Introduction
Mobulid rays were largely under-studied during the 20th century, and despite progress in the last decade, large knowledge gaps in their life history and ecology still exist (Stewart et al., Reference Stewart, Jaine, Armstrong, Armstrong, Bennett, Burgess, Couturier, Croll, Cronin, Deakos, Dudgeon, Fernando, Froman, Germanov, Hall, Hinojosa-Alvarez, Hosegood, Kashiwagi, Laglbauer and Stevens2018). The family Mobulidae previously consisted of 11 species in two genera, but taxonomic and genetic evidence now suggest the family be consolidated into a single genus of nine species (Notarbartolo di Sciara et al., Reference Notarbartolo di Sciara, Adnet, Bennett, Broadhurst, Fernando, Jabado, Laglbauer and Stevens2019; Hosegood et al., Reference Hosegood, Humble, Ogden, de Bruyn, Creer, Stevens, Abudaya, Bassos-Hull, Bonfil, Fernando, Foote, Hipperson, Jabado, Kaden, Moazzam, Peel, Pollett, Ponzo, Poortvliet and Carvalho2020). All species are planktivorous with some of the most conservative life history strategies and slowest population growth rates of all elasmobranchs (Dulvy et al., Reference Dulvy, Pardo, Simpfendorfer and Carlson2014; Rambahiniarison et al., Reference Rambahiniarison, Lamoste, Rohner, Murray, Snow, Labaja, Araujo and Ponzo2018). Mobulids are primarily threatened by interactions with fisheries, both as bycatch and direct capture (Croll et al., Reference Croll, Dewar, Dulvy, Fernando, Francis, Galván-Magaña, Hall, Heinrichs, Marshall, Mccauley, Newton, Notarbartolo-Di-Sciara, O'Malley, O'Sullivan, Poortvliet, Roman, Stevens, Tershy and White2016). Trade in and demand for mobulid gill plates in Asian markets has driven targeted fisheries around the world, especially in Africa and Asia (Ward-Paige et al., Reference Ward-Paige, Davis and Worm2013; Croll et al., Reference Croll, Dewar, Dulvy, Fernando, Francis, Galván-Magaña, Hall, Heinrichs, Marshall, Mccauley, Newton, Notarbartolo-Di-Sciara, O'Malley, O'Sullivan, Poortvliet, Roman, Stevens, Tershy and White2016). The conservation status of mobulid rays was recently assessed by the IUCN and all species were categorized as Endangered or Vulnerable to extinction (e.g. Marshall et al., Reference Marshall, Barreto, Bigman, Carlson, Fernando, Fordham, Francis, Herman, Jabado, Liu, Pardo, Rigby, Romanov and Walls2019).
Mobula tarapacana (Philippi, 1892) is one of the largest mobulid species, reaching a disc width of up to 370 cm (Marshall et al., Reference Marshall, Barreto, Bigman, Carlson, Fernando, Fordham, Francis, Herman, Jabado, Liu, Pardo, Rigby, Romanov and Walls2019). It is known to make regular deep dives of 800 m with maximum depths reaching nearly 2000 m (Thorrold et al., Reference Thorrold, Afonso, Fontes, Braun, Santos, Skomal and Berumen2014). Most data on M. tarapacana comes from research at oceanic island aggregation sites, specifically the Azores, St Helena and the Saint Peter and Saint Paul Archipelago (Sobral & Afonso, Reference Sobral and Afonso2014; Mendonça et al., Reference Mendonça, Macena, Afonso and Hazin2018; Beard et al., Reference Beard, Henry, Cherrett and Dovev2021), or from fishing markets in Asia (Lewis et al., Reference Lewis, Setiasih, Fahmi, Dharmadi, O'Malley, Campbell, Yusuf and Sianipar2015; Rambahiniarison et al., Reference Rambahiniarison, Lamoste, Rohner, Murray, Snow, Labaja, Araujo and Ponzo2018).
Mobula tarapacana is thought to have a circumglobal, yet patchy distribution in tropical, subtropical and temperate waters. Establishing clear geographic ranges is essential for accurate assessments and targeted management actions (Lawson et al., Reference Lawson, Fordham, O'Malley, Davidson, Walls, Heupel, Stevens, Fernando, Budziak, Simpfendorfer, Ender, Francis, Notarbartolo di Sciara and Dulvy2017). While M. tarapacana is reported extant in the Gulf of Mexico (Childs, Reference Childs1997; Jones et al., Reference Jones, Driggers, Hannan, Hoffmayer, Jones and Raredon2020) and the Atlantic coast of South America (Notarbartolo-di-Sciara & Hillyer, Reference Notarbartolo-di-Sciara and Hillyer1989), no extant population has been reported off the Atlantic coast of the USA. Recent efforts to evaluate the distribution of the closely related giant manta ray (Mobula birostris, Walbaum 1792) revealed numerous sightings of M. tarapacana by aerial surveys (Farmer et al., Reference Farmer, Garrison, Horn, Miller, Gowan, Kenney, Vukovich, Willmott, Pate, Webb, Mullican, Stewart, Bassos-Hull, Jones, Adams, Pelletier, Waldron and Kajiura2022). Here, we provide sightings data from multiple sources that confirm M. tarapacana within the western North Atlantic Ocean off the US coast.
