Once considered a condition of hyperactive boys, our knowledge and understanding of attention deficit hyperactivity disorder (ADHD) and has dramatically evolved.Reference Biederman, Mick and Faraone 1 Landmark studies by Biederman, Kessler, Faraone, and others have changed and deepened our understanding of ADHD to include a condition which not only affects boys but quite often affects girls. 1–5 The evolution of symptoms across the lifespan and the concomitant neurologic changes which underlie this symptomatic expression has similarly evolved.Reference Shaw, Eckstrand and Sharp 6 Studies by Dalsgaard and others have brought to light the significantly increased morbidity and mortality associated with preschoolers, children, and adults struggling with ADHD and associated conditions.Reference Dalsgaard, Østergaard and Leckman 7, Reference Dalsgaard8
This article will help clinicians:
-
(1) Develop a deeper understanding of the evolution of ADHD symptoms and functional struggles across the age span.
-
(2) Become familiar with rating scales that evaluate ADHD symptoms, behavioral challenges, and functional difficulties in ADHD.
-
(3) Learn how to optimize outcomes and manage breakthrough symptoms.
-
(4) Understand the onset, offset, and duration of action of various ADHD medications.
-
(5) Develop strategies to adjust or combine medications to address breakthrough symptoms in the morning, afternoon, or evening.
Consequences of ADHD Across the Lifespan
ADHD is a common neurodevelopmental disorder affecting approximately 8% to 11% of school-aged children, both in the United States and throughout the rest of the developed world.Reference Cortese 9 ADHD has come to be recognized as a condition which affects both boys and girls and often persists from childhood to adolescence and into adulthood. 10 The classic triad of hyperactivity, impulsivity, and inattention captures many of the core aspects of ADHD but fails to capture some of the difficulties surrounding executive function and emotional reactivity which collectively account for much of the social, educational, occupational, and emotional impairment of the disorder. In early childhood and throughout grade school ADHD is diagnosed more frequently in boys than girls; possibly relating to the higher expression of overt hyperactive symptomatology which is found in preadolescent boys.Reference Greene, Biederman and Faraone 5 ADHD patients with predominantly inattentive symptoms are often overlooked, misdiagnosed, or diagnosed 2 to 3 years later in life in that the overt outward marker of hyperactivity has often been used to identify this condition.
Our basic understanding of the neurophysiology of ADHD has advanced dramatically with studies by investigators such as Nora Volkow, who highlighted the differences in dopamine transporter activity in children with ADHD, Philip Shaw who tracked maturation of the prefrontal cortex in children with ADHD, and studies looking at the influence of genetic heritability and environmental factors.Reference Faraone and Mick 2 , Reference Volkow, Wang and Newcorn 11 These studies, taken in total, show that ADHD is a highly genetic neurologic condition which affects numerous cortical and subcortical pathways that coordinate information processing, impulsivity, emotional modulation, and neurochemical pathways that modulate communication between these cortical regions. ADHD has an overall genetic heritability of approximately 75%, that is, three out of four times there will be a genetic family history. There is also an important impact of environmental factors and the influence of the environment upon our genes with epigenetic changes occurring in a number of our children exposed to environmental stressors such as prenatal nicotine exposure, lead exposure, or severe psychosocial trauma.Reference Banerjee, Middleton and Faraone 12 , Reference Zhu, Lee, Spencer, Biederman and Bhide 13
Long-term follow-up longitudinal studies have shown that ADHD children are at greater risk of academic difficulties, emotional dysregulation with oppositional defiant disorder tendencies and emotional volatility. Longitudinal imaging studies have shown a 2 to 3 year delay in maturation of the prefrontal cortex and associated pathways in children with ADHD as compared to non-ADHD controls.Reference Shaw, Eckstrand and Sharp 6 It is therefore not surprising that ADHD symptomatology continues to evolve throughout childhood, adolescence, and into early adulthood as the underlying neurophysiology of the brain continues to mature and is influenced by outside environmental demands such as high school, college, employment, and the social demands of adulthood.
