Most neonates have the potential for interatrial shunting, in most instances because of persistent patency of the oval foramen, usually described as patent foramen ovale. Reference Homma, Messé and Rundek1,Reference Ozcelik, Atalay, Tutar, Ekici and Atasay2 The oval foramen provides an interatrial communication during fetal life such that oxygenated blood from placenta is shunted to the left atrium, thereby bypassing the pulmonary circulation. The morphology is such that a flap valve derived from the primary atrial septum is able to prevent postnatal shunting by dint of fusion with the rims of the foramen when the increase in pulmonary venous return has increased left atrial pressure. The result is the formation of the oval fossa, with the original primary septum forming its floor. Reference Jensen, Spicer, Sheppard and Anderson3 Closure normally occurs early after birth, but in a significant number of infants, however, the closure is not complete during the first few years of life. In approximately 25% of the adult population, the foramen retains its patency throughout life. Reference Homma, Messé and Rundek1,Reference Kheiwa, Hari, Madabhushi and Varadarajan4,Reference Hagen, Scholz and Edwards5 In a minority of individuals, nonetheless, deficiencies in the floor of the fossa can result postnatally in the presence of a true atrial septal defect of the “ostium secundum” type. Reference Gittenberger-de Groot, Calkoen, Poelmann, Bartelings and Jongbloed6,Reference Franklin, Béland and Colan7
The differentiation between patency of the oval foramen and atrial septal defect can be challenging. Currently, there is no generally accepted method to classify the different types of interatrial communications. Reference Scheuerle8 The morphological differentiation based on non-invasive imaging is most apparent in the newborn. It tends to become less apparent with growth. Most previous studies examining interatrial communications in newborns do not distinguish between patency of the oval foramen and atrial septal defect, Reference Hansen and Oxhoj9,Reference Senocak, Karademir, Cabuk, Onat, Koc and Duman10 while others classify the communications based only on the size of the defect. Reference Yildirim, Aydin, Demir, Aydin, Ucar and Kilic11–Reference Connuck, Sun and Super13
Some of the interatrial communications are of no or minimal haemodynamic importance, while others are larger and associated with significant shunting. Even those that are not haemodynamically important are associated with poor long-time outcome, reflecting lower exercise capacity and higher risk of atrial fibrillation, stroke, psychiatric disorders, pneumonia, and even premature death as compared to the background population. Reference Nyboe, Karunanithi, Nielsen-Kudsk and Hjortdal14–Reference Karunanithi, Nyboe and Hjortdal20 In order to study interatrial communications and their significance over time, an unambiguous classification of interatrial communications is necessary.
The aim of our study, therefore, was twofold: firstly, we aimed to develop an echocardiographic classification of neonatal interatrial communications using standard echocardiograms obtained in a cohort of unselected newborns, basing our approach on existing knowledge regarding the embryological development of the atrial septum. Secondly, we wanted to study the performance of our new classification in terms of intra- and interobserver variability.
Methods
This study is part of the Copenhagen Baby Heart Study. This prospective multi-centre population study focuses on cardiovascular health, based on prenatal inclusion of more than 25,000 participants. Inclusion in the study was offered to parents of children born between April, 2016 and October, 2018 in the three main maternity wards in the Copenhagen metropolitan area of Denmark. A detailed description of the study design and cohort overview has previously been published. Reference Sillesen, Raja and Pihl21,Reference Vøgg, Basit and Raja22 Participation included an echocardiogram performed within 30 days after birth.
The study complies with the Declaration of Helsinki and was approved by the Regional Ethics Committee (H-16001518), Capital Region of Denmark. Written informed consent was obtained from all parents prior to inclusion.
Population
For the construction of our algorithm, we reviewed 682 echocardiograms collected consecutively between 21 May and 16 June, 2017. Of these, 187 (27.4%) were subsequently excluded because of suboptimal image quality. This left 495 echocardiograms with acceptable image quality for inclusion. None of the included echocardiograms showed any sign of severe or moderate CHD as defined by Hoffmann et al. Reference Hoffman and Kaplan23
For our reliability study, we chose 50 echocardiograms among the 495 included in the study. We ensured by primary review that at least two examples of all subtypes were included.
