Donovan, McIntosh, Lam, Vazquez, Kapczinski, McIntyre, Kozicky, Kanba, Lafer, Suppes, Calabrese, Vieta, Malhi, Post and Berk2018).
Our finding showed that valproate-treated group did not differ from lithium-treated group in the risk of unnatural death. This concurs with some Collins and McFarland, Reference Collins and McFarland2008; Hayes et al., Reference Hayes, Pitman, Marston, Walters, Geddes, King and Osborn2016; Smith et al., Reference Smith, Sondergard, Lopez, Andersen and Kessing2009) but not all studies (Antolín-Concha et al., Reference Antolín-Concha, Lähteenvuo, Vattulainen, Tanskanen, Taipale, Vieta and Tiihonen2020; Crump et al., Reference Crump, Sundquist, Winkleby and Sundquist2013; Song et al., Reference Song, Sjölander, Joas, Bergen, Runeson, Larsson, Landén and Lichtenstein2017), which revealed that lithium was associated with a reduced suicide risk relative to valproate. Although studies which compared patients or treatment-periods with versus without mood-stabilizing medications generally reported a lower risk estimate for lithium than for valproate (i.e., relative to no treatment) (Chen et al., Reference Chen, Tsai, Chen, Pan, Su, Chen and Kuo2023; Toffol et al., Reference Toffol, Hätönen, Tanskanen, Lönnqvist, Wahlbeck, Joffe, Tiihonen, Haukka and Partonen2015; Tsai et al., Reference Tsai, Cheng, Chou, Lin, McInnis, Chang and Lan2016), the substantially overlapping 95% CIs for the risk estimates of lithium and valproate in fact suggested non-significant risk difference between these two mood stabilizers. Nonetheless, caution should be exercised when comparing our findings with those of prior research specifically focusing on suicide risk as our data had included all sorts of unnatural death causes, despite suicide likely being the major contributor. Previous investigation on suicide risk between lithium- and valproate-treated patients had included both completed and attempted suicides in the composite suicidal behaviour outcome (Song et al., Reference Song, Sjölander, Joas, Bergen, Runeson, Larsson, Landén and Lichtenstein2017). Likewise, our data revealed a comparable risk of natural deaths in lithium and valproate exposure groups. This is contrary to an earlier register-based study demonstrating a lower risk of natural-cause mortality in BD patients treated with lithium than those with valproate within 90 days of commencement of mood-stabilizer treatment (Smith et al., Reference Smith, Austin, Kim, Eisen, Kilbourne, Miller, Zivin, Hannemann, Sauer and Valenstein2015). However, this association became non-significant when the observation period was extended to 180 days and 365 days after treatment initiation (Smith et al., Reference Smith, Austin, Kim, Eisen, Kilbourne, Miller, Zivin, Hannemann, Sauer and Valenstein2015). In fact, in our crude analyses (i.e., the unweighted sample), valproate was related to a greater likelihood of all-cause and natural-cause mortality, relative to lithium. The non-significant associations in our PS-weighted models may imply that the overall confounding effect might initially bias against valproate. The finding of a significant difference in natural-cause mortality risk between lithium and valproate in the previous study might be subject to residual confounding (Smith et al., Reference Smith, Austin, Kim, Eisen, Kilbourne, Miller, Zivin, Hannemann, Sauer and Valenstein2015). On the other hand, we had included lithium users in the lithium-treated group regardless of their subsequent discontinuation of lithium treatment, which, however, was found to be linked to increased mortality risk (Bocchetta, Reference Bocchetta2005; Müller-Oerlinghausen et al., Reference Müller-Oerlinghausen, Wolf, Ahrens, Glaenz, Schou, Grof, Grof, Lenz, Simhandl, Thau, Vestergaard and Wolf1996; Smith et al., Reference Smith, Austin, Kim, Eisen, Kilbourne, Miller, Zivin, Hannemann, Sauer and Valenstein2015). The mortality risk of lithium exposure group may thus have been overestimated, masking the actual between-drug difference with valproate. Yet, our sensitivity analysis restricting to patients with a high degree of adherence to mood stabilizers (i.e., MPR ≥90%) yielded a similar result, indicating that such potential bias (due to raised mortality risk related to lithium discontinuation in lithium exposure group) is minimized. Of note, despite its comparable mortality risk as lithium, substantial evidence has shown that foetal exposure to valproate is associated with increased risk of major congenital malformations (Jentink et al., Reference Jentink, Loane, Dolk, Barisic, Garne, Morris and de Jong-van den Berg2010; Veroniki et al., Reference Veroniki, Cogo, Rios, Straus, Finkelstein, Kealey, Reynen, Soobiah, Thavorn, Hutton, Hemmelgarn, Yazdi, D’Souza, MacDonald and Tricco2017) and adverse neurodevelopmental outcomes including cognitive impairment, growth retardation and behavioural disturbances such as autistic spectrum disorder and attention-deficit/hyperactivity disorder (Baldwin and Amaro, Reference Baldwin and Amaro2020; Veroniki et al., Reference Veroniki, Cogo, Rios, Straus, Finkelstein, Kealey, Reynen, Soobiah, Thavorn, Hutton, Hemmelgarn, Yazdi, D’Souza, MacDonald and Tricco2017). In fact, various treatment guidelines have recommended valproate should be avoided in women of childbearing age (Anmella et al., Reference Anmella, Pacchiarotti, Cubała, Dudek, Maina, Thomas and Vieta2019; NICE, 2014; Yatham et al., Reference Yatham, Kennedy, Parikh, Schaffer, Bond, Frey, Sharma, Goldstein, Rej, Beaulieu, Alda, MacQueen, Milev, Ravindran, O’Donovan, McIntosh, Lam, Vazquez, Kapczinski, McIntyre, Kozicky, Kanba, Lafer, Suppes, Calabrese, Vieta, Malhi, Post and Berk2018). In the current study cohort, valproate-treated patients represented the largest exposure group among five studied mood stabilizers, with 58% of them being women. The comparatively high prescription rate of valproate in women with BD highlights an urgent need for a comprehensive review of local current prescribing practice and implementation of regulatory measures to enhance adherence to clinical guidelines to minimize valproate use in female BD patients during their reproductive years.