By adolescence, there is an increased rate of drug and alcohol abuse, an increased rate of motor vehicle and traffic-related difficulties and a dramatically increased rate of teenage pregnancy for ADHD individuals compared to controls.Reference Cortese 9 Followed on into adulthood, ADHD individuals have lower occupational and economic performance, have increased difficulty with financial management, have increased rates of psychiatric comorbidity such as depression, alcohol, and drug abuse, intermittent explosive disorder, and a variety of anxiety disorders.Reference Barkley, Murphy and Fischer 14 ADHD adults have increased rates of marital dysfunction and divorce, dramatically worsened abilities to maintain ongoing friendships and significantly lower evaluations of self-esteem and self-worth.Reference Wilens and Biederman 15
Can Treatment Make a Difference?
Although classic ADHD studies have shown that both behavioral and medical interventions can improve core ADHD symptoms, there has been debate about the overall long-term functional impact of ADHD interventions. Studies by Biederman, Wilens, and others have shown that consistent ADHD treatment decreased the rate of drug and alcohol abuse later in life.Reference Wilens and Biederman 15 Numerous studies in analog classroom settings have shown improved performance on task-related activities such as completing math problems and improvement on behavioral tasks measured by rating scales such as the SKAMP (Swanson, Kotkin, Atkins, M-Flynn, and Pelham).Reference Wigal, Gupta and Guinta 16
Studies by Dalsgaard and others have shown that ADHD is associated with significantly increased morbidity and mortality.Reference Dalsgaard, Østergaard and Leckman 7 , Reference Dalsgaard 8 These studies have found increased rates of accidental injury requiring medical treatment and injury requiring emergency room intervention. Utilizing the Danish birth registry, Dalsgaard found that on a nationwide basis ADHD individual had dramatically increased mortality rates compared to the general population. ADHD preschoolers had an 86% increased mortality rate, school-aged students had a 58% increased mortality rate, and adults with ADHD had a 325% increased mortality rate relative to non-ADHD individuals within the same population.Reference Greene, Biederman and Faraone 5 These studies went on to analyze whether ADHD treatment within the overall population of Denmark impacted these adverse outcomes. They found that ADHD treatment was associated with an overall decrease in accidental injury and medical utilization due to accidents and trauma. On a nationwide basis, they found a 25% to 37% decrease in emergency room utilization among ADHD individuals receiving treatment versus those not receiving treatment.Reference Dalsgaard, Østergaard and Leckman 7
Similarly, a 2 year follow-up study of ADHD adults in the United States found that women had a 27% reduction and men had 34% reduction in motor vehicle trauma rates in the months where they took their medications as compared to the months where they were unmedicated. Treatment was even more impactful in those with high impulsivity with men who ride motorcycles having a 49% reduction in trauma rates in the months where they took their medications.Reference Chang 17
Diagnosis and Recognition of Symptoms
ADHD is a pervasive condition with symptoms that start in childhood or adolescence and involve a core of 18 classic symptoms. 10 According to DSM V criteria, children with ADHD must have a persistent pattern of inattention and/or hyperactivity-impulsivity that interferes with functioning with ≥6 of nine inattentive symptoms for an inattentive presentation, ≥ 6 of nine hyperactive/impulsive symptoms for a hyperactive/impulsive presentation or ≥6 inattentive and six hyperactive/impulsive symptoms to meet criteria for a combined presentation. Longitudinal studies of ADHD children followed into adulthood by Barkley found that ADHD adults had difficulty remembering symptoms dating back to early childhood and therefore the DSM V diagnostic criteria have been modified.Reference Barkley, Murphy and Fischer 14 For a diagnosis in late adolescence or adulthood, several symptoms must have been present before age 12 and there must continue to be ≥5 of nine symptoms in either the inattentive, hyperactive/impulsive, or in both domains for diagnosis.
