Introduction
The Reed Parrotbill Paradoxornis heudei David, 1872 is a reedbed-inhabiting passerine distributed in China, Mongolia, and Russia (BirdLife International 2008) and, because of habitat loss, this species is currently listed as ‘Near Threatened’. However, these threats have not been clearly defined (BirdLife International 2008), and more relevant ecological knowledge is required to enhance the management of reedbeds and promote the conservation of this species.
Reed Parrotbills are found in the Shanghai municipality. Since there are no clearly defined measures for reedbed management in this region, almost all the reedbed areas are harvested by local farmers during the period between November-December and April. This practice can have a negative impact on reed-inhabiting arthropods (Schmidt et al. Reference Schmidt, Lefebvre, Poulin and Tscharntke2005) and bird communities (Poulin and Lefebvre Reference Poulin and Lefebvre2002). Graveland (Reference Graveland1999) showed that the densities and breeding success of some reedbed-nesting passerines were greater in unharvested areas, in comparison with the corresponding values in harvested areas. Therefore, we hypothesized that reed harvesting would have a negative impact on Reed Parrotbills. Another potential threat for this species is a reduction in the area of suitable habitat due to invasion by Smooth Cordgrass Spartina alterniflora. Smooth Cordgrass shows various properties relevant to ecological engineering, such as a great capacity for mitigating erosion and trapping sediments. This species has been deliberately introduced into coastal and estuarine regions of China and is currently flourishing in the region from Guangxi to Tianjin (Chen et al. Reference Chen, Li, Zhong and Chen2004, Chung Reference Chung2006). Owing to its stronger reproductive capacity and wider ecological niche, Smooth Cordgrass has rapidly expanded and outcompeted the native species (Li and Zhang Reference Li and Zhang2007) and is currently found in monospecific patches that were previously occupied by native species such as Common Reed Phragmites australis. In 1985, the coverage of Smooth Cordgrass in six Chinese counties was approximately 260 ha (Chung Reference Chung, Mitsch and Jorgensen1989), and by 2000 this had increased to more than 112,000 ha (An et al. Reference An, Gu, Zhou, Wang, Deng, Zhi, Li, Chen, Yu and Liu2007). Observations during winter indicated that Reed Parrotbills are present in areas covered by Common Reed, and the birds avoid Smooth Cordgrass (Ma et al. Reference Ma, Gan, Choi, Jing, Tang, Li and Chen2007). Therefore, we also hypothesize that Smooth Cordgrass expansion is a serious threat for Reed Parrotbill because of large-scale habitat losses.
In 2007, we conducted a study on Reed Parrotbills to determine the impact of both annual reed harvesting and Smooth Cordgrass distribution on the individual densities of birds during the nesting period. We used two methods (individual observations and nest-detection based estimates) and compared the results to estimate bird densities. We also analyzed the reedbed vegetation cover to define the vegetation requirements for nesting Reed Parrotbills.
Methods
Study areas
The study was conducted in the Chongming Dongtan National Nature Reserve (121°50′–122°05′E 31°25′–31°38′N), which is a complex of coastal wetland ecosystems listed as a Ramsar Wetland of International Importance. The reserve is situated on the eastern end of the Chongming Island in the Yangtze Estuary and constitutes the largest wetland area in the Shanghai municipality.
We selected two 4.5 ha (300 m × 150 m) sample areas located in the reedbed of the reserve, namely, the harvested and non-harvested areas (Figure 1). In the harvested area, the reeds were harvested annually and in the non-harvested area, the reeds had not been harvested since 2004. The reedbed contained three major types of vegetation cover arising from different invasion stages: monospecific stands of Common Reed, mixed areas with Common Reed and Smooth Cordgrass, and monospecific stands of Smooth Cordgrass. As it was difficult to distinguish the exact boundaries between monospecific stands of Common Reed and mixed areas, we pooled them together to finally define two types of vegetation cover (Table 1 and Figure 2).

Figure 1. (a) Location of Chongming Island, (b) Chongming Dongtan Nature Reserve (grey area on part b), and (c) the non-harvested (NHA) and harvested (HA) reedbed sample areas.