Methods
We assembled confirmed M. tarapacana sightings from: (1) New York State Energy Research and Development Authority (NYSERDA) and Bureau of Ocean Energy Management (BOEM) aerial digital surveys conducted by Normandeau Associates/APEM; (2) National Oceanic and Atmospheric Administration (NOAA) aerial surveys conducted by Southeast Fisheries Science Center (SEFSC); (3) VACAPES NFC U.S. Navy Monitoring Program aerial surveys conducted by HDR Environmental, Operations and Construction, Inc. (HDR EOC); (4) NOAA Pelagic Longline (PLL) Observer programme data; and (5) incidental sightings by divers and opportunistic samplers. We define ‘sighting’ as the identification of one or more individuals of M. tarapacana observed together.
Normandeau associates/APEM NYSERDA and BOEM aerial digital surveys
NYSERDA and BOEM contracted with Normandeau Associates Inc. in collaboration with APEM Ltd to conduct aerial digital surveys to assess the abundance and spatial distribution of birds, marine mammals, sea turtles, cartilaginous fish, and other taxa in areas considered for offshore energy development (Halpin et al., Reference Halpin, Read, Fujioka, Best, Donnelly, Hazen, Kot, Urian, LaBrecque, Dimatteo, Cleary, Good, Crowder and Hyrenbach2009; APEM & Normandeau Associates, 2018, 2019a, 2019b, 2020, 2021; Jervis & Phillips, Reference Jervis and Phillips2021). NYSERDA conducted offshore aerial digital surveys sampling within the New York Offshore Planning Area, which spans >43,745 km2. NYSERDA surveys were conducted four times a year over three years, between August 2016 and May 2019. Eight BOEM aerial digital surveys sampled the offshore waters of North Carolina and South Carolina in 2018 and 2020.
Each resulting dataset consists of high-resolution (1.5 cm at the sea surface) aerial digital imagery. For both survey efforts, images were collected with downward-facing cameras from a flight altitude of 414.5 m. Animal targets were extracted from imagery in the NYSERDA and BOEM data using a combination of detection software and manual review. Two authors (CH, JP) re-evaluated taxonomic identifications of all large rays in Normandeau's ReMOTe dataportal to confirm M. tarapacana identification. Characteristics used to identify M. tarapacana from other ray species were presence of cephalic fins, relatively large size, uniform olive-green to golden-brown dorsal colouration and the sicklefin shape of trailing edge of pectoral fins (Stevens et al., Reference Stevens, Fernando and Notarbartolo di Sciara2018). Only clear photos where the ray was near the surface and diagnostic features clearly visible were included in the dataset.
To measure disc width (DW) and disc length (DL) of detected M. tarapacana, we used the Normandeau ReMOTe data portal's measuring tool. This measurement tool takes the mean known resolution at the sea surface of the specific camera by which the animal was detected and sums the number of pixels across the user-defined measurement. Measurements are unable to compensate for the unknown depth of the animal and the potential distortion in the image from water refraction, therefore an element of minor error is associated with all subsurface measurements. Measurements (DW and DL) were only taken on photos where the tips of both pectoral fins were clearly visible. However, DW is usually measured at full extension of pectoral fins and we were unable to account for any fin flexion in DW measurements. Any flexion of fins will cause DW to be underestimated, so these DWs can be considered a minimum estimate. DW is plotted against DL and fitted with a linear regression curve. A disc ratio was obtained by dividing DW by DL. All measurements are reported as mean with standard deviation.
NOAA aerial surveys
SEFSC conducts visual aerial surveys in the Atlantic and Gulf of Mexico to assess the spatial distribution and abundance of marine mammals and sea turtles (see Figure 2A, B in Farmer et al., Reference Farmer, Garrison, Horn, Miller, Gowan, Kenney, Vukovich, Willmott, Pate, Webb, Mullican, Stewart, Bassos-Hull, Jones, Adams, Pelletier, Waldron and Kajiura2022). These aerial survey programmes covered the continental shelf and the inner continental slope in western Atlantic Ocean waters off the US East Coast, including the Gulf of Mexico, from New Jersey (40°N 74°W) to southern Texas (24.3°N 94.8°W).