Improving the Accuracy of ADHD Diagnosis and Treatment
Clinical data and research evidence have highlighted the need to shift from a subjective to a more objective measure of ADHD diagnosis and treatment. A variety of fairly sensitive and specific ADHD rating scales have been developed to better define and measure ADHD symptoms both during initial diagnosis and throughout ongoing treatment. While in no way substituting for a thorough clinical evaluation, these scales help to:
-
(1) Better quantify and define ADHD impairments during the initial diagnosis.
-
(2) Measure ADHD symptoms at various time points throughout the day.
-
(3) Track ADHD symptoms to make sure that overall symptomatic improvement, symptomatic remission, and functional normalization have been optimized for each patient.
-
(4) Save time by providing an objective measure of symptom severity before meeting with a patient.
Why Should a Clinician Use ADHD Rating Scales?
Just as it is helpful for an internist to know an individual’s blood pressure or hemoglobin A1c when diagnosing and optimizing treatment for a hypertensive or diabetic patient, ADHD rating scales can be similarly useful in the management of our patients.
The ADHD-Rating Scale (ADHD-RS), the Vanderbilt, and the Conner’s are examples of ADHD scales that have been shown to be consistent measures of core ADHD symptoms and are sensitive to treatment effect. 18–21 Various ratings of executive function such as the Behavior Rating Inventory of Executive Function, can be utilized to measure executive function deficits which frequently cause impairment for ADHD individuals.Reference Guy, Isquith and Gioia 22
Another group of scales has been developed to measure ADHD symptoms and how they fluctuate throughout the day. Analog Classroom and Analog Workplace settings have been developed to measure ADHD symptoms and evaluate how long different treatment modalities improve symptoms over the course of the day. In these settings the 13 item SKAMP is used to measure attention, behavior, and deportment in children and the Permanent Product Measure of Performance measures a child or adult’s ability to sit and complete a series of basic math equations during 10 minutes and these tests are then repeated at various time points throughout the day.Reference Wigal, Gupta and Guinta 16 , Reference Wigal and Wigal 23 The Before School Functioning Questionnaire (BSFQ) has been developed to help clinicians better evaluate ADHD symptoms and functional impairments which cause significant disruption during the morning hours.Reference Wilens, Hammerness and Martelon 24 The Parent Rating of Evening and Morning Behavior (PREMB) evaluates behavioral problems both in the morning (PREMB-AM) and evening (PREMB-PM) in children.Reference Greene, Biederman and Faraone 5
Rating scales cannot only be used to detect breakthrough symptoms for pharmacotherapeutic adjustment but can also be used to detect symptomatic or functional issues for psychotherapeutic intervention. Family training, daily structure, and behavior modification can all be optimized by using appropriate rating scales to monitor associated struggles throughout the day.
Goals for Treatment
ADHD medications have some of the highest effect sizes of any medical intervention.Reference Faraone and Glatt 25 Studies also demonstrate, however, that response to specific medications is highly individual. Studies show that some patients respond equally well to either class, while other individuals have a preferential response to either a methylphenidate preparation or an amphetamine preparation.Reference Arnold 26 Individuals who have experienced adverse effects or have failed stimulants may go on to either tolerate or preferentially respond to non-stimulant medications.Reference Cutler, Brams and Bukstein 27
Studies with all three classes of medications, methylphenidates, amphetamines, and non-stimulants, have highlighted that it is all too easy for clinicians to settle for partial improvement while still leaving patients with ongoing symptomatology.Reference Mattingly and Anderson 28 Clinical response has often been defined as a 25% to 30% symptom improvement, but this still leaves patients with ongoing significant symptomatic and functional impairment. Interestingly, clinicians interpret 30% symptom improvement as “much” or even “very much improved” on global clinical measures. 29–32
Patients and clinicians may be tempted to be satisfied with 30% improvement, even though the evidence is clear that such minimal improvement almost inevitably means continued functional impairment. For most patients, further improvement is possible. Long-term trials demonstrate that nearly 75% to 80% of patients can achieve >50% symptom reduction and achieve symptomatic remission with overall Attention Deficit Hyperactivity Disorder Raring Scale- ADHDRS scores of less than 18 (meaning that ADHDRS scores are mild or less on average).Reference Mattingly, Weisler and Young 33 , Reference Mattingly, Childress and Nordbrock 34
Most individuals with ADHD will have moderate to moderately severe symptoms when first presenting for diagnosis or treatment with corresponding ADHD-RS scores in the 30 seconds to low 40 seconds (18 symptoms rated 0 to 3, 0 none, 1 mild, 2 moderate, and 3 severe).Reference Mattingly and Anderson 28
A recent study with MPH DR/ER dosed in the evening for ADHD children demonstrated that ADHD symptoms could be decreased from 42 to 11 after 6 weeks of dose titration, with an average final dose of 60 mg per day. To put this in clinical perspective; these children were highly symptomatic with ADHD scores that were moderate or severe on all 18 items and by the end of 6 weeks their ADHD symptoms were mild or none on average. This and other studies point to the importance of appropriate titration to optimize outcomes in our patients with ADHDReference Mattingly and Anderson 28 (Figure 1).