Figure 2. Locations of monospecific Smooth Cordgrass stands, mixed areas of Common Reed and Smooth Cordgrass and Reed Parrotbill nests in the (a) non-harvested and (b) harvested sample areas.
Table 1. Vegetation covers of the reedbed study areas (ha).

Habitat use and selection
We determined the bird abundance in each sample area using counts of both nests and individual birds.
Nest counts. Nest searches were undertaken between May and July. We identified nests by systematically searching through the reeds, which were divided into 3 m wide transects covering the entire sample area, every two weeks (a total of three investigations were performed for each sample area). To determine the number of individuals in each habitat, we considered that each nest was occupied by two individuals.
Individual counts. The individual numbers were directly determined by using a point-transect method conducted between 20 June and 1 August. The counts (n = 5 in each sample area) were made at intervals of 10 ± 2.6 days. In each sample area, six 0.5 ha sites (50 m × 150 m) were delimited and observed from the dike during 10 minute observation periods using binoculars. Only individuals perched on vegetation were counted and the habitat type for each observation noted as mixed reed/cordgrass or monospecific cordgrass. All observations were made by the same observer from 06h00 to 10h00 on days when there was no rain and little wind.
A chi-square goodness-of-fit test was used to identify whether there was any significant association between the presence of nests and any particular habitat (Neu et al. Reference Neu, Byers and Peek1974). We did not perform a chi-square test with the individual counts, because the expected frequencies were not higher than 5, which is a prerequisite for performing this test (Cochran Reference Cochran1952). Since we did not count any individuals in the Smooth Cordgrass patches, we used a Student’s t-test to compare the means of individuals in harvested and non-harvested areas.
Vegetation sampling
In each sample area, we defined two 300 m parallel transects that were separated by a width of 50 m. Then, 50 cm × 50 cm quadrats were installed at 50 m intervals along each transect (14 quadrats in each sample area). Between 23 May and 15 June, we measured the number, height, and diameter of both dry and green reed and cordgrass stems.
Since reed growth occurring between 23 May and 15 June could have had an impact on the results, we estimated the bias due to reed growth and obtained two measurements of green reed stems in the harvested area quadrats (22–23 May and 13–15 June). We compared the mean heights in the two quadrat sets by using an independent-sample 2-tailed Student’s t-test. The total mean of the green stem heights on the harvested area was 67.1 ± 18.8 cm (n = 11) in May and 71.8 ± 20.1 cm (n = 11) in June. The height difference was not significant (t test; t 20 = –0.57; P = 0.57), suggesting that the differences between quadrats cannot be explained by reed growth during the study period. For comparison with other areas, we used data obtained in the month of June for the harvested area.
Between 10 May and 24 June, these measurements were also performed on quadrats installed around the 13 nests built during that period. We analyzed all the nest sites discovered on the harvested area during this period (n = 3) and the 10 nest sites found on the non-harvested area. This group of nests and the surrounding quadrats were defined as the NEST group (n = 13 in total). For each vegetation-structure parameter, we used 1-way analysis of variance (ANOVA) to test the significant differences among groups (harvested, nonharvested, and NEST). Normal distributions were examined by a Q–Q plot graphical method, which showed that all the data were normally distributed. When the results of Levene’s test showed homogenous variances, significant differences between groups were analyzed using a Tukey HSD posthoc test. In the cases of non-homogenous variances, a Games-Howell test was used. We used principal component analysis (PCA) to define the principal vegetation factors impacting Reed Parrotbill nest-site selection. In PCA, we used measurements of the previously described vegetation components on each of the 41 quadrats, namely, the harvested (n = 14), nonharvested (n = 14), and NEST (n = 13) quadrats. After projection of each quadrat on the chosen axis, we determined by eyes the number and characteristics of vegetation categories revealed with PCA analysis.
All statistical analyses and graphs were performed using SPSS 13.0 software. Maps were created using ArcGis 9.0 Service Pack 3. The significance level for all tests was maintained at P < 0.05. The mean values were presented along with the standard deviation (SD) values, unless otherwise stated.
Results
Habitat use and selection
Using the nest-count method, individuals were not uniformly distributed among the habitats and we estimated that 12 individuals had nested in the harvested area and 52 individuals had nested in non-harvested area (Table 2 and Figure 2, χ23 = 44.5; P < 0.001). Fewer birds then expected were observed in the harvested area (both habitats) and in the monospecific stands of Smooth Cordgrass in the non-harvested area.