Echocardiography
A standard transthoracic echocardiographic examination was performed by a physician or sonographer within 30 days of birth using Vivid E9 ultrasound equipment (GE Healthcare, United States of America) using 6S-D and 12S-D transducers. The protocol (Supplemental table S1) included three subxiphoid images showing the atrial septum. These were two images in an oblique view in between a transverse and a sagittal plane in which the atrial septum was best visualised using cross-sectional images and colour Doppler, along with an additional image in a sagittal plane in which the superior caval vein was also visualised using colour Doppler. Standard settings of the images included three cardiac cycles per recorded loop, and frame rates were above 80 frames per second and Nyquist value of 0.94. Offline analysis was performed using EchoPAC software version 113.
Construction of the algorithm
The algorithm (Fig 1a) was designed to classify interatrial communications in newborns. It was constructed based on the existing anatomical and embryological knowledge, combined with the technical and practical challenges related to transthoracic echocardiography in unsedated newborns.

Figure 1. Diagnostic algorithm for classification of interatrial communications on transthoracic echocardiography in newborns. PFO = patency of the oval foramen; ASD = atrial septum defect. Letters in panel A corresponds to detailed description in panel B.
The included echocardiograms were systematically reviewed with a focus on the subxiphoid images of the interatrial septum, and the findings were discussed by four echocardiographic experienced reviewers (KI, NV, LM, and SC).
The existence of an interatrial communication was identified as the presence of a colour Doppler signal of a blood flow across the atrial septum in combination with one of the following: a visible communication in the atrial septum on cross-sectional images or if no visible communication was present, flow acceleration in the colour Doppler signal. For both criteria, conformity was required between the site of the visible communication and the point where the flow was seen to cross the septum, or where the acceleration in flow was observed. The combinations of criteria were included to avoid including artefacts as interatrial communications. Such artefacts include dropout on the cross-sectional images, which might wrongly be interpreted as septal deficiency, as well as noise in the colour Doppler signal, which could wrongly be interpreted to represent flow across the septum through a defect not visualised on the cross-sectional image. We assumed that blood flow through a small restrictive defect, not seen on cross-sectional images, would result in flow acceleration visible in the colour Doppler signal. All these considerations were taken into account in the definitions and in the algorithm as shown in boxes A–C of Figure 1a. If the criteria were not met, the echocardiogram would be classified as “No patency of the oval foramen/atrial septal defect.”
For the echocardiograms, who met our definition of the presence of an interatrial communication, the following echocardiographic criteria were systematically documented:
-
1) Colour flow crossing the septum.
-
2) A visible communication seen on the cross-sectional images at the same point where the flow visualised by the colour Doppler signal crosses the septum.
-
3) Acceleration on the colour Doppler signal where the flow crosses the septum.
-
4) Normal location of defect defined as a location in the superior two-thirds of the septum.
-
5) A channel-like structure formed by overlap of the flap valve of the fossa derived from the primary atrial septum and the rims of the fossa. A communication was not deemed to be “channel-like” if the flap valve of the fossa did not overlap its rims, leaving an opening between them, or if the flap valve was positions on the right atrial aspect of the rims.
-
6) Multiple interatrial communications.
We also measured the size of any non-channel-like defect when visible on cross-sectional imaging from the frame in which the communication was largest.
We then incorporated these six criteria, along with a size limit for the defect of 4 millimetres, corresponding to the 95th percentile of the measured size of the communications, into the algorithm in such a way so the interatrial communications are classified into two main categories: patency of the oval foramen and atrial septal defect. More than one communication, an abnormal location, or a defect size larger than or equal to 4 mm distinguished the atrial septal defects from the patency of the oval foramen. We chose these structural criteria on the basis that knowledge of atrial septal development has shown the flap valve of the fossa to be derived from the primary atrial septum, and to overlap the rims of the oval foramen when normally developed. When the flap valve, and hence the primary septum, is deficient, it will not overlap the rims of the fossa, then being unable postnatally to close the foramen. We then considered visible overlapping between the flap valve and the rims of the fossa, with the flap valve on the left side of the fossa, thus creating a channel-like communication, to be proof of normal septal development, but with persistent patency of the oval foramen. This arrangement is classified as a “Channel-like patency of the oval foramen” in the algorithm (Fig 2b). We deemed the communications present as the result of deficiency of the flap valve to represent a continuum from small openings as normal variants to large openings as septal deficiencies. As the cut-off in this continuum, as already stated, we used the limit of 4 mm, corresponding to the 95th percentile of the measured size of the communications in the cohort. Those septal deficiencies of less than 4 mm have been classified as “Size-defined patency of the oval foramens” in the algorithm (Fig 2c), while deficiencies measured at 4 mm or greater are classified as “Size-defined atrial septal defects” (Fig 2d).