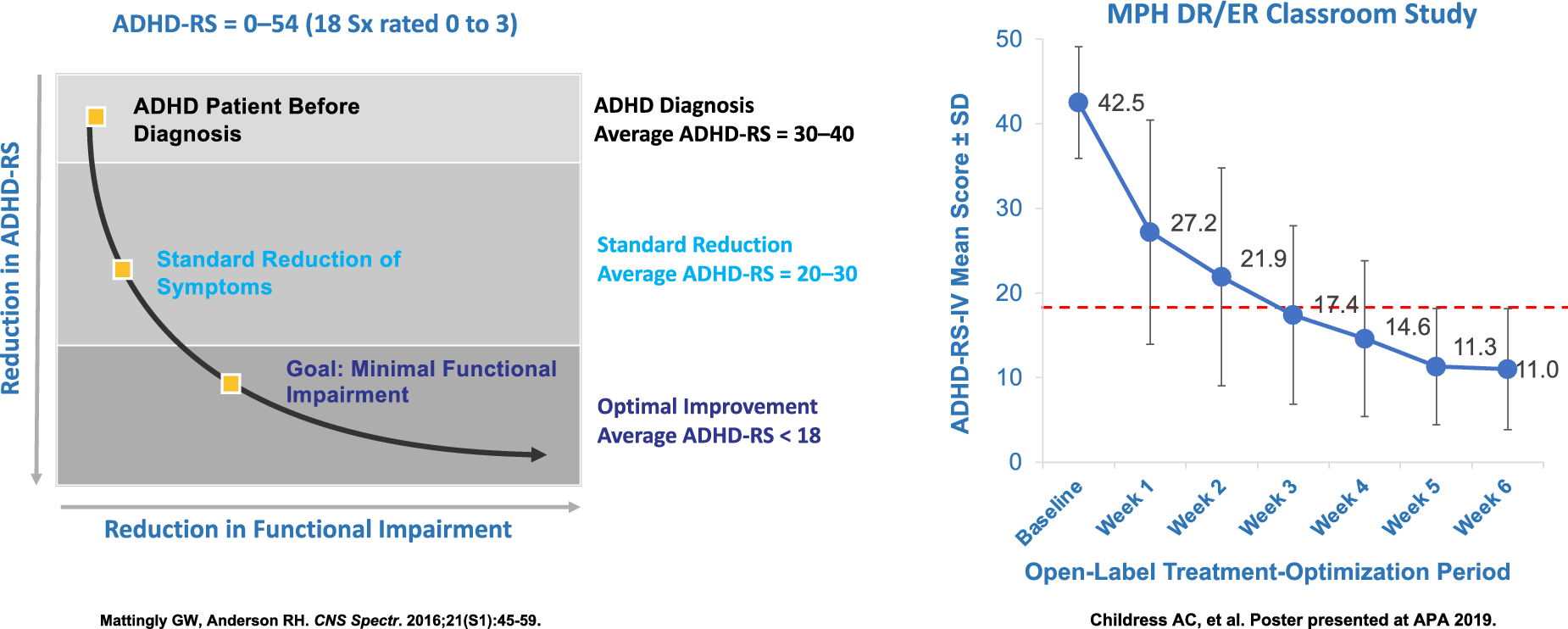
Figure 1. Optimizing symptom reduction results after 6 weeks of treatment.
