INTRODUCTION
In Denmark and Australia, as in other developed countries, shigellosis is fairly rare [1] (www.germ.dk); infections are usually associated with travel overseas, or men who have sex with men. Foodborne outbreaks of Shigella spp. are uncommon and mostly caused by foodstuffs imported from endemic countries [Reference Kapperud2–Reference Frost6]. Shigella sonnei is the predominant species isolated in most developed countries and in rapidly industrialized countries such as Thailand where, with an incidence of 60/100 000 population [Reference Chompook7], shigellosis remains a considerable burden.
In August 2007, concurrent outbreaks of S. sonnei infection were reported in Denmark and Australia. In Denmark, on 16 August, the Regional Food Control Authority East (Fødevareregion Øst) and the Statens Serum Institut (SSI) became aware of a cluster of S. sonnei infections in employees of two companies through the Danish clinical notification system. The patients had eaten in their workplace canteens, which were served by the same catering company. From preliminary trawling interviews with symptomatic employees, imported baby corn or sugar snaps were hypothesized as being possible vehicles. In Australia, on 14 August, the Queensland Health Department was notified about a large outbreak of multi-resistant shigellosis involving more than 40 symptomatic individuals in a film production crew. The Australian public health authorities made a connection with the Danish outbreak after noting identical antibiotic resistance patterns in the Australian outbreak strain to the one Denmark had published as part of a rapid communication at the end of August [Reference Lewis8]. Multi-disciplinary outbreak teams were formed in Denmark and Australia to identify the cause of the outbreaks and to establish whether they were linked by a common food supplier.
We report the results of investigations into these outbreaks of shigellosis in Denmark and Australia in August 2007 which were found to be associated with the consumption of raw baby corn imported from a common supply chain in Thailand. We also discuss the effectiveness of the prevention and control measures implemented.
METHODS
Epidemiological investigations
Case finding
Cases were ascertained through the national surveillance systems in Denmark and Australia. A confirmed case-patient was defined as any person with multi-drug resistant (resistant to tetracycline, ampicillin, sulphonamides, cephalothin, and streptomycin) S. sonnei infection acquired in Denmark or Australia in August 2007 excluding those who had travelled to an endemic area in the 3 days before onset of symptoms or those that could be explained by an alternative exposure. All S. sonnei case-patients in Australia, and a proportion of case-patients in Denmark, were interviewed by the respective health departments with a standardized questionnaire to identify possible risk factors for infection, clinical details and other related cases. We constructed a combined epidemic curve for Denmark and Australia using date of onset of illness. International awareness was raised to determine if other countries were experiencing similar outbreaks (Table 1).
Table 1. Time line of major actions and international communications
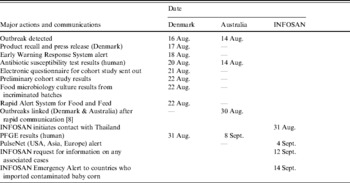
INFOSAN, International Food Safety Authorities Network.
Retrospective cohort study – Denmark, company A
In Denmark, a retrospective cohort study was undertaken in one of the larger workplaces (185 employees) affected by the outbreak. A link to an electronic self-administered questionnaire (defgo.net) was emailed to all employees on 19 August. The questionnaire enquired if the person had eaten in the workplace canteen on weekdays between 6 and 10 August and whether they had eaten any of a list of foods served in the canteen on 6 and 7 August, the two days when suspected baby corn and sugar snaps had been served. In this study the case definition was people who had eaten in the company canteen and who presented with diarrhoea, nausea or abdominal pain in August 2007. Respondents were excluded if they had not eaten in the canteen between 6 and 10 August or if they had travelled to an endemic area in the week before onset of illness.
We used Stata software, version 9 (Stata Corporation, College Station, TX, USA) to perform the analyses. Data were stratified by canteen day and food-specific attack rates and relative risks of illness associated with canteen days and consumption of different foods in the canteen, and 95% confidence intervals (CI) were calculated using Fisher's exact test. For each canteen day, significant variables (based on a P value of 0·05) were fitted into a model and we performed multivariable logistic regression using stepwise exclusion.
Laboratory methods
Faecal samples
Clinical laboratories in Denmark and Australia cultured faecal samples and S. sonnei isolates were tested for susceptibility to antibiotics by the agar tablet diffusion method performed in accordance with the manufacturer's instructions (Neo-sensitabs User's Guide, A/S Rosco Diagnostica, Taastrup, Denmark). Pulsed-field gel electrophoresis (PFGE) was undertaken on selected isolates in both Denmark and Australia using the same running conditions and two enzymes (XbaI and BlnI) according to the PulseNet protocol [Reference Ribot9].