Several limitations of the study should be noted when interpreting the study results. First, data on socio-economic status, educational level, lifestyle variables such as physical activity, dietary patterns and smoking, history of abuse and previous self-harm behaviours were not adequately recorded in the medical-record database and thus were not included in the analyses for confounder adjustment. Second, similar to most other pharmacoepidemiological studies, patients’ adherence to prescribed mood stabilizers was derived from dispensing records, which may overestimate the actual intake of medications in the study cohort. Third, medication dose of mood stabilizers was not available, precluding the evaluation on the dose-dependent effect of mood stabilizers and its potential association with incremental mortality risk. Fourth, missing data on patients’ death causes may compromise the accuracy in evaluating natural- and unnatural-cause mortality. Fifth, as information on specific natural and unnatural causes was not available, we were not able to investigate the mortality risk in relation to more specific death causes such as cardiovascular-mortality and suicide. Sixth, the study data did not contain more specific illness-related information denoting disorder subtypes (type I or II), clinical polarity, presence of psychotic features and symptom severity of BD (though we used history of psychiatric admission as a proxy indicator of illness severity), failure of initial pharmacological treatment or treatment refractoriness or other psychiatric comorbidities (e.g., anxiety disorders). Hence, we were not able to explore whether the observed associations of mortality risk with mood-stabilizer exposure are similar in relation to the specific illness-related features of BD. Seventh, information about exposure to benzodiazepines, which has been shown to be associated with elevated mortality risk in patients with schizophrenia in previous research (Fontanella et al., Reference Fontanella, Campo, Phillips, Hiance-Steelesmith, Sweeney, Tam, Lehrer, Klein and Hurst2016; Tiihonen et al., Reference Tiihonen, Mittendorfer-Rutz, Torniainen, Alexanderson and Tanskanen2016) (albeit with some evidence suggesting no or minimal increased mortality risk associated with benzodiazepine use in adults [Patorno et al., Reference Patorno, Glynn, Levin, Lee and Huybrechts2017]), was unavailable in our dataset, precluding us from adjusting for its potential confounding effect in our BD cohort. Of note, our PS weighting model, incorporating a wide array of potential confounding variables including psychotropics other than the five studied mood stabilizers (e.g., antidepressants, other mood-stabilizing anticonvulsants and other antipsychotics), yielded satisfactory post-weighting between-group balance. Notwithstanding, future research should take into consideration potential confounding effect of benzodiazepine use in mortality risk analysis for BD patients, especially those with chronic, high-dose utilization patterns. Eighth, the study dataset did not include information about formulations of antipsychotics, thus analyses examining the potential differential associations of oral vs. long-acting injectable antipsychotics with mortality risk could not be performed. Ninth, patients’ data were retrieved from medical-record database of public healthcare services managed by HA, and patients who were under private psychiatric care were not included in the study. However, HA is the predominant provider of psychiatric services to individuals with severe mental disorders in HK, and hence the risk of selection bias or missing treated cases of BD was minimized.
In conclusion, this population-based cohort study showed that BD patients in olanzapine and risperidone exposure groups had significantly higher mortality risk than those in lithium exposure group, while lithium, valproate and quetiapine were associated with comparable mortality risk. The association between olanzapine and an increased risk of natural-cause mortality relative to lithium was consistently affirmed in sensitivity analyses. Our findings thus provide evidence further supporting the adherence to treatment guidelines recommending lithium as the first-line maintenance treatment for BD. More research examining underlying factors or mechanisms that contribute to excess mortality related to individual SGA agents relative to lithium, with adequate sample size and data on more specific natural and unnatural death causes, is required to provide clinically useful data to inform risk-benefit evaluation on the use of these alternative mood stabilizers to improve treatment outcome and to minimize avoidable premature mortality in BD patients.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796024000337.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgements
The authors would like to thank the colleagues in the Hospital Authority of Hong Kong for their kind assistance in data extraction for the current investigation.
Author contributions
Authors WCC and CSMW designed and conceptualized the study. Author JKNC conducted statistical analysis and wrote the first draft of the manuscript. Authors WCC, JKNC and CSMW interpreted the study data. Authors JKNC and WCC revised and finalized the manuscript. All authors provided critical feedback to the manuscript and have approved the final manuscript.
Financial support
The study was supported by Hong Kong Research Grants Council (grant number: 17127417) and the State Key Laboratory of Brain & Cognitive Sciences, the University of Hong Kong. Additional financial support to undertake this research was provided by the Research Fund Award 2022, Schizophrenia International Research Society.
Competing interests
The authors declared no conflicts of interest in relation to the subject of this study.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2000.





