INTRODUCTION
Salmonella enterica subspecies enterica serovar Infantis (S. Infantis) is one of the five most common Salmonella serotypes worldwide [Reference Galanis1]. S. Infantis outbreaks were related to exposure to various food items including scrambled eggs, ham and dressed broiler-fryer chickens [Reference Kohler2–Reference Woodburn4] as well as exposure to pet treats and cattle feedlot runoff [Reference Clark5, Reference Miner, Fina and Piatt6].
The incidence rates of salmonellosis and S. Infantis in Israel were 33·5 and 11·8/100 000 in 2009, respectively [7]. The proportion of S. Infantis of all Salmonella isolates rose from 3·8% in 2004 to 44·3% in July 2009, becoming the most prevalent serotype in the country [Reference Bassal8]. The rising number of S. Infantis infections was not associated with reported outbreaks but rather with an increase in sporadic cases. The aim of the present study was to identify risk factors for sporadic S. Infantis infections in Israel.
MATERIALS AND METHODS
Salmonella isolates from all bacteriological laboratories in Israel are forwarded by law to the National Reference Laboratory of the Ministry of Health for further characterization. A matched case-control study was conducted by the Israel Center for Disease Control (ICDC) from February to July 2009. All culture-confirmed symptomatic patients diagnosed as having S. Infantis infection during that study period were included in the current study. Each study patient (case) was matched by gender, age and neighborhood with a healthy control through the Israeli Population Register. After obtaining oral informed consent, a comprehensive structured questionnaire was administered by telephone to all the cases and controls. For cases and controls aged <18 years, parents were interviewed. The collected data included information on symptoms of illness, medications, comorbidities, demographic characteristics, breastfeeding and formula use (for infants), contact with animals, and exposure to various food items (including eggs, poultry and meat, dairy products, fruits and vegetables), food handling and water consumption.
Data analysis
Differences in proportions between cases and controls were assessed using the McNemar test. Differences in numeric variables were assessed using the paired t test. Conditional logistic regression models were used for the univariate and multivariate analyses. Matched odds ratios (mORs) and 95% confidence intervals (CIs) were calculated for all variables. Multivariate analysis included variables which were significantly associated with illness in the univariate analysis. Analyses were conducted separately for patients aged ⩽1 year and >1 year. Data analysis was performed with SAS software release 9·1·3 (SAS Institute Inc., USA). For all statistical tests, a two-sided P value of <0·05 was considered statistically significant.
RESULTS
Between 1 February 2009 and 26 July 2009, 303 culture-proven cases of S. Infantis were reported from the Salmonella Reference Laboratory to the ICDC. Of those, 274 cases were interviewed for an overall response rate of 91·6%. The reasons for not interviewing 29 cases were as follows: two patients died, two were not Israeli citizens, six were unable to cooperate because of advanced age or health status, seven were unreachable and 12 refused to participate. The cases were interviewed within 34·6 days after obtaining the stool culture, and the controls were interviewed within 5·6 days after the matched case was interviewed. Eleven cases were asymptomatic and therefore not included in the analysis, leaving a total of 263 cases and 263 controls in the final analysis.
Infants aged ⩽1 year
There were 77 cases and 77 controls in this part of the study. The mean age was 0·56 years for the cases and 0·54 years for the controls [standard error (s.e.) 0·03 for both groups] and 45 (58·4%) were males. There were no significant differences in the distribution of country of birth, religion, mother's education and monthly income between the two groups (Table 1). The main symptoms of S. Infantis infection were diarrhoea and fever (Table 2). In the univariate analysis, consumption of milk formula was a risk factor for S. Infantis infection while breastfeeding and a larger family size were significant protective factors (Table 3 a). In the multivariate analysis, breastfeeding (mOR 0·24, 95% CI 0·10–0·59, P < 0·01) and more children in the family (mOR 0·75, 95% CI 0·59–0·95, P = 0·02) were significant protective factors against S. Infantis infection (Table 4 a).
Table 1. Demographic characteristics of cases and controls according to age group
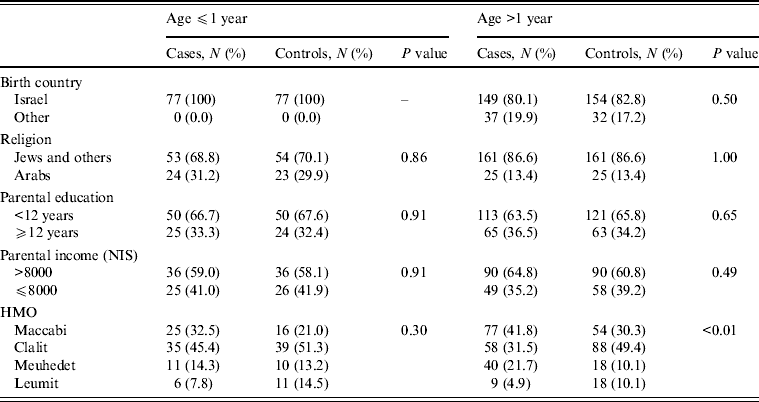
NIS, New Israeli Shekel; HMO, health maintenance organization.