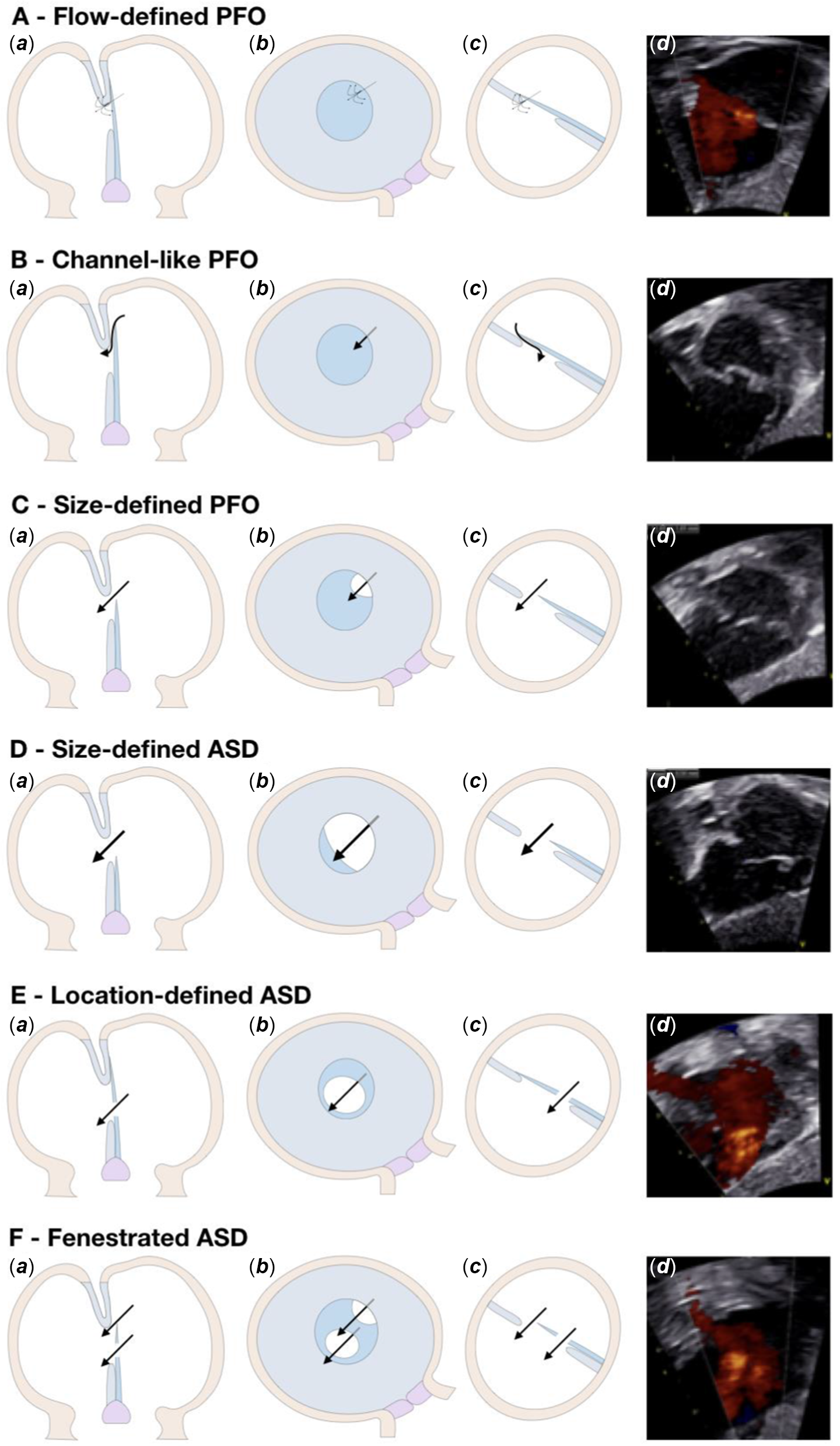
Figure 2. Subtypes. PFO = patency of the oval foramen; ASD = atrial septum defect. ( a ) Trans-sectional view; ( b ) view from right atrium; and ( c ) and ( d ) oblique view in between a transverse and a sagittal plane where the atrial septum is best visualised on transthoracic echocardiography.