Food samples
In Denmark, samples of baby corn and sugar snaps from the batches implicated in the outbreak were collected from importers, retail shops, canteens and two individual consumers positive for S. sonnei infection and were sent to the Danish Veterinary and Food Administration regional laboratories for examination.
The samples were investigated for Shigella spp. by enrichment in Gram-negative (GN) broth and GN broth supplemented with ampicillin, streptomycin and tetracycline. The enriched GN broths were seeded on xylose lysine deoxycholate agar and SSI blood agar plate [Art. no. 22880 (14 cm)] and investigated for Shigella. The GN broths were additionally investigated for Shigella by polymerase chain reaction (PCR). DNA was purified from 1 ml of GN broth using the MagneSil KF Genomic System kit (Promega, USA). The PCR analysis was performed as previously described [Reference Sethabutr10]. The samples were further examined for the presence of coliform bacteria [11] and Salmonella [12] by the methods described by the Nordic Committee on Food Analysis. The Salmonella NMKL method was supplemented with a secondary selective enrichment media, modified semi-solid Rappaport–Vassiliadis Medium [Reference De Smedt13].
In Australia, there was no leftover baby corn from the original consignment or brand available. However, other Thai brands of baby corn on sale were examined for E. coli using Petrifilm (AOAC Official method 991.4), for Salmonella spp. (AS5013.10/ISO 6579:1:2004) [14] and for Shigella spp. (ISO/DIS 21567) [15]. DNA-based testing for Shigella spp. was also performed [Reference Sethabutr10, Reference Houng, Sethabutr and Echeverria16] on the Shigella enrichment broths.
Environmental investigations
We traced back the supply chain of baby corn and sugar snaps in Denmark through the wholesaler who supplied the catering firm. Once epidemiological evidence pinpointed baby corn as the source of the outbreaks and the link to the Australian outbreak had been made, investigators conducted trace-back on sources of baby corn.
The Thai Ministry of Agriculture along with the Ministry of Public Health investigated hygiene standards in packing sheds, collection houses and farms in the supply chain. Environmental samples were taken from the floor, food-contact surfaces, packing processors and water sources in two suspected packing sheds. Staff were interviewed about hygiene and tested for Shigella spp. The International Food Safety Authorities Network (INFOSAN) (see http://www.who.int/foodsafety/fs_management/infosan/en/index.html) liaised with the Thai authorities to identify where the supply chain was common to both countries.
RESULTS
Epidemiological investigation
Case finding
In Denmark, between 1 August and 30 September 2007, the SSI received notification of 241 S. sonnei isolates. Twenty-six case-patients were excluded because of travel [Reference Davis17], alternative exposures [Reference Molbak and Neimann4] or being secondary cases [Reference Naimi5], leaving 215 primary domestic case-patients. Case-patients were reported from the whole of Denmark, but most (181/215, 84%) were reported from Zealand. The median age was 37 years (range 0–92 years) and 74% (160/215) were female. For cases for which the date of symptom onset was available (153), the outbreak began on 5 August and continued until 20 August 2007 (Fig. 1). In-depth interviews were undertaken of 56 case-patients, all of whom reported diarrhoea, with 48% (27/56) experiencing bloody diarrhoea. Sixteen percent (9/56) of cases were known to have been admitted to hospital and 88% (49/56) recalled eating baby corn.

Fig. 1. Epidemic curve of confirmed Shigella sonnei infection in Denmark (![]() ) and Australia (□), August 2007 (n=163) by date of onset.
) and Australia (□), August 2007 (n=163) by date of onset.
In Australia, Queensland Health identified a total of 12 cases of S. sonnei infection with the same multi-resistant antibiotic profile as the Danish isolates. The median age was 33 years (range 18–76 years) and 58% (7/12) were female. All 12 reported either consuming baby corn or eating at a venue where Thai baby corn was frequently served. The onset dates for 10 case-patients ranged from 9 to 27 August 2007 (Fig. 1). Four confirmed case-patients were part of a larger outbreak involving a film production crew where there were a further 43 epidemiologically linked patients with symptom onset between 9 and 14 August.