In addition to overall symptom improvement, our treatments must deliver symptomatic improvement at time points where functional impairment is occurring for our patients. Studies by Sallee, Whalen, Mattingly, and others have shown that clinicians primarily focus on consequences of ADHD during school and work while overlooking impairments that occur at the beginning and end of the day. 35–37 Sallee et al found that 79% of ADHD caregivers have discussed early morning functional impairments such as getting out of bed, getting dressed, self-hygiene, eating breakfast, packing their backpack, and being able to catch the bus as being some of the most impairing issues for their children with ADHD. Nearly half of these caregivers reported getting early to administer ADHD medication before their child’s normal wake time to compensate for functional difficulties experienced before their child’s medication was otherwise taking effect.Reference Mattingly, Culpepper and Babcock 32
Comorbid Conditions
As now noted in DSM 5, ADHD frequently presents with a constellation of associated emotional, behavioral, and cognitive challenges. 10 Young children with ADHD frequently have associated learning disorders or developmental disabilities such as difficulties with sensory integration, problems with working memory, speech, and language delay, and difficulties with reading comprehension. A baseline battery of neurocognitive testing to detect specific learning challenges should be considered in all school-age children with ADHD. Recognition of specific learning challenges can help to better identify therapeutic interventions and academic accommodations which can be of great benefit for children struggling with ADHD and associated learning difficulties.
Emotional Impulsivity, Oppositional Behavior, and Poor Frustration Tolerance
Many children, adolescents, and adults with ADHD struggle with symptoms associated with poor frustration tolerance. These symptoms can present in childhood as difficulty waiting, impatience with delays, or excessive frustration when asked to shift from a preferred to a nonpreferred task.Reference Mattingly, Wilson and Ugarte 36 Such frustration often leads to a sequence of escalating emotional and behavioral dyscontrol, “meltdowns” out of proportion to the task at hand, and emotional fragmentation.Reference Whalen, Henker and Jamner 37 , Reference Mattingly, Surman and Mao 38
Oppositional thoughts and behaviors can trouble ADHD individuals of any age. 39–41 Difficulties shifting from preferred to non-preferred tasks and impaired recognition of the emotional impact of their behavior on others frequently lead to such oppositional patterns in ADHD individuals.
Appropriate medication selection can improve not just core ADHD symptoms but can also improve morning function, morning behavioral issues, and evening behavior.Reference Mattingly, Surman and Mao 38 , Reference Faraone 42 The BSFQ allows clinicians to monitor functional issues that a parent or caregiver are noting to be disruptive for a child or their family in the morning before school. The PREMB allows a clinician to disruptive behaviors in both the morning or evening. Morning behaviors include getting up in the morning, getting ready for school, arguing with siblings, or being late for school or the bus. Evening behaviors include doing homework, eating dinner with the family, getting ready for bed, and falling asleep (Tables 1 and 2; Figure 2).
Table 1. FDA-Approved Methylphenidate a Formulations for ADHD

Abbreviations: ADHD, attention-deficit/hyperactivity disorder; FDA, U.S. Food and Drug Administration; HCL, hydrochloride; NA, not available; ODT, orally disintegrating tablet.
a The American Academy of Pediatrics recommends utilizing methylphenidate as the first choice for preschool-aged children.Reference Wigal and Wigal 23
b Methylin is bioequivalent to Ritalin,Reference Greene, Biederman and Faraone 5 but it has not been tested independently in a classroom study.