Fenestrations of the flap valve can lead to isolated defects or can be combined with either patency of the oval foramen or an incompetent valve. On this basis, we determined that the location of the communication had to be in the central to superior part of the fossa to be considered a normal variation. Shunting across the inferior part was then deemed to be abnormal. Since only one channel is present when the fossa is normally developed, multiple communications were considered pathologic. In the setting of these criteria, our algorithm classified the communication as a “Location-defined atrial septal defect” (Fig 2e), or a “Fenestrated atrial septal defect” (Fig 2f).
The term “Flow-defined patency of the oval foramen” (Fig 2a) accounted for the arrangement in which no visible communications was seen on the cross-sectional image, but flow acceleration in the colour Doppler signal was present as a single communication normally located within the oval fossa.
Reliability study
The reliability study was performed with two groups of readers, each consisting of four experts in paediatric echocardiography. Readers in the first group were introduced to the algorithm prior to the analysis, being shown examples of scans selected to represent each subtype and description. Specific potential pitfalls related to the algorithm, which had come to our attention during the construction of the algorithm, were brought to the attention of the reviewers in Supplemental table S2 and Supplemental Fig. S1–S4, which accompanied the algorithm. The group analysed the echocardiograms twice, providing a time interval of at least 4 weeks between the two readings to avoid recall bias. On each assessment, the experts were asked to categorise the echocardiograms into one of the subtypes defined in the algorithm. The readers making up the second group were not introduced to the algorithm. They were simply asked to categorise each echocardiogram with the “traditional” definition for either patency of the oval foramen, atrial septal defect, or no interatrial communication based on their existing knowledge and consistent with their own clinical practice.
Statistics
Continuous demographic variables are presented as mean ± standard deviation when normally distributed and otherwise as median [interquartile range]. Continuous echocardiographic variables are presented as median and range. Categorical variables are presented as numbers and percentages of the total. Interobserver variability in the reliability study is reported using Fleiss’s kappa coefficients. Reference Landis and Koch24 Intraobserver variability is reported as a mean of Fleiss’s kappa coefficients obtained from comparisons for each reader with their reports in the two reviews. Data analysis was performed in the statistical program R Studio (version 1.0.153, using packages lpSolve (version 5.6.13) and irr (version 0.84)).
Results
The novel algorithm
As indicated, the study was based on 495 echocardiograms obtained from The Copenhagen Baby Heart Study. The median age of the cohort was 11 [8;13] days, and 255 (51.5%) were females. Mean gestational age at delivery was 40 ± 1.4 weeks, and mean birth weight was 3523 ± 511 grams.
In 414 (83.6%) newborns, we found an interatrial communication, and the findings from their echocardiograms are displayed in Table 1. A visible communication on cross-sectional images was seen in 276 (66.7%) newborns. In the remaining 138 (33.3%), the communication was only visible by colour Doppler with flow acceleration. Of those with a visible communication on cross-sectional images, which were located in the superior to central part of the septum, 191 (46.1%) had a single communication without any overlap between the flap valve and its rims of the fossa. The median size of the visible non-channel-like communications seen in the absence of overlapping was 2 mm, ranging between 0.5 mm and 6 mm. The calculated 90th percentile was 3 mm, with the 95th percentile being 4 mm, and the 98th percentile 5 mm. In 73 (17.5%) of the newborns, we observed a channel-like structures, and multiple communications were found in 8 (1.9%). A single communication located in the inferior part of the septum was found in four (1.0%) newborns.
Table 1. Findings of echocardiographic criteria of interatrial communications

Of the 414 newborns with an interatrial communication in our cohort, use of the algorithm showed that 386 (93.2%) had patency of the oval foramen and 28 (6.8%) had an atrial septal defect. Among those with patency of the oval foramen, 138 (33.3%) were classified as Flow-defined patency of the oval foramen, 73 (17.6%) as Channel-like patency of the oval foramen, and 175 (42.3%) as Size-defined patency of the oval foramen. For the atrial septal defects, 16 (3.9%) were classified as Size-defined atrial septal defects, 4 (1.0%) as Location-defined atrial septal defects, and 8 (1.9%) as Fenestrated atrial septal defects (Table 2). No reduction in the prevalence of interatrial communications was seen when comparing newborns examined during the first 2 weeks of life to those examined during week 3–4 (Supplemental table S3).