Table 2. Characteristics of cases infected with Salmonella Infantis according to age group

ED, Emergency department.
Table 3. Risk factors for Salmonella Infantis infection

mOR, Matched odds ratio; CI, confidence interval; s.e., standard error.
Table 4. Multivariate analysis of risk factors for Salmonella Infantis infection

mOR, Matched odds ratio; CI, confidence interval.
Patients aged >1 year
The older group consisted of 186 cases and 186 controls with a mean age of 22·8 years (s.e. = 1·9 for both groups) and 100 (53·8%) were males. There were no significant differences in the demographic characteristics between cases and controls (Table 1). Symptoms of S. Infantis infection were mainly diarrhoea, stomach ache and fever (Table 2).
Table 3 b summarizes the risk and protective factors associated with S. Infantis infection in the univariate analysis. Consumption of eggs and thawing chicken in water were risk factors, while consumption of ice cream, carrots, green leafy foods, drinking only tap water (and not mineral water), observing a religious lifestyle and the presence of a high number of children in the household were protective factors. In the multivariate analysis, the consumption of eggs (mOR 1·87, 95% CI 1·00–3·49, P = 0·05) was a significant risk factor and thawing chicken in water (mOR 2·55, 95% CI 0·94–6·91, P = 0·07) was a borderline risk factor, while the consumption of carrots (mOR 0·46, 95% CI 0·26–0·83, P < 0·01), drinking only tap water (mOR 0·44, 95% CI 0·22–0·85, P = 0·02), observing a religious lifestyle (mOR 0·40, 95% CI 0·21–0·74, P < 0·01) and large family size (mOR 0·72, 95% CI 0·58–0·88, P < 0·01) were significantly protective (Table 4 b).
DISCUSSION
In the present study we examined risk factors for Salmonella Infantis infection. Our analysis yielded no definitive risk factor that could explain the increase in S. Infantis cases occurring in Israel since 2008. S. Infantis is currently the most prevalent serotype nationwide, and its replacement of S. Enteritidis and S. Typhimurium as the leading serotype in Israel may be explained by the actions taken to reduce the prevalence of the latter in poultry [Reference Bassal8]. Those actions included tight surveillance of all breeding flocks and hatcheries, culling or treatment of all flocks infected with S. Enteritidis or S. Typhimurium, improvement of biosecurity and infrastructure, and systematic immunization against S. Typhimurium and S. Enteritidis initiated in 1994 and 1996, respectively [Reference Bassal8].
Our present study revealed significant protective factors for S. Infantis infection. Breastfeeding in infants aged ⩽1 year decreased the estimated risk for acquiring infection by fourfold. Breastfeeding had been found to be protective against Salmonella infections in several earlier studies [Reference Rowe9, Reference Jones10]. In one case-control study conducted by FoodNet sites for children aged <12 months, consumption of breast milk was found to be protective against Salmonella compared to the consumption of any other fluid (mOR 0·05, 95% CI 0·00–0·30). That study reported that breastfeeding could prevent as many as 74–100% of all Salmonella cases [Reference Rowe9]. Similar results were found in a case-control study that examined the association between sporadic Salmonella and different exposures, where the OR for breastfeeding was 0·5 (95% CI 0·3–0·6) [Reference Jones10]. The biological explanation for the beneficial effect of breastfeeding may be the presence of phagocytes as well as specific secretory IgA in the colostrum and breast milk [Reference Orlando11, Reference Johnson12]. Another explanation is that infants who are breastfed, are less likely to become exposed to contaminated food or bottled items.