Table 2. FDA-Approved Amphetamine Formulations for ADHD

Abbreviations: ADHD, attention-deficit/hyperactivity disorder; FDA, U.S. Food and Drug Administration; HCL, hydrochloride; NA, not available; ODT, orally disintegrating tablet.
a Adzenys XR-ODT and Adzenys ER are bioequivalent to extended-release mixed amphetamine salts (ie, Adderall XR),Reference Faraone 42 , Reference Mattingly, Wilson and Rostain 85 but have not been tested independently in a classroom study.

Figure 2. Improving function and behavior.
Immediate-Release, Extended-Release, Delayed-Release, or Combinations; Choosing Between Medication Options
The last several years have seen an explosion in stimulant delivery systems available for ADHD treatment. Stimulants are now considered the first-line pharmacologic treatment option for individuals with ADHD.Reference Mattingly, Wilson and Rostain 85 , Reference Briars and Todd 86 ADHD treatment has progressed from a decision between short-acting methylphenidate (Ritalin, Methylin…) versus short-acting amphetamine (Dexedrine, Adderall…), each of which required dosing several times per day in order to maintain therapeutic efficacy.
Various strategies have been developed by pharmaceutical manufacturers to avoid the mid-day and intraday dosing. Early modifications involved slow-release wax matrix technologies (Ritalin SR…) that while extending the duration of action continued to have difficulties with variable release patterns from day to day and from patient to patient depending on pH-related factors, gastric motility, and meal effects.
The next set of sustained-release medications involved beaded technologies (Adderall XR, Focalin XR…) where a certain percentage of medication was released in short-acting immediate release beads while another percentage of beads were coated with a pH-dependent layer that would begin releasing approximately 4 hours later in the less acidic small intestine providing clinical efficacy for approximately 8 to 10 hours. These beaded technologies allowed for adjustment of the percent immediate-release versus sustained-release beads (30/70, 40/60, or 50/50) in order to have greater delivery in the morning or increased medication delivery in the afternoon. A triple bead mixed amphetamine salt compound has been approved for adolescent and adults with ADHD. This compound (Mydayis) was shown to achieve a 16 hour duration of action in clinical trials.
A more recent adaptation of the beaded technology involves a multi-layered release technology where each bead has an immediate-release outer layer with an extended release inner layer. These pH-dependent layers then dissolve as each bead passes through various points in the intestinal tract. This multilayered release profile results in a biphasic pharmacokinetic curve with an immediate first peak 2 hours post dose and a second peak 8 hours post dose with an MPH compound (Aptensio) achieving 12 hour duration of effect in clinical trials. A longer duration multilayered MPH formulation (Adhansia) has been approved with duration of improvement of 13 hours in pediatric trials and up to 16 hours in adult trials.
Beyond Beads
The OROS capsule (Concerta) provides a novel delivery system with ongoing continuous release. After ingestion, stomach fluid is absorbed through osmotic pores in one end of the capsule causing medication to be excreted through a laser-drilled hole at the other end of the capsule. The release occurs over 10 to 12 hours but many patients experience a shorter duration of action.
A prodrug version of amphetamine was developed by binding lysine to amphetamine. Lisdexamfetamine (Vyvanse) is a hydrophilic “biologically inactive” prodrug which is not able to cross the bi-lipid blood–brain barrier. Lisdexamfetamine is enzymatically cleaved into free amphetamine and free lysine by enzymes in the cytosol of the human red blood cells providing sustained symptomatic improvement for 13 hours in children and 14 hours in adults. Lisdexamfetamine can be dissolved in fluid such as juice and administered in a liquid dose.
Transdermal
The ADHD field has one transdermal methylphenidate (Daytrana) option which provides continuous transdermal release of methylphenidate from the moment the patch is applied with continued efficacy until approximately 2 hours after the patch is removed. 61 , Reference Mattingly 87 This allows flexibility of daytime dosing with shorter or longer wear times depending on the individual needs of the patient. Unfortunately, adverse effects such as rash and skin discomfort often hinder the use of this transdermal technology, although it should be noted that a transdermal amphetamine based patch currently in development may minimize some of the associated skin reactions.