Table 2. Number of Reed Parrotbill nests observed and predicted to be present in the available habitats according a uniform nest distribution.

The mean of the individual counts in areas with the presence of the Phragmites in the harvested area (4.0 ± 1.6; n = 5) was significantly lower than that in non-harvested (11.8 ± 3.5; n = 5) (t test; t 8 = 4.55; P = 0.002). No individual was observed perching in monospecific patches of Smooth Cordgrass in either area, and no nests were found within these patches.
Vegetation structure
There were no significant inter-quadrat differences in green reed density (ANOVA; F 2,37 = 2.59; P = 0.09), green reed diameter (ANOVA; F 2,33 = 1.28; P = 0.29), and green Smooth Cordgrass density (ANOVA; F 2,37 = 2.72; P = 0.08). However, the non-harvested quadrats had the lowest green reed densities, and the nest quadrats had the lowest green Smooth Cordgrass densities. The high standard deviations reflect high heterogeneities for both green reed and green cordgrass densities, probably explaining the absence of many significant differences. The green reed heights, dry reed heights, densities, and diameters varied between the quadrat types (ANOVA, respectively F 2,33 = 10.89, P < 0.001; F 2,24 = 9.14, P = 0.001; F 2,37 = 13.43, P < 0.001; F 2,24 = 4.09, P < 0.03). The green reed heights, dry reed heights, densities and diameters for the nest and non-harvested area quadrats were found to be statistically similar. However, the green reed heights, dry reed heights, and densities in the harvested area quadrats were significantly lower than the corresponding values in the nest quadrats (Figure 3). The dry Smooth Cordgrass densities varied between areas (ANOVA; F 2,37 = 7.16; P = 0.002), and were significantly higher on the non-harvested quadrats than on the nest harvested quadrats. We did not detect any significant differences between the dry smooth cordgrass densities in the nest and harvested groups (Figure 3).

Figure 3. Vegetation structure surrounding nests (NEST), nonharvesting (NHA) and harvesting (HA) areas. The letters a and b denote significant differences between areas (Tukey HSD or Games-Howell test, significance level: P < 0.05). Error bars represent the standard deviations.
The Common Reed densities for both green and dry stems on all nest and non-nesting non-harvested area quadrats were similar, and these reed densities differed from the vegetation components in non-nesting harvested areas which had high green stem and low dry stem densities. Reed heights of both green and dry stems were significantly related to nest presence. These nests tended to be located in the areas containing the highest stems.
PCA on the vegetation requirements for nesting
The PCA analysis revealed two principal components with eigenvalues greater than 1. The analysis explained 76% of the variation in the vegetation data, i.e., 51% and 25% on axes 1 and 2, respectively (Table 3). The height and diameter of both green and dry reed stems were strongly positively correlated with the first component; in contrast, dry reed stem density also lesser positive influence and green reed stem density was not significantly associated with the first component. The density of green cordgrass density was negatively associated with the first component. The first axis was therefore defined by both the vegetation type and the height and diameter of the reeds. The dry stem densities of both Common Reed and Smooth Cordgrass were strongly positively correlated with the second component. In contrast, green reed stem density was negatively correlated with this component. Therefore, this axis represented the maturity level of the reedbed.
Table 3. Results of Principal Components Analysis showing the first 3 axes of vegetation structure.
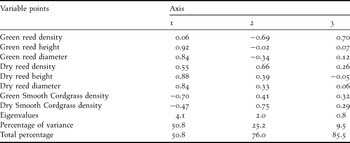
Quadrats situated low on the first axis tended to have a vegetation structure with high Smooth Cordgrass density and low reed height and diameter. Quadrats situated high on the first axis tended to have low Smooth Cordgrass density and high reed height and diameter. For the second axis, a high score reflected an aged vegetation structure with high densities of dry stems and low densities of green stems, while low scores reflected a young vegetation structure with high densities of green reed stems and low densities of dry stems (Figure 4).

Figure 4. Projection of reedbed-vegetation quadrat data (NEST, quadrats surrounding nests; HA, quadrats on harvested area; NHA, quadrats on non-harvested area) on the 2 first axes of PCA.