An EU Early Warning Response System report and the INFOSAN Emergency Alert (Table 1) did not identify further associated cases.
Retrospective cohort study – Denmark
About 69% (117/170) of employees at the workplace the week of the outbreak responded to the web-based cohort study in Denmark. Ninety-five questionnaires were returned from people who had eaten in the canteen 1–10 August and, of these, 27 persons met the case definition. There was a higher, although non-significant, relative risk (RR) of illness in people who had eaten in the canteen on 6 August (RR 1·3), 7 August (RR 5·1) or 8 August (RR 1·8) (Table 2). The attack rate for eating baby corn was 56% (15/27) on 6 August (RR 4·0, 95% CI 1·8–8·9) and on 7 August (RR 3·7, 95% CI 1·6–8·1) (Table 3). Baby corn was the only food item to be found significantly associated with illness on 7 August (P<0·001). Three food items were found to be significantly associated with illness on 6 August: baby corn (P<0·001), peas (P=0·002) and cauliflower (P=0·04). In a multivariable analysis baby corn remained as the only variable independently associated with illness (odds ratio 6·0, P=0·03).
Table 2. Risk of illness by exposure to canteen on 1–10 August 2007 in company A, Copenhagen, Denmark

AR, Attack rate; RR, risk ratio; CI, confidence interval.
Table 3. Risk of illness by exposure in canteen on 6 and 7 August 2007 in company A, Copenhagen, Denmark

AR, Attack rate, RR, risk ratio
(* P<0·05); CI, confidence interval.
Laboratory investigation
Characterization of human isolates
S. sonnei isolates from confirmed case-patients in Denmark and Australia were resistant to five antibiotics: tetracycline, ampicillin, sulphonamides, cephalothin, and streptomycin, but susceptible to nalidixic acid, ciprofloxacin, chloramphenicol, mecillinam, and gentamicin. Three isolates from Danish patients from the same time period were only resistant to tetracycline, sulphonamides, and streptomycin. Those isolates lacking resistance to ampicillin and cephalothin had a PFGE profile missing one band compared to the outbreak strain and therefore these patients were not considered part of the outbreak.
PFGE testing of the human isolates from the Danish outbreak showed that most had a profile that was indistinguishable from that of human isolates from the outbreak in Australia (Fig. 2). The PFGE profile was made available to PulseNet Europe, United States and Asia for use by other countries with cases of shigellosis that were potentially linked to this global outbreak.

Fig. 2. PFGE patterns of Shigella sonnei isolates digested with restriction enzyme XbaI. Lanes 2–4, 6–9, S. sonnei isolates from patients affected in the Danish outbreak; lanes 1, 5 and 10, Salmonella Braenderup strain used as molecular marker.
Food samples
Microbiological testing of seven different batches (121 samples) of implicated baby corn from different sources (importer, retail shops, canteen, patient's home, etc.) in Denmark detected high levels of E. coli [range 260–2700 colony-forming units (c.f.u.)/g]. Salmonella enterica subsp. enterica, serovars Hvittingfoss and Weltevreden were found in the two last batches to be imported into Denmark prior to a national recall, of which only a small proportion of the consignment entered the market. Shigella spp. were not detected in any batches. However, two batches imported at the beginning of the outbreak were unavailable for testing as they had already been consumed.
In Australia, although there was no leftover baby corn from the original consignment or brand for laboratory testing, microbiological sampling of other Thai brands of imported baby corn that were available at the time of the investigation showed several samples to be contaminated with high levels of E. coli (range 100–350 c.f.u./g). Shigella flexneri was detected by PCR and confirmed by sequencing in one of the baby corn samples that had high counts of E. coli (350 c.f.u./g).
Environmental investigations
The Danish Veterinary and Food Administration issued a recall of baby corn and sugar snaps on 17 August which led to the recovery of around 3·5 tonnes of baby corn. No further case-patients were reported within 3 days of this recall. A press release was issued advising the public to cook or blanch exotic vegetables before consumption. Once we identified baby corn as the food vehicle, an EU Rapid Alert for Feed and Food was issued on 21 August. The baby corn originated from an exporter in Thailand and trace-back uncovered a complicated supply chain (Fig. 3). During the time period covered by the recall (16 July–16 August 2007) about 16 tonnes of the incriminated baby corn was imported into Denmark, most of which was distributed to the final consumer. The implicated shipments were covered by a supplier declaration that the exporter adhered to all legislation relevant to food safety issues, although there was no analytical documentation available on the relevant individual shipments of baby corn. Two wholesalers distributed the baby corn throughout the country and some was sold on from Denmark to Sweden. It became apparent that the supply chain in Thailand had been extended in July 2007 to include a new packing shed (B) due to an increased demand for baby corn. Due to the timing of the outbreak which coincided with the introduction of corn from this source, investigations subsequently concentrated on packing shed B and the farms and collecting houses supplying it.