Microparticles
A variety of pH based ion-exchange polymers have been developed to allow ion exchange of stimulant molecules with underlying microparticle polymers. These pH based microparticle polymer technologies have allowed the development of sustained-release orally disintegrating tablets (ODT). Sustained release microparticle-based ODTs, MPH (Cotempla ODT) or amphetamine (Adzenys ODT) as well as a liquid suspension amphetamine (Adzenys) are approved for treatment of ADHD. Each ODT has stimulant molecules ionically bound to between 100 000 and 200 000 microparticles. A portion of these microparticles begin releasing stimulant immediately when they encounter ions in gastric lumen and some of these micorparticles are pH coated such that they will not release stimulant until they have passed into the small intestine.
Another microparticle multilayered technology allows liquid suspension or chewable tablets. Methylphenidate (Quillivant and Quilichew) and amphetamine (Dyanavel) preparations are approved for treatment of ADHD. These liquid formulations offer sustained-release preparations that can be titrated in small increments for children that are especially sensitive to side effects.
Nighttime Dosing
A unique microparticle MPH medication (Jornay PM) has been developed to improve ADHD symptoms both in the morning upon awakening yet also provide sustained duration to improve ADHD symptoms throughout the day until bedtime. This delayed release extended release “DR/ER” ADHD medication is designed to be taken around 8 pm in the evening. The outer delayed release layer does not allow the medication to begin absorption until 5 to 6 am. The inner extended release layer provides gradual stimulant release into the early evening. This technology demonstrated improvement in morning behavior, before school functioning, reduction in core ADHD symptoms, and improvement in evening behavior.
Nonstimulants
Three noradrenergic non-stimulants are currently approved in the United States for treatment of ADHD. The options either block reuptake of norepinephrine transporters (atomoxetine, Strattera) or directly stimulate the norepinephrine alpha 2a receptor (guanfacine-XR, Intuniv, and clonidine-XR, Kapvay). These options may be utilized for individuals who cannot tolerate the dopaminergic side effects of stimulants, require 24 hour symptomatic coverage or who have significant concerns about abuse or diversion of stimulants. In addition, both guanfacine-XR and clonidine-XR are approved for use in combination with a stimulant for children who have break through symptoms on a stimulant alone.
A fourth novel nonstimulant (viloxazine XR) is set for tentative approval by the FDA in early 2021. Viloxazine, considered a “multimodal agent” in that it both blocks the norepinephrine reuptake pump and directly modulates several serotonin receptors with elevation of intrasynaptic serotonin levels. Viloxazine will likely initially be approved for children and adolescents with positive adult ADHD studies reported in December 2020 (Table 3).
Table 3. FDA-Approved Nonstimulant Medications for ADHD

Abbreviations: ADHD, attention-deficit/hyperactivity disorder; FDA, U.S. Food and Drug Administration; HCL, hydrochloride; NA, not available.
a Time to onset of the full effect of nonstimulant medications is extended compared to stimulant medications due to long titration periods.Reference Childress 43 , Reference Briars and Todd 86 , Reference Goodman, Starr, Ma, Rostain, Ascher and Armstrong 97
b The duration of effect of atomoxetine has not been formally measured as in studies of stimulation medications. Evidence from clinical studies suggests that one-daily dosing of atomoxetine is associated with efficacy into the evening. 48
c Pending FDA approval. 49
Adjusting Medication to Optimizing Outcomes
Quite often a patient will return stating that “I’m doing better” after starting an ADHD treatment. The question is are they normalized, are they partially better but still symptomatic, are their symptoms still causing functional difficulties at various points throughout the day or in certain areas of their life. Are they partially better but not well?