While rays were not the target taxa of these surveys, pilots on 1996–1998 Gulf of Mexico surveys noted locations of ‘golden mantas’. Only one photo was available for verification of golden mantas being M. tarapacana. Given that these rays were specifically designated as ‘golden’ (uniform brownish colour being an identifying characteristic of the species) and that the photo also confirmed species identification, we are confident that these records are M. tarapacana. Similarly, between 2019–2021, observations of M. tarapacana were recorded by trained observers with the SEFSC's Atlantic aerial survey programme (Palka et al., Reference Palka, Aichinger Dias, Broughton, Chavez-Rosales, Cholewiak, Davis, DeAngelis, Garrison, Haas, Hatch, Hyde, Jech, Josephson, Mueller-Brennan, Orphanides, Pegg, Sasso, Sigourney, Soldevilla and Walsh2021) following training and distribution of a large ray aerial survey guide in 2019 (NOAA Fisheries, 2019).
HDR Environmental, Operations and Construction, Inc.
HDR Environmental, Operations and Construction, Inc. (HDR EOC) led VACAPES NFC (Norfolk Canyon) visual aerial surveys as part of the US Navy Monitoring Program in 2018–2019. HDR EOC conducted marine species monitoring surveys beginning in April 2018 through August 2019 in the NFC study area off the coast of Virginia Beach, VA. Twenty-three days of aerial surveys were completed between 9 April 2018–12 August 2019. Survey results were submitted to the OBIS-SEAMAP database (Halpin et al., Reference Halpin, Read, Fujioka, Best, Donnelly, Hazen, Kot, Urian, LaBrecque, Dimatteo, Cleary, Good, Crowder and Hyrenbach2009; Cotter, Reference Cotter2019, Reference Cotter2020). Species identification was confirmed by trained observers (T. Pusser and M. Cotter, pers. comm. to N. Farmer, 5/2022).
PLL Observer data
PLL Observer data were collected by trained fisheries observers aboard pelagic longline vessels in the Atlantic and Gulf of Mexico. Prior to July 2019, fisheries observers did not classify rays according to species; therefore, all pre-2020 M. tarapacana observations were verified by associated videos and photos of capture. Beginning in 2020, fisheries observers began identifying and collecting bycatch data on large mobulid rays. Not all of these records included photos, but of the four that did, all were verified as M. tarapacana by an author (CJ). Due to issues with confidentiality, location is reported as within particular fishing zones as opposed to exact GPS coordinates (see map of longline fishing areas in Supplemental Figure).
Incidental observations
Incidental observations were sightings reports initially sent to NOAA or the Marine Megafauna Foundation (MMF) as manta rays (Mobula birostris), but later identified as M. tarapacana based on photographs. All photographs were confirmed as M. tarapacana by at least one author. One opportunistic sighting of M. tarapacana by a scuba diver was reported to MMF in 2018. A second sighting was initially reported as a ‘manta ray’ by the Gulf Coast Research Laboratory of the University of Southern Mississippi from a plankton survey in the Gulf of Mexico in 2019, but photos later confirmed the species as M. tarapacana. Additionally, in 2006 a commercial saturation diver's downline was entangled by an M. tarapacana off Louisiana in the northern Gulf of Mexico (https://www.longstreath.com/community/incidents/?page=3). A video of the encounter (https://www.youtube.com/watch?v=959CWu0w8dc) was used to confirm the species identification. Finally, a boater observed at least 8 M. tarapacana in the Florida Keys in May 2022.
Results
In total, 361 individual M. tarapacana were identified from 180 sightings in the five datasets in the waters off the US East Coast and Gulf of Mexico between 1996 and 2022 (Table 1, Figure 1). The majority (88%, N = 316) of individuals were seen in June through August. Most sightings (75%, N = 136) were of lone individuals, but group sizes ranged up to at least 24 individuals, with small groups of 2–4 individuals being the most common group size (70%, N = 31 out of 44 groups observed).