Fig. 3. Flowchart of supply chain of baby corn in Thailand, Denmark, Australia and other countries supplied.
In Australia, the implicated baby corn was part of a small consignment (~260 kg) imported in late July by a single wholesaler in Queensland from a different Thai exporter from the one that exported baby corn to Denmark. There was one common packing shed (B) that supplied baby corn to both wholesalers in Denmark and Australia. At the time the link to baby corn was established in Australia, no product recall was considered necessary as there was no evidence that the outbreak was ongoing, and no baby corn from the implicated batch was left in the marketplace.
Thai authorities reported that tap water supplied by the local authority was used to wash the baby corn in packing shed B for 2–3 min. Although chlorine was added, with concentrations up to 100 ppm, this is below recommended levels (200 ppm) for disinfection. Furthermore, this packing shed received baby corn from several collecting houses. A visit to one collecting house revealed that baby corn was placed directly on the ground and that the peeling and de-silking process was done by local adult villagers without strict hygienic processes. Environmental samples from surfaces and machines were negative for Shigella spp. in packing shed B. The distribution records from packing shed B were reviewed and indicated that incriminated batches of baby corn could have been distributed to three additional countries other than Denmark and Australia. The World Health Organization informed these three countries through an INFOSAN Emergency Alert and the International Health Regulations (IHR) Event Information Site (information available at http://www.who.int/csr/ihr/en/) so that appropriate action could be undertaken. Health authorities in the three countries did not identify any associated case-patients with the imported batches of corn.
DISCUSSION
We investigated a large outbreak of shigellosis in Denmark and Australia that was caused by baby corn imported from a common packing shed in Thailand. A high percentage of the case-patients in both Denmark and Australia recalled eating baby corn. In a cohort study conducted in Denmark, consumption of baby corn was the only exposure that remained significant in a multivariable model. Evidence of internationally distributed baby corn as the source was strengthened by the fact that the food recall was highly effective in terminating the outbreak. The Danish habit of eating whole or chopped raw baby corn in salads plus the larger consignment imported is thought to account for the larger outbreak in comparison to Australia; and for the absence of cases in other countries which imported the incriminated baby corn. Eating raw baby corn is not part of the culinary tradition in Asian countries and is also unusual in Europe.
Other large S. sonnei outbreaks have been linked to the consumption of raw vegetables including the consumption of lettuce in England [Reference Frost6] and from salad [Reference Martin3, Reference Davis17] shredded cabbage [Reference Satchell18] and parsley [Reference Naimi5] in America. Baby corn from Thailand also caused a S. sonnei outbreak in Denmark in 1998 [Reference Molbak and Neimann4]. The finding of high levels of E. coli in baby corn from Thailand during a survey of Danish foreign vegetables and berries conducted the following year resulted in the Danish Veterinary and Food Administration enforcing an order that shipments, which arrive chilled by air freight, should be accompanied by a certificate documenting the bacteriological status of the goods.
Many Shigella outbreaks are attributed to poor food-handler hygiene [Reference Davis17, Reference Dunn19, Reference Reller20] or are from produce contaminated at source [Reference Martin3, Reference Naimi5]. Due to the isolation of multiple agents and high concentrations of faecal indicator organisms, our outbreak was likely to have involved some form of sewage contamination. Contamination of the food source was most likely to have occurred at one of the packing or collecting houses which had sub-hygienic conditions. As the chlorine level in water used for washing baby corn in the implicated packing shed was not high enough, any contamination introduced earlier in the supply chain would not have been eliminated. Shigella has a very low infectious dose (10–500 cells [Reference Kothary and Babu21]) and raw vegetables may become heavily contaminated. It is questionable whether washing vegetables and fruit (e.g. cantaloupe) with an uneven surface with water alone is sufficient to reduce bacterial contamination to a safe level [22].