We observed four categories in the projection of reedbed vegetation quadrat (Figure 4). The first category contained quadrats with high Smooth Cordgrass densities with practically no Common Reed. Both harvested and non-harvested areas contained areas with this type of vegetation cover, but no nesting quadrats were identified within this vegetation category. Thus, although areas with high Smooth Cordgrass densities were observed, no nests were found to be built in these areas.
The second category contained non-harvested areas quadrats with the oldest vegetation structures. These quadrats had the highest densities of dry stems and the lowest densities of green stems. There were no nesting quadrats in this vegetation category, indicating that the nests were not present in the oldest reed growth areas.
The third category contained quadrats where the vegetation was youngest with highest densities of green reed stems and lowest densities of dry stems. This category included quadrats from the harvested area, none from the non-harvested area and three of the 13 identified nesting quadrats.
The fourth category contained quadrats where reed stems were tallest and the Smooth Cordgrass densities were lowest, in comparison with the corresponding values for the other categories. These quadrats also had relatively equal densities of dry and green stems. This last category, which was present only on the non-harvested area samples, contained 10 of the 13 nests found in the study.
Annual impact of reed harvesting
Counts of both nests and individual birds showed that adult densities in annually harvested areas were lower than those in non-harvested areas. The most frequently used nesting areas were those with the tallest reed stems and where the densities of both dry and green reed stems were similar. The results of the study suggest that the vegetation height in harvested areas was too low to provide suitable habitat for bird nesting. Moreover, the lower densities of individuals in the harvested reedbed can be explained by the fact that the quantities of arthropods that constitute potential food sources for Reed Parrotbills seem to be lower in harvested areas (Xiong et al. Reference Xiong, Wu, Gao, Zhou and Liu2007). It can be suggested that a no-harvesting approach, which will create older areas with high dry-stem densities, may be suitable for bird nesting. However, our study did not support such a hypothesis. We found that the Reed Parrotbills avoided the oldest stem regions while nesting and this behaviour can be attributed to the fact that the stems of both old and young reeds at these sites were too low for nesting. If unharvested, reed stems remain intact for 2–3 years and gradually break up (Haslam Reference Haslam1972, Hawke and Jose 1996). Consequently, in areas with a high density of dry stems, the reed stems have a low mean height. Since the green stem densities on unharvested areas are lower than those in harvested areas, the low mean height of reed stems cannot be compensated for by green stem heights.
It appears that reed height strongly influences the nesting of the Reed Parrotbill, as has been observed for Acrocephalus warblers by Leisler et al. (Reference Leisler, Ley and Winkler1989). Areas harvested annually do not provide suitable nesting habitats due to the low reed height and probably the almost complete absence of dry stems. However, in our opinion, the study indicates that some reed harvesting is necessary to provide young reedbeds and maintain an appropriate balance between densities of green and dry stems and a suitable vegetation height, a combination that is strongly preferred by the species for nesting.
Impact of Smooth Cordgrass invasion
Several studies have shown that Smooth Cordgrass invasion altered the structure of the trophic functional groups of benthic nematode communities (Chen et al. Reference Chen, Li, Hu, Chen and Wu2007) and macroinvertebrates (Chen et al. Reference Chen, Fu, Wang, Li, Wu and Chen2005), reduced the species richness and density of shorebirds by reducing habitat quality (Chen et al. Reference Chen, Li, Hu, Chen and Wu2007) and reduced the quality of breeding areas of Saunder’s Gull (Jiang et al. Reference Jiang, Hou, Chu, Qian, Wang, Zhang and Zheng2010). Our results complement these studies by showing that Smooth Cordgrass invasion in reedbeds decreases the habitat quality for Reed Parrotbills. The absence of both observed individuals and nests in monospecific Smooth Cordgrass areas indicates that the Reed Parrotbill is strongly dependent on Common Reed. Our results are in accordance with those provided by Gan et al. (Reference Gan, Zhang, Ma, Chen and Li2006), who showed the same avoidance of Smooth Cordgrass patches during winter. According to these authors, higher stem densities and biomass values in monospecific patches of Smooth Cordgrass appear to hamper bird movement through stems. Our results also showed that a majority of the nests were built in taller vegetation patches. In mixed areas, the tallest reed stems were localized in areas with the lowest Smooth Cordgrass densities, probably because in mixed areas Smooth Cordgrass alters the growth of the Common Reed. Consequently, the vegetation cover for nesting is extremely low. Thus, it is likely that the rapid expansion of Smooth Cordgrass in reedbeds in Chongming Dongtan Nature Reserve and generally in the Shanghai municipality appears to be a serious threat for Reed Parrotbill habitats and for the survival of the species.