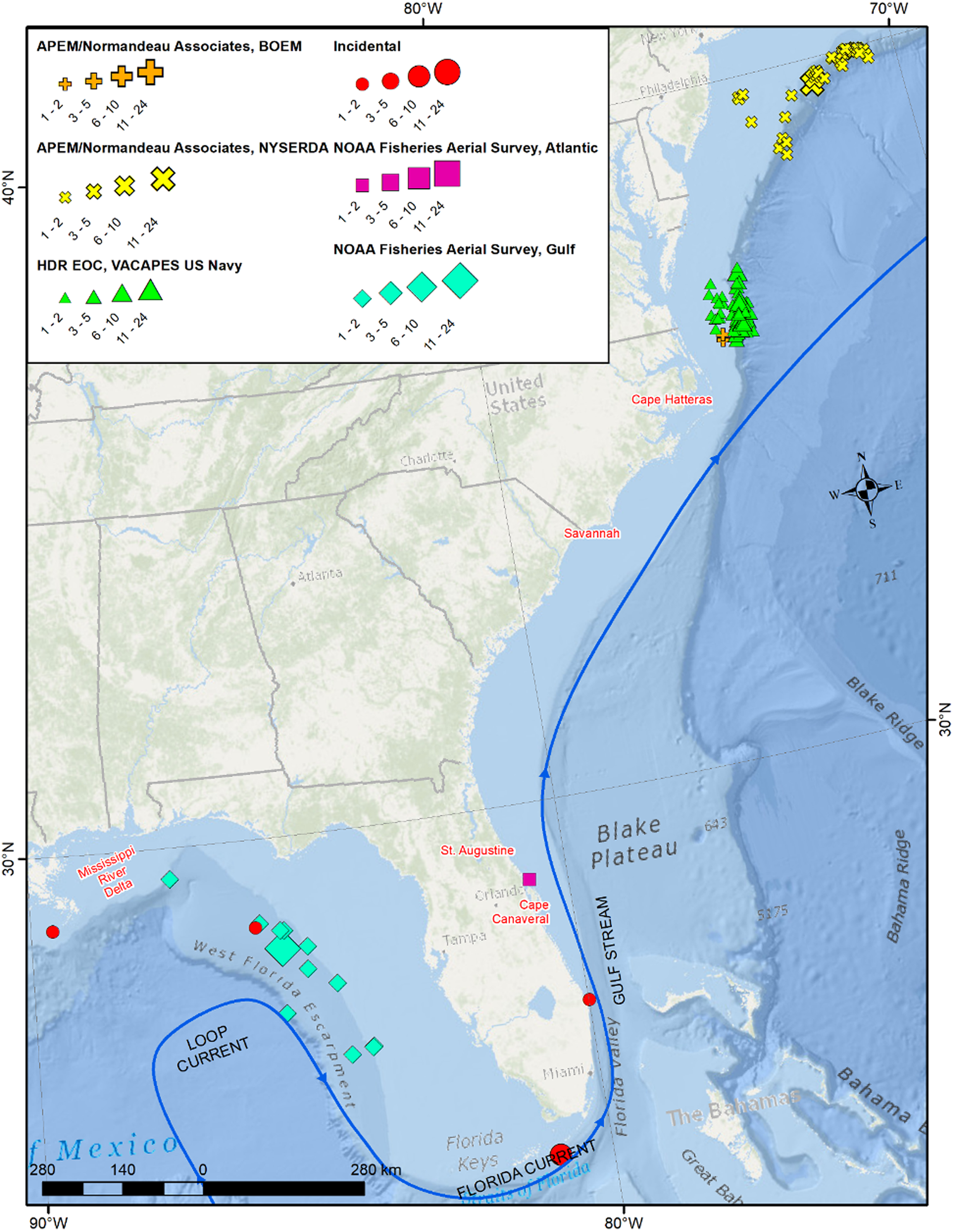
Figure 1. Mobula tarapacana locations from confirmed aerial survey and incidental sightings, scaled to the number of individuals sighted. Pelagic Longline Observer data excluded to protect confidentiality.
Table 1. Total Mobula tarapacana individuals from: (1) Bureau of Ocean Energy Management (BOEM) aerial digital surveys and New York State Energy Research and Development Authority (NYSERDA) and other aerial surveys conducted by Normandeau Associates/APEM, (2) VACAPES NFC U.S. Navy Monitoring Program aerial surveys conducted by HDR Environmental, Operations and Construction, Inc (HDR EOC), (3) Incidental sightings by divers and opportunistic samplers, (4) National Oceanic and Atmospheric Administration (NOAA) aerial surveys conducted by Southeast Fisheries Science Center (SEFSC), and (5) NOAA NEFS Pelagic Longline (PLL) Observer programme data [by fishing area: Mid-Atlantic Bight (MAB), South Atlantic Bight (SAB), Northeast Coastal (NEC), Florida East Coast (FEC) and Gulf of Mexico (GOM)]

Normandeau Associates/APEM definitively identified 101 M. tarapacana individuals in 61 sightings in their aerial digital surveys for NYSERDA and BOEM. In the NYSERDA data, 61 images contained a ray that was definitively identified as M. tarapacana (Figure 2A–C). Another 72 images were identified as potentially M. tarapacana, but submergence of ray or poor-quality photos prohibited accurate identification. In 10 of the definitively identified images, the ray selected by the software was nearby one or more ray(s). It was only possible to definitively identify 10% (5 of 50) nearby rays as M. tarapacana, but given that M. tarapacana is often seen in groups (Beard et al., Reference Beard, Henry, Cherrett and Dovev2021) it is probable that other rays, of similar size and colouration, were the same species. When including these nearby rays, the NYSERDA data identified a total of 101 M. tarapacana. The 10 observed groups consisted of 2–21 individuals, with an average of 5 per group (± 5.8 rays).