Table 2. Suggested diagnostic criteria for patency of the oval foramen and trial septal defect subtypes in newborns and reported distribution between subtypes
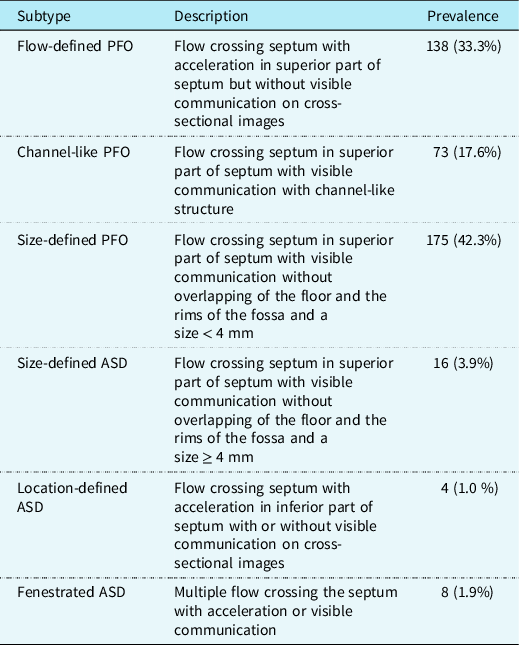
PFO = patency of the oval foramen; ASD = atrial septum defect.
Had the defect size upper limit been based on the 90th percentile at 3 mm, the number of communications classified as atrial septal defects would have been 44 (10.6%). Had the 98th percentile at 5 mm been used, then 20 (4.8%) newborns would have been classified as atrial septal defects
Reliability study
In the reliability study, we included a different cohort of 50 newborns, with a median age of 11 [8;13] days of whom 18 (36.0%) were females. Mean gestational age at delivery was 39.6 ± 1.5 weeks, and mean birth weight was 3457 ± 477 grams.
Interobserver variability in the first group of readers, who used the algorithm, was 0.66 for the main categories (patency of the oval foramen, atrial septal defect, and no patency of the oval foramen/atrial septal defect), interpreted as substantial agreement. The value was 0.46 for subtypes, interpreted as moderate agreement. Intraobserver variability for the same group was 0.82 for main categories, interpreted as almost perfect agreement and 0.65 for subtypes, interpreted as substantial agreement. The average performance amongst the group was 35.5 (71.0%) categorised as patency of the oval foramen, 8 (16.0%) as atrial septal defects, and 6.5 (13.0%) as no patency of the oval foramen/atrial septal defect.
In the second group of readers, who categorised into main categories without using the algorithm, basing their choice on existing knowledge and in consistency with their clinical practice, the interobserver variability was 0.20, interpreted as slight agreement. The group categorised on average 30.3 (60.5%) as patency of the oval foramen, 12.0 (24.0%) as atrial septal defect, and 7.8 (15.5%) as no patency of the oval foramen/atrial septal defect.
Discussion
In this study, we developed an algorithm based on echocardiographic criteria for the differentiation between patency of the oval foramen and atrial septal defect, and for defining subtypes in newborns. As expected, the majority of interatrial communications were classified as patency of the oval foramen. We found good reproducibility when the interatrial communications were classified using the algorithm. The usefulness of the algorithm was thus substantiated by the fact that only slight agreement on categorisation was achieved by those who did not use it.