We were unable to recover S. sonnei from baby corn samples tested in Denmark and Australia. This was not too surprising as the isolation of Shigella spp. from food is generally considered to be difficult, particularly when the bacteria are present in low numbers [Reference Wachsmuth, Morris and Doyle23]. In any case, contamination levels may have been low or specific batches contaminated with Shigella spp. may not have been examined. In Denmark, although incriminated batches were tested, two large batches distributed early in the outbreak were not available for testing and it is possible that the contamination with S. sonnei was limited to these. In Australia, incriminated batches were not available for testing; however, S. flexneri DNA was detected in a batch of a different brand of imported baby corn from Thailand. Multiple other pathogens were isolated from incriminated batches which suggests gross levels of faecal contamination. Salmonella Hvittingfoss and Weltevreden, serotypes uncommon in Denmark, but prevalent in Thailand [Reference Bangtrakulnonth24], were isolated from batches of baby corn imported towards the end of the recall period. Only a small portion of these batches reached the consumer and this might explain why we did not see an increase in these Salmonella serotypes in the population. As a result of these findings, the Danish Food Control Authority will conduct a survey of the microbiological quality of imported baby corn during 2009 which may indicate that improved knowledge and tighter controls are required, for example, with regard to certificates of microbiological status accompanying individual baby corn shipments.
S. sonnei outbreak isolates in Denmark and Australia demonstrated the same uncommon antibiotic resistance pattern (ASTSuTmSp), providing further evidence of a common source. A high percentage of S. sonnei strains in Thailand exhibit multiple antibiotic resistance [Reference Chompook7]. PFGE, being a more discriminatory technique, reinforced the evidence for a common source when the Danish and Australian profiles were found to be indistinguishable. As so few strains (23/20 000 entries since 1999) in the PulseNet US database had previously demonstrated this PFGE pattern (P. Gerner-Smidt, personal communication) it might be said with a high degree of confidence that this pattern could be used to identify the outbreak strain. PulseNet international was an effective way of rapidly sharing the profile of this outbreak strain.
This outbreak highlights the importance of timely communication in helping to identify when contaminated food enters international trade (Table 1). The two outbreaks became linked after rapid publication of the Danish outbreak [Reference Lewis8] which emphasizes the importance of these informal public citings in addition to the formal European and worldwide communication channels (e.g. EWRS, RASFF, PulseNet international, INFOSAN and other WHO processes). In addition, foodborne outbreaks represent a complicated event whereby timely multi-lateral rather than bi-lateral communications are required between health, food, veterinary and environmental organizations in all countries involved as appropriate [Reference Kirk25–Reference Unicomb27]. During this outbreak, Thai authorities collaborated closely with the WHO through INFOSAN and the International Health Regulations, and the affected countries, from an early stage. In this new era of global outbreaks, we encourage communication between countries with an understanding that openness can lead to a timely response, improved public health and prevent future repetition of events [Reference Molbak and Neimann4].
This outbreak also highlighted the issue of the level of evidence required to initiate a food recall in the absence of microbiological confirmation of contaminated food. It is likely that we prevented additional cases of illness of Shigella spp. and outbreaks of Salmonella infections through the early product recall, particularly as baby corn has a long shelf life (3 weeks). In Denmark, it is the responsibility of the authorities to determine what level of evidence they find acceptable for a recall in each individual scenario. The decision in this case was based on convincing epidemiological evidence in spite of the fact that there was no microbiological evidence for the presence of Shigella or other pathogenic species in food available at this time. In many countries microbiological evidence is required by law before a recall can be issued, which often leads to a delay in terminating outbreaks [Reference Naimi5, Reference Webby28]. We believe international discussions and a consensus are required on this issue.
Although it is the responsibility of the supplier to ensure hygiene standards are maintained throughout the production chain, it is important for catering companies and consumers to be aware that raw vegetables are high-risk products for Shigella spp. It is recommended that exotic raw vegetables are blanched or cooked before consumption. With increased imports of exotic foods, open and rapid communications are required worldwide to improve standards of fresh produce and tackle outbreaks quickly.
ACKNOWLEDGEMENTS
The authors thank C Kjelsø, M Howitz, K Qureshi and L. Vestergaard for assisting with the outbreak investigation at the Statens Serum Institut. Thanks are also due to the Queensland Outbreak Investigation team, OzFoodNet epidemiologists and staff at ESR New Zealand. The cohort study in Denmark was funded by the Statens Serum Institut.
DECLARATION OF INTEREST
None.