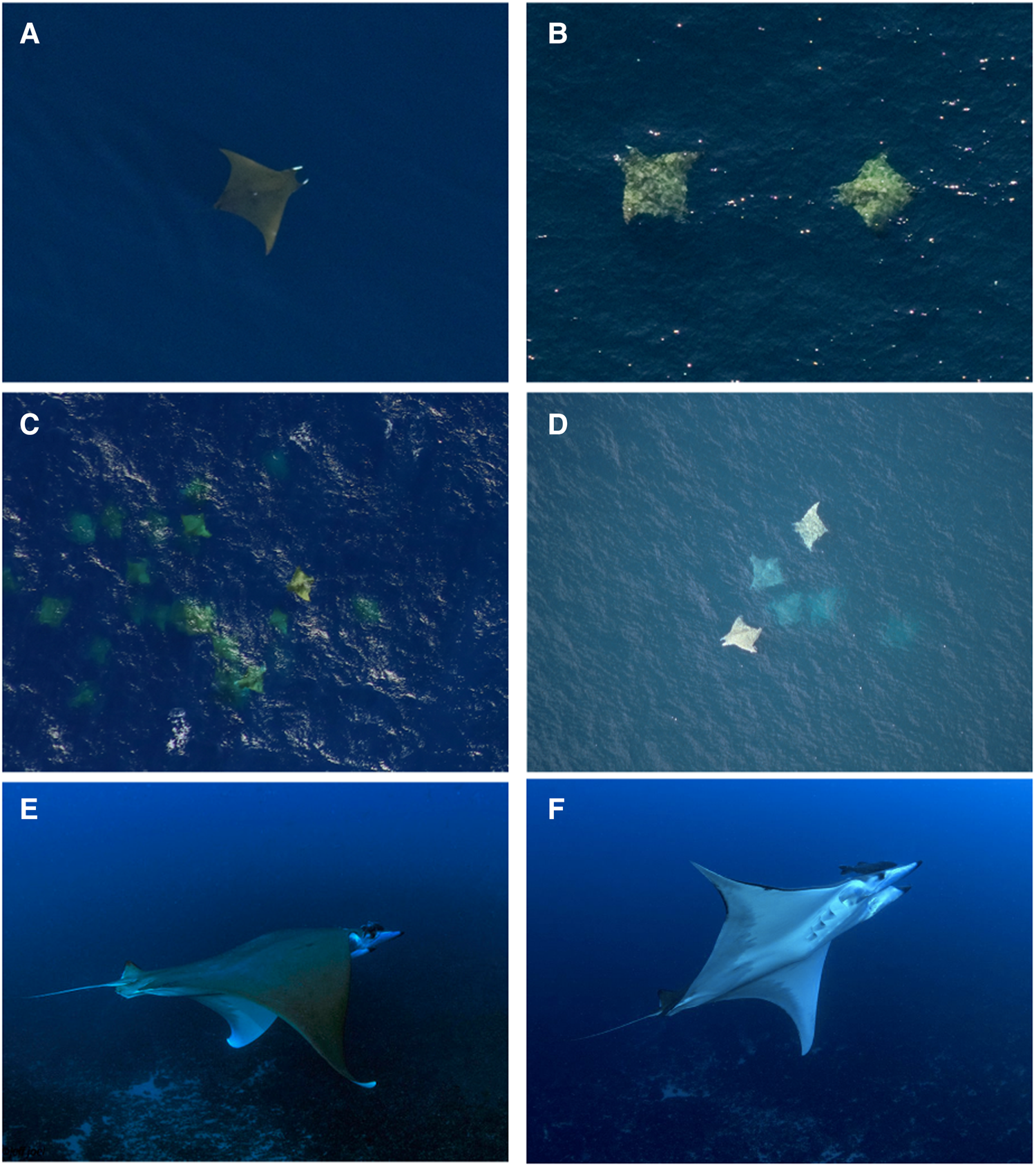
Figure 2. Photos of M. tarapacana from NYSERDA aerial surveys (A, B and C), SEFSC marine mammal aerial survey (D), and Florida scuba diver Jeff Joel (E and F).