A new diagnostic algorithm
Based on the known knowledge concerning the development of the atrial septum, patency of the oval foramen in the newborn period can be considered as a normal physiological phenomenon. While most interatrial communications in this period represents patency of the oval foramen, a proportion will be atrial septal defects that are consequence of true deficiency of the flap valve of the fossa, which is derived from the primary atrial septum. Both types of interatrial communications can readily be detected on transthoracic echocardiography in newborns, but distinguishing them is difficult due to the lack of well-defined and internationally accepted criteria. With this deficiency in mind, we set out to create a diagnostic algorithm. When designing the algorithm, it proved challenging to establish a cut-off which reliably differentiated between persistent patency as opposed to true septal deficiency. A size limit of 3 mm was previously suggested, but no justification had been provided for this choice. Reference Yildirim, Aydin, Demir, Aydin, Ucar and Kilic11,Reference Azhari, Shihata and Al-Fatani25 The main argument for using 3 mm as the cut-off value is data indicating the rates of subsequent spontaneous closure are high when communications are measured of less than 3 mm. Some studies, nonetheless, have reported significant rates of spontaneous closure for communications larger than 3 mm. Reference Senocak, Karademir, Cabuk, Onat, Koc and Duman10,Reference Radzik, Davignon, van Doesburg, Fournier, Marchand and Ducharme26 Other studies have used 5 mm as cut-off in combination with enlargement of the right heart chambers, but again without any argumentation for this choice. Reference Ooshima, Fukushige and Ueda12 In our study, we used the 95th percentile of the size of the defects measured in our test sample of 191 newborns with non-channel-like communications. This permitted us to identify 4 mm as the differentiating value. Using this value yields a frequency of atrial septal defects of 6.8%, as compared to 10.6% for a size limit of 3 mm. Reference Senocak, Karademir, Cabuk, Onat, Koc and Duman10,Reference Radzik, Davignon, van Doesburg, Fournier, Marchand and Ducharme26 When using 5 mm as the size limit, 4.8% were classified as atrial septal defects. Reference Ooshima, Fukushige and Ueda12 We recognise the optimal cut-off should reflect clinical importance rather than statistical considerations and will thus need to be further evaluated based on follow-up studies of closure rates and other clinical outcomes throughout life. Prior echocardiographic studies of interatrial communications in newborns have often included selected groups of patients, either referred for cardiac evaluation Reference Hansen and Oxhoj9,Reference Bierman and Williams27 or admitted to a neonatal ward. Reference Fukazawa, Fukushige and Ueda28,Reference Zhou and Guntheroth29 Our algorithm is based on an unselected group of newborns, which allows to better identify normal variations. It also provides a validated tool to study the prevalence of the different types of interatrial communications in the general population.
One single study has previously tried to establish diagnostic criteria of interatrial communications. This study used single-gated pulsed echocardiography and concluded it to be impossible to differentiate between patency of the oval foramen and small atrial septal defect. Reference Oberhoffer and Lang30 This study along with multiple other studies examining interatrial communications in newborns Reference Hansen and Oxhoj9,Reference Senocak, Karademir, Cabuk, Onat, Koc and Duman10,Reference Ooshima, Fukushige and Ueda12,Reference Connuck, Sun and Super13,Reference Radzik, Davignon, van Doesburg, Fournier, Marchand and Ducharme26,Reference Bierman and Williams27,Reference Zhou and Guntheroth29 were performed more than 20 years ago. The echocardiographic technology and the image quality at these times cannot be compared to the modern standards in echocardiographic apparatus. In our study, we used modern ultrasound equipment representing the standard used in contemporary medical practice.
Another previous study examined criteria used to differentiate between patency of the oval foramens and atrial septal defects by means of a survey of paediatric cardiologist. This revealed a great variability in clinical definitions used by the physicians, identifying problems for research based on the differentiation. Reference Scheuerle8 Our reliability study supports these findings, since weak interobserver agreement was found between the experts who categories the examinations based on the “traditional method” according to their existing knowledge, and consistent with their own clinical practice.
Potential pitfalls to be aware of when using the algorithm
Potential echocardiographic pitfalls in the application of the algorithm are listed in Supplemental table S2 and Supplemental Fig. S1–S4. These include image dropouts within the septum, which might be mistaken for defects. Reference Pollick, Sullivan, Cujec and Wilansky31 We have tried to address this caveat in our definition of the presence of an interatrial communication. It is also of importance to recognise other flow profiles which may be present in the atria, Reference Oberhoffer and Lang30 especially when an eustachian valve or Chiari network is present and may cause turbulence.