Implications for management
The Environmental Protection Bureau of China has already listed Smooth Cordgrass as a harmful exotic species. Measures to control this species have drawn significant attention from biologists and ecologists from China and abroad, who have experimented with physical, chemical, biological, and ecological methods (Farber et al. Reference Farber, Costanza and Wilson2002, Fisher et al. Reference Fisher, Di Tomaso and Gordon2005, Wang et al. Reference Wang, Qin, Wan, Zhou, Zai and Yan2008). However, Huang et al. (Reference Huang, Zhang and Yuan2007) showed that the expansion rate of Smooth Cordgrass is 3–5 times higher than that of Common Reed and predicted that range expansion of Smooth Cordgrass will continue. The conservation of biodiversity should entail maintenance of genetic diversity (De Salle and Amato Reference De Salle and Amato2004), which is presumably correlated with adaptability (Frankham Reference Frankham1995) and survival (Vrijenhoek Reference Vrijenhoek, Loeschcke, Tomiuk and Jain1994). In Dongtan, since genetic exchanges with other Reed Parrotbill populations are unknown and probably very low, maintaining a high population size is crucial for avoiding a decrease in genetic diversity. However, in Chongming Dongtan Nature Reserve, the area of Common Reed has fallen by 55% in the five years since Smooth Cordgrass was introduced (Huang et al. Reference Huang, Zhang and Yuan2007). Thus, Smooth Cordgrass expansion constitutes a real problem for Reed Parrotbill conservation. Outside the nature reserve, reedbeds without Smooth Cordgrass have been identified in fishponds which are being considered for development as a green park. In order to counter habitat disappearance and maintain a viable population size, we recommend that it is necessary to maintain and manage the reedbed habitats in these fishponds until expansion of Smooth Cordgrass in the nature reserve has been stopped.
In order to maintain a balance between maturation and rejuvenation of reedbeds and ensure suitable nesting habitats for the Reed Parrotbill, we also recommend rotational harvesting in the regions inside and outside the nature reserve. This management system can provide heterogeneous habitats that will satisfy the needs of various species and increase the conservation value of reedbed habitats (Poulin and Lefebvre Reference Poulin and Lefebvre2002). Furthermore, on unharvested areas, dry stems crack and break, fall to the ground, and constitute a litter layer that needs several years to decompose (Haslam Reference Haslam1972). Litter accumulation leads to reedbed drying, which permits other species to colonize (Haslam Reference Haslam1972) and, over time, litter accumulation results in the loss of suitable habitat for the Reed Parrotbill. Harvesting prevents this natural process by removing litter. Güsewell et al. (Reference Güsewell, Le Nédic and Buttler2000) indicate that a harvested reedbed reaches a height similar to that of a non-harvested reedbed after the third year. Since dry stems remain 2–3 years after growth, a harvesting-cycle rotation with a duration of four years or less can be considered as best practice.
Acknowledgments
This study was supported in part by grants from the Technology and Science Ministry Key Project of China during the Eleventh Five-Year Period (No. 2006BAC01A14), the Shanghai Municipal Science and Technology Committee of China (No. 08DZ1203202 and No. 08231200701) and the Project Arcus 2006 Languedoc-Roussillon/China Arcus Chongming Project. Many thanks to members of the Shanghai Key Laboratory of Urbanization and Ecological Restoration of School of Life Sciences, East China Normal University, and particularly Zheng-huan Wang for helping field investigation planning, Xiao-jun Xu and Bin Dong for their assistance with the collection of the field data. We appreciate the improvements in English usage made by Phil Whitford through the Association of Field Ornithologists’ programme of editorial assistance.