Despite equivalent survey effort in the autumn and winter months (Figure 2F in Farmer et al., Reference Farmer, Garrison, Horn, Miller, Gowan, Kenney, Vukovich, Willmott, Pate, Webb, Mullican, Stewart, Bassos-Hull, Jones, Adams, Pelletier, Waldron and Kajiura2022), M. tarapacana were only observed during July and August in NYSERDA surveys. Twenty-nine rays were observed in August 2016, 24 in August 2017 and 8 in July and August of 2018. Twenty-two (36%) of photos were appropriate to take measurements of DW and DL. Mean DW was 268 cm (±25; range 232–316 cm), mean DL was 196 cm (±16, range 165–222 cm) and mean disc ratio was 1.4 (±0.1, range 1.2–1.6; Figure 3). The relationship between DW and DL was described by the following linear regression equation (Figure 3):
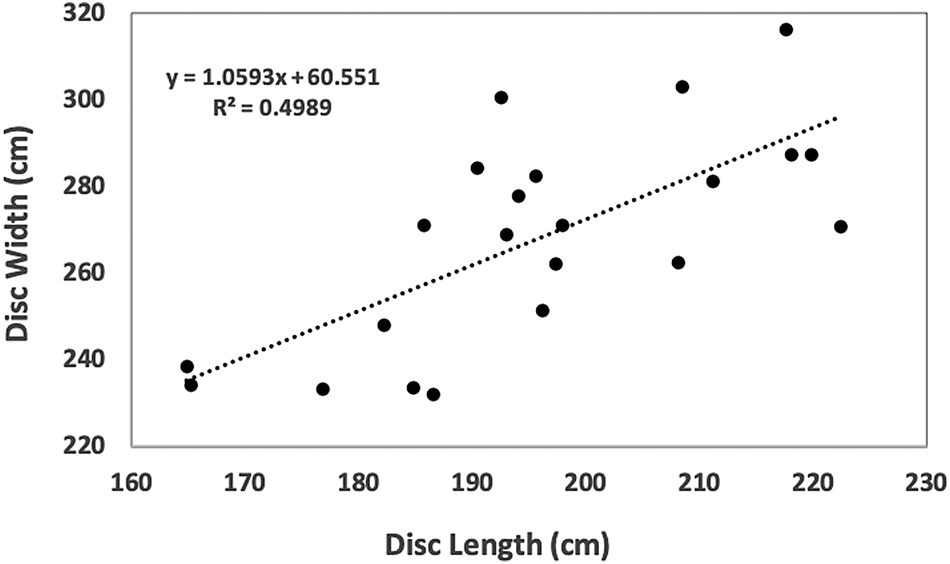
Figure 3. Mobula tarapacana (N = 22) disc width plotted against disc length. Measurements are from NYSERDA aerial photo data, measured using the Normandeau data portal measuring tool.
In the BOEM aerial digital survey data, 2 M. tarapacana individuals in separate sightings were definitively identified, with another 6 rays identified as potentially M. tarapacana. Both definitively identified rays were observed in the afternoon of 25 May 2018. Only a photo of a single individual was good enough to allow measurement and this ray had a DW of 272 cm, DL of 205 cm and disc ratio of 1.3.
NOAA Fisheries SEFSC Gulf of Mexico marine mammal aerial surveys reported 12 sightings of M. tarapacana with a total of 27 individuals (Figure 2D). All observations were of a solitary individual, except for a group of 15 observed in July 1996 and a pair of M. tarapacana observed in March 1997. All sightings occurred in the months of March (42%, N = 5) or July (58%, N = 7). Another solitary individual was observed in December 2019 off the Atlantic coast of Florida by the SEFSC programme.
Trained observers with HDR EOC reported 70 sightings of M. tarapacana with a total of 188 individuals. Observations were noted April through October with most (60%, 42 of 70 sightings) noted in June (Table 1). Forty-four per cent (31 of 70) of sightings by HDR EOC were of more than one M. tarapacana with groups sizes ranging from 2–24 (average of 2.7 ± 3.7 rays).
The PLL Observer programme reported 31 M. tarapacana. Fourteen individuals were incidentally captured in the Mid-Atlantic Bight, 2 in the South Atlantic Bight, 3 in the North-east Coastal, 7 in the Florida East Coast and 5 in the Gulf of Mexico (Table 1).
A single M. tarapacana was observed on 10 July 2018 at 10:00 am by a scuba diver in Jupiter, Florida, USA (Figure 2E, F). The ray was at a depth of 40 m and the surrounding water temperature was measured at 27.8°C. The ray circled the diver for less than 2 minutes and then departed to the south. A second incidental observation was made during a NOAA plankton survey in the Gulf of Mexico on 30 May 2019. Additionally, a single M. tarapacana became entangled at 61 m depth in a commercial diver's downline for ~30 s at West Delta 104 offshore Louisiana on 2 September 2006, just a week after a diver was killed by rapid ascent during a similar incident with a large ray (https://www.longstreath.com/community/incidents/?page=3). Finally, a group of at least 8 M. tarapacana were observed by a boater on 7 May 2022 in 134 m water depth off Marathon in the Florida Keys (Figure 1).