Study strength and limitations
Since we used standard paediatric echocardiographic methods, the algorithm should be easily applicable to general clinical practice. The diagnostic classification is based mainly on subxiphoid images, which provides the best views for visualising the atrial septum in infants and children. Reference Silvestry, Cohen and Armsby32 At present, the classification of interatrial communications in neonates and infants is often based on individual judgement, based on the experience of the operator. The introduction of an algorithm, therefore, has the potential to improve the reproducibility of classification of interatrial communication and thereby enable identification of clinically relevant interatrial communications. We acknowledge that additional views could be used for identifying and describing interatrial communications, especially in older children and adults in whom subxiphoid images are more challenging. In infants and neonates, however, these additional views often are not needed for the purpose of imaging the atrial septum. The main limitation of the algorithm is that it does not take account of defects outside the oval fossa. We recommend, for a full assessment of the atrial septum, to include sweeps from posterior to anterior in the coronal plane and from right to left in the sagittal plane. This allows visualisation of the entire atrial septum in all three orthogonal planes. For practical reasons, it was difficult to include this additional feature in our current protocol as we had to make choices on the images, and we could include in the prospective data capturing. Thus, at present, our algorithm is designed only for classification of defects involving the oval fossa. It cannot be used for classification of other atrial septal defects such as sinus venosus defects or coronary sinus defects nor for the “ostium primum” atrioventricular septal defects.
In this study, 187 of the examinations were excluded due to poor image quality. The study was a part of the Copenhagen Baby Heart Study, where newborns underwent a standard echocardiography, that is, the focus was not solely the atrial septum. The views necessary to use the algorithm, nonetheless, are standard. They are not technically different to obtain. We are convinced, therefore, that in a real-life setting, where the examiner could focus on the atrial septum and re-examine if necessary, the success rate will be markedly higher. We included newborns examined up to 30 days after birth and recognise that some patency of the oval foramens may have closed during that period. However, the assessment of the prevalence of interatrial communications during the period did not support that presumption.
We chose a relatively high Nyquist limit to minimise the risk for false-positive Doppler signals but acknowledge the increased risk for not detecting flows with low velocity.
An elongated cresent-shaped patency of the oval foramen could be misinterpreted as a fenestrated atrial septum defect, if the echocardiographic image cuts the cresent so that it appears to be two atrial septum defects. This problem could potentially miscatogerise a patency of the oval foramen as an atrial septum defect.
Aneurysms of the atrial septum may be present in the newborn. Reference Ozcelik, Atalay, Tutar and Ekici33 Aneurysms were seen rarely in our cohort and were not systematically registered why we cannot describe if the presence of atrial septum aneurysms effects the utility of the algorithm.
We could not provide follow-up data of our study group to look at the closure rate of the defects over time, which is needed to verify the utility of this classification for clinical use. A longitudinal study with a larger cohort would allow determination of which children require follow-up and especially who do not require follow-up. This will promote optimisation of health care resources and development of practice guidelines.
Clinical impact
With a new diagnostic algorithm for classification of interatrial communications, we are providing a method with the potential to fill a gap that has been pointed out by others occupied with this area. Reference Ozcelik, Atalay, Tutar, Ekici and Atasay2,Reference Yildirim, Aydin, Demir, Aydin, Ucar and Kilic11,Reference Hoffman and Kaplan23,Reference Fukazawa, Fukushige and Ueda28 An unambiguous phenotypic classification of interatrial communications is essential to enable appropriate studies of interatrial communications. It is a prerequisite for the assessment of clinical outcomes and for the identification of which subtypes can be considered as normal and which represents possible pathology that require further follow-up and interventions. This tool can be used for future studies looking at the prevalence and distribution of different subtypes of interatrial communications in the neonatal period.
Conclusions
We have developed a classification system for describing neonatal interatrial communications using echocardiographic criteria, which can form the basis for a common echocardiographic definition of interatrial communications. The potential utility of the algorithm was validated by the finding of an improved reproducibility and a far higher agreement on categories when using the algorithm than without. We suggest that this method should be used in the future for classification of neonatal interatrial communications in research as well as in clinical practice.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122003365
Acknowledgements
None
Financial support
The Copenhagen Baby Heart Study receives financial support from the Danish Heart Association, the Danish Children’s Heart Foundation, Candy’s Foundation, the Toyota Foundation, the Herlev-Gentofte Hospital Research Foundation, and the Gangsted Foundation. The funders have no role: in the design of the study in the collection, analysis, or interpretation of data; in the writing of manuscripts; or in decisions to publish results.
Conflicts of interest
None.