Although larger families are usually of lower socioeconomic status, which is commonly considered a risk factor for enteric diseases [Reference Hasin13], we demonstrated that a large family size was protective in both age groups. A similar result was reported in a matched case-control study performed in children aged <5 years in Tanzania, where a large number of siblings was shown to be protective against diarrhoeal diseases [Reference Gascon14]. The researchers attributed the decrease in risk with increasing number of siblings to maternal experience affecting hygiene practices [Reference Gascon14]. A Danish study which examined the association between socio-demographic variables and enteric diseases due to Salmonella serotypes other than S. Typhimurium and S. Enteritidis in adults found that a large number of children in a household was protective against those diseases [Reference Simonsen, Frisch and Ethelberg15]. Those researchers proposed that when there are children at home, kitchen hygiene may become more important and the diet may contain fewer risky food items [Reference Simonsen, Frisch and Ethelberg15]. They also noted that the threshold for parents to seek medical care due to mild gastrointestinal symptoms may be higher with later-born than firstborn children [Reference Simonsen, Frisch and Ethelberg15]. If so, the lower risk in members of households with ⩾2 children may be, at least in part, the result of underreporting of disease in families with several children [Reference Simonsen, Frisch and Ethelberg15]. Our finding may also be explained in a number of ways: first, a large number of people in the household increases the chances of exposure to Salmonella and subsequent development of a high natural immunity against this bacterium as a result of a previous exposure. Second, there may be frequent exposures to low infective doses which do not cross the high threshold necessary to cause a disease but are sufficient to create natural immunity.
Consumption of eggs was a significant risk factor in our older age group. Previous studies demonstrated that consumption of improperly cooked eggs, such as scrambled and fried, were risk factors for infection with Salmonella serotypes [Reference Hedberg16, Reference Hennessy17]. Several reports on diarrhoeal outbreaks that were related to Salmonella serotypes were associated with the consumption of eggs [Reference Badrinath18, Reference Salmon19]. However, we could not identify an association with the method of preparation (scrambled, hardboiled, soft-boiled, omelette, fried), place of purchase (grocery, supermarket, private hutch, market) or amount of eggs that were consumed. Our finding was supported by data from the Central Salmonella Laboratory that reported in 2009 that 32·5% and 36·2% of human and animal Salmonella cases, respectively, were S. Infantis, and chickens were the main source of S. Infantis infection in animals (96·6%) [7].
Thawing chicken in water was a risk factor (with borderline significance) for S. Infantis infection in our cases. This way is quicker than thawing at room temperature and in the refrigerator [Reference Roberts20]. The outer layer of the chicken, especially that of a whole chicken, is defrosted more rapidly than the inner layer, and cooking a chicken which is not fully defrosted and at an insufficiently high temperature does not destroy Salmonella [Reference Roberts20]. It is also possible that using running water or still water in the thawing process may facilitate cross-contamination with Salmonella.
Our results revealed that eating carrots was protective against S. Infantis infection. The anti-bacterial activity of carrots is presumably due to apolar components. Free saturated fatty acid (dodecanoic acid) and methyl esters of saturated fatty acids (dodecanoic and pentadecanoic acids) were identified in purified active extracts of carrots and may be related to anti-bacterial activity [Reference Babic21]. Treatments with carrot extracts, especially the alcoholic extract, were reportedly efficient in inhibiting the growth of Pseudomonas aeruginosa [Reference Abdula Ali22]. However, the protective effect may be attributed to consumption of carrots and other vegetables which, as part of a healthy lifestyle, may prevent exposure to potential risk factors.
Observing a religious lifestyle was protective against S. Infantis infection. Religious Jews routinely separate dairy products and meat products, a practice that may prevent cross-contamination. However, another possibility for this finding is under-representation of observant individuals in the cases in our study because they are less likely to visit a physician. Another explanation, which was confirmed by our database, is the fact that religious families are larger than secular families [23].
Drinking tap water was found protective against S. Infantis compared to bottled water and filtered water. In Israel, 80% of tap water is supplied by one company [24]. Tap water is closely regulated for safety by the Ministry of Health from the production site to the customer's residence. Bottled water is regulated only at the manufacturer's plant. In addition, individuals who consume filtered water are recommended to replace the filters every 6 months (for a considerable amount of money), and there is no data as to how many comply with these recommendations. This may explain the advantage of drinking tap water.
Our study has a number of possible limitations. First, there may have been recall bias since cases were interviewed on an average of 34·6 days after obtaining the stool sample. Due to the long time interval elapsing, cases are less likely to remember their exposure. However, since the difference between cases and controls was only 5·6 days, we conclude that there is no reason to believe there was a differential misclassification between cases and controls. Matched ORs may thus be an underestimate of the true association. Second, controls were matched to cases by age, gender and neighbourhood. This may have led to overmatching, and important risk factors may have been obscured.
In conclusion, Salmonella infections may be prevented by good hygiene habits. Breastfeeding should be more vigorously encouraged by the Israeli public health authorities. The health authorities must raise the level of public awareness regarding the importance of hygienic care in the kitchen and of the importance of properly cooking eggs as well as of thawing chicken before preparation and cooking it properly.
ACKNOWLEDGEMENTS
We thank Nadia Pekurovski for her assistance in the data collection of the case-control study.
DECLARATION OF INTEREST
None.