Discussion
While Mobula tarapacana is assumed to have a circumglobal distribution, there is a lack of data confirming this (Marshall et al., Reference Marshall, Barreto, Bigman, Carlson, Fernando, Fordham, Francis, Herman, Jabado, Liu, Pardo, Rigby, Romanov and Walls2019). This study expands the known range of M. tarapacana further north in the western Atlantic, spanning from Florida to offshore of New York. While M. tarapacana has previously been reported in the Gulf of Mexico (Childs, Reference Childs1997; Jones et al., Reference Jones, Driggers, Hannan, Hoffmayer, Jones and Raredon2020), we also include additional confirmation of this with the PLL Observer data, SEFSC aerial visual survey data and a recorded interaction with a commercial saturation diver. The previous northernmost confirmed record of M. tarapacana in the western Atlantic was from the north-western Gulf of Mexico, specifically the Flower Garden Banks off the coast of Texas (28°N, Childs, Reference Childs1997), though the Azores population resides at 38°N. Our data slightly extend the northern range of M. tarapacana in the Gulf of Mexico to 29°N, and in the Atlantic to 40°N. In the Pacific, the northernmost record is Japan (45°N, Tomita et al., Reference Tomita, Kawai, Matsubara and Nagata2013).
Despite year-round effort of NYSERDA aerial digital surveys, M. tarapacana were only observed in the summer months of July and August. Average water temperature in July and August for this time period were 22.8°C and 23.9°C, respectively (NOAA buoy 44025: 40.251°N 73.164°W). Additionally, pelagic longline captures only occurred in the North-east Coastal (NEC) fishing area during August and September. Further south in the Mid-Atlantic Bight (MAB) and off the coast off Virginia and North Carolina, M. tarapacana were only observed in the months of April through October. Similar seasonal trends are found in the Azores where M. tarapacana are only found in the boreal summer months with water temperatures ranging from 17.7–20.7°C (Morato et al., Reference Morato, Afonso, Lourinho, Nash and Santos2003; Sobral & Afonso, Reference Sobral and Afonso2014), and satellite tagged M. tarapacana all moved south from the Azores after the summer aggregation (Thorrold et al., Reference Thorrold, Afonso, Fontes, Braun, Santos, Skomal and Berumen2014). Though M. tarapacana can tolerate cold water (4°C) on deep dives (2000 m), they bask in warm, daylit waters before and after doing so (Thorrold et al., Reference Thorrold, Afonso, Fontes, Braun, Santos, Skomal and Berumen2014).
No seasonal trends were detected off the southern coast of the USA, with M. tarapacana being observed in both summer and winter months. Mobula tarapacana are sighted year-round in St Helena in the South Atlantic Ocean where temperatures range between 19°C (winter) and 25°C (summer, Brown et al., Reference Brown, Beard, Clingham, Frickle, Henry and Wirtz2019). Mobula tarapacana are also sighted year-round in the Saint Peter and Saint Paul Archipelago (SPSPA, an island group 3000 km north-west of St. Helena), but were more frequently sighted in the months of January–June. (Mendonça et al., Reference Mendonça, Macena, Afonso and Hazin2018). These months of higher frequency M. tarapacana sightings in the SPSPA were attributed not to water temperature, but to probable greater food availability (Mendonça et al., Reference Mendonça, Macena, Afonso and Hazin2018). The degree to which M. tarapacana movements in the western Atlantic are driven by temperature and/or productivity could be clarified with satellite telemetry and distribution modelling.
Accurately measuring mobulid rays in situ is difficult without capture, and many studies estimate in-water size by comparison of an object of known length (Mendonça et al., Reference Mendonça, Macena, Afonso and Hazin2018, Reference Mendonça, Macena, Araújo, Bezerra and Hazin2020). Precise measurements can be more reliably collected on DL than DW due to the difficulty in getting a photo when pectoral fins are at exact full extension. The DW can then be estimated from a DW–DL relationship, such as that provided in the Results section above (Figure 3). However, caution should be taken when applying this equation to M. tarapacana measurements due to the moderate (0.5) correlation coefficient.