Studies around the world have demonstrated the need to gradually adjust the dose and titrate for optimal effect. Many consumer databases have shown that quite often ADHD medications are being prescribed at doses below what was shown to be the optimal effective dose. The rule of thumb is to titrate until there are no breakthrough symptoms or residual functional difficulties or one encounters a dose-limiting side effect or has reached the maximum dose. One should then consider trying an alternative molecule-MPH, amphetamine, or nonstimulant or an alternative formulation of the same molecule that may address breakthrough symptoms at the beginning, the middle, or the end of the day.
Breakthrough Symptoms
End of the day breakthrough symptoms one should consider:
-
(1) Increase dose of current medication to see if it will provide longer coverage.
-
(2) Change to a formulation with a longer duration-
-
a. short acting to intermediate-MPH to MPH XR, AMPH to AMPH XR
-
b. intermediate to long-MPH XR to OROS MPH, AMPH XR to Lisdexamphetamine
-
c. long to longer-OROS MPH to MPH XXR or MPH DR/ER, Lisdexamphetamine to AMPH XXR
-
-
(3) Consider adding a nonstimulant-atomoxetine, guanfacine, clonidine, viloxazine.
-
(4) Consider using a long-acting stimulant and adding a short-acting stimulant at the end of the day.
-
(5) Consider layering 2 doses of long-acting stimulant-one in the morning and one around noon.
Beginning of the day breakthrough symptoms one should consider:
-
(1) Give the morning dose as early as possible.
-
(2) Adding a bit of short-acting stimulant in the morning with the long-acting stimulant.
-
(3) Using a long-acting stimulant that releases more medication in the IR component.
-
(4) Adding a nonstimulant with 24 hour coverage-atomoxetine, viloxazine.
-
(5) Switching to 8 pm dosing of MPH DR/ER to allow blood level ascendency to coincide with morning awakening.
Summary
Our knowledge and understanding of the underlying neurobiology and symptomatic expression of ADHD has advanced dramatically over the past decade. Associated with these advances has been a similar explosion of new formulations for individualization of treatment based on our patient’s needs. Optimized treatment is enhanced by measuring and tracking ADHD symptoms with the goal of treating to symptomatic remission with minimal functional impairment. Individual clinical presentation and patient response guide a clinician’s choice between chemical classes of medications: methylphenidate, amphetamine, or non-stimulant. Within both classes of stimulant, we now have delivery systems that tailor the release kinetics to each individual patient with immediate-release, 8-hour sustained-release, 12 hour sustained-release, and 13 to 16 hour sustained-release. In addition, we now have an evening dosed DR/ER methylphenidate that captures morning symptoms with sustained symptom control throughout the day and into the evening. A deeper understanding of the functional difficulties encountered by ADHD patients throughout their lives, coupled with more consistent use of ADHD rating scales enables clinicians to choose between a wide variety of medication delivery systems in order to optimize the outcome for each of their patients. 98–103
Disclosures
Greg W. Mattingly serves as a Speaker for Abbvie, Alkermes, Eisai, Ironshore, Janssen, Lundbeck, Otsuka, Sunovion, Takeda, and Tris; as a consultant for Abbvie, Acadia, Akili, Alkermes, Axsome, Eisai, Intracellular, Ironshore, Janssen, Lundbeck, Otsuka, Neos, Purdue, Rhodes, Sage, Sunovion, Takeda, and Teva; and is a researcher for Abbvie, Acadia, Akili, Alkermes, Axsome, Boehringer, Emalex, Idorsia, Janssen, Lundbeck, Medgenics, Purdue, NLS-1 Pharma AG, Otsuka, Reckitt Benckiser, Roche, Sage, Sunovion, Supernus, Takeda, and Teva. Joel L. Young, MD serves as a Speaker for Corium, Ironshore, Janssen, Otsuka, Sunovion, Supernus, and Takeda. He is a Researcher for Janssen and Otsuka. He serves on the Advisory Boards of Adlon, Alkermes, and Corion.