Mobula tarapacana mature at a DW of 270–280 cm for females and 240–250 cm for males (Rambahiniarison et al., Reference Rambahiniarison, Lamoste, Rohner, Murray, Snow, Labaja, Araujo and Ponzo2018; Stevens et al., Reference Stevens, Fernando and Notarbartolo di Sciara2018). While we were unable to determine sex from aerial digital survey data, 77% (N = 17) of the rays for which measurements were possible had DW greater than 240 cm. Minimum DW measured was 232 cm. These DWs indicate that the M. tarapacana offshore of New York are adults and sub-adults; however, it is important to note that we were only able to measure rays in 22 (36%) of the photos in the NYSERDA dataset. In addition, while we only used photos that clearly showed body margins of the ray and that appeared to be at or near full extension of the pectoral fins, there is likely some measurement error due to the subsurface location of the rays and the bending of the pectoral fins.
While most studies of M. tarapacana occur in remote ocean island groups, this study elucidates a potential seasonal population of M. tarapacana for future study off the coast of the continental USA, specifically New York. Future studies of M. tarapacana in the western Atlantic should examine whether the offshore area off New York may represent an important foraging or reproductive habitat, as well as describe their horizontal and vertical migrations and habitat use. Genetic and telemetry studies should also investigate the connectivity between eastern and western Atlantic populations of M. tarapacana.
Understanding spatial distribution is key to management and conservation of endangered species. This study provides insight into where M. tarapacana may overlap with fisheries and are at greater bycatch risk. Prior to July 2019, fisheries observers in the USA did not classify mobulid ray bycatch according to species. With future bycatch data accurately representing mobulid species catch, we can quantify which fisheries affect M. tarapacana, and make management decisions accordingly. Also, recent advances in the distribution modelling of manta rays (Farmer et al., Reference Farmer, Garrison, Horn, Miller, Gowan, Kenney, Vukovich, Willmott, Pate, Webb, Mullican, Stewart, Bassos-Hull, Jones, Adams, Pelletier, Waldron and Kajiura2022) can be applied to M. tarapacana to further elucidate environmental drivers of species distribution. This may increase efficiency for research programmes and potentially reveal areas likely to contain previously undiscovered M. tarapacana aggregations.
This study highlights how incidental observations and observer data can provide important data on rare, vulnerable and difficult to study species. Recently, similar methods were used to describe the spatio-temporal distribution of Mobula mobular in the Eastern Atlantic (Lezama-Ochoa et al., Reference Lezama-Ochoa, Lopez, Hall, Bach, Abascal and Murua2020), as well as Mobula thurstoni habitat use in a marine protected area in Brazil (Bucair et al., Reference Bucair, Mendonça, Araújo, Rangel and Gadig2022). We hope this encourages other researchers and managers to examine regional databases for information on other data-poor species.
Supplementary material
The supplementary material for this article can be found at https://www.iccat.int/Documents/CVSP/CV055_2003/n_4/CV055041576.pdf
Acknowledgements
SEFSC aerial survey data for the Gulf of Mexico were collected during a study funded in partnership with the US Department of the Interior, Bureau of Ocean Energy Management through Interagency Agreement M17PG00013 with the US Department of Commerce, National Oceanic and Atmospheric Administration (NOAA). S. Venables provided helpful comments on a first draft of this manuscript. Many thanks to L.A. Dias, M. Cotter and T. Pusser for their support in the development of this manuscript. Many thanks to S. and D. McKee and J. Joel for sharing their observations.
Author contributions
JP formulated the research question, JP, JRW, CJ, CH and NF all collected data and designed the study, JP and CH analysed the data and JP wrote the manuscript. All authors read and approved of the manuscript.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
All authors declare that they have no competing interests.
Data availability
Data collected by Normandeau-APEM are freely available. Imagery may be reviewed on the Normandeau ReMOTe web site (https://remote.normandeau.com/remote_about.php). All other aerial digital data may be found at the links below:
NYSERDA:
Digital Aerial Baseline Survey of Marine Wildlife in Support of Offshore Wind Energy – OPA 2016
https://seamap.env.duke.edu/dataset/1817
Digital Aerial Baseline Survey of Marine Wildlife in Support of Offshore Wind Energy – WEA 2016
https://seamap.env.duke.edu/dataset/1818
Digital Aerial Baseline Survey of Marine Wildlife in Support of Offshore Wind Energy – OPA 2017
https://seamap.env.duke.edu/dataset/1994
Digital Aerial Baseline Survey of Marine Wildlife in Support of Offshore Wind Energy – OPA 2018
https://seamap.env.duke.edu/dataset/2073
BOEM:
Ecological Baseline Studies of the U.S. Outer Continental Shelf Option Year 1
https://seamap.env.duke.edu/dataset/2065
Ecological Baseline Studies of the U.S. Outer Continental Shelf Option Year 2
https://seamap.env.duke.edu/dataset/2161
Other data are available on request due to privacy/ethical restrictions.






