Over 13 000 laboratory-confirmed cases of rotavirus diarrhoea are reported in England and Wales each year. The estimated community rate of rotavirus disease is 7·1/1000 person-years [Reference Wheeler1]; in cases of gastroenteritis, rotavirus infection is identified most commonly in children aged <5 years [Reference Tompkins2]. The risk factors associated with rotavirus disease in children in England, as identified by a case-control study, include living in rented council housing, accommodation with <5 rooms, contact with another person with gastroenteritis; and in infants, not being exclusively breastfed [Reference Sethi3].
In Europe rotavirus disease exhibits a distinct seasonal pattern, the causes of which are not well understood [Reference Koopmans and Brown4]. A winter/early spring peak in reported cases is typical, but the exact timing of the peak varies between countries. In England and Wales it occurs in late February/early March [Reference Van Damme5]. Protective immunity to rotavirus is relatively complex, with various factors contributing to immunity: maternal antibodies confer some protection for newborns, neonatal infection is believed to offer partial protection against disease, and previous infections progressively reduce a child's risk of subsequent rotavirus infection and disease [Reference Offit6]. The incidence of clinical disease peaks between ages 6 and 24 months [7].
Given these immunological and seasonal characteristics, we hypothesized that a child's risk of rotavirus diarrhoea depends partly on the child's season of birth. We investigated whether the season of birth is related to the risk of rotavirus diarrhoea in England and Wales.
The Health Protection Agency (HPA) collects reports of laboratory-confirmed rotavirus infections from laboratories across England and Wales. These laboratories receive stool specimens for testing from clinicians seeing patients in hospital or primary healthcare facilities in both urban and rural settings. Most laboratories offer rotavirus testing all year round for stool specimens from gastroenteritis cases in children aged <5 years. Enzyme immunoassays are the diagnostic methods of choice for the majority of laboratories, and no major changes in testing policy have occurred since their widespread introduction in 1993.
Weekly counts (by date the stool specimen arrives at the laboratory) of laboratory-confirmed rotavirus reports from children aged <5 years born between 1998 and 2007 were extracted from the national database. Before 1998 there was limited recording of date of birth, so children born before 1998 were excluded from this analysis. Numbers of monthly live births in England and Wales during this period were obtained from the Office for National Statistics [8].
Season of birth was divided into winter (December, January, February), spring (March, April, May), summer (June, July, August) and autumn (September, October, November). Seasonal birth cohorts for each year of birth between 1998 and 2007 were formed by combining all the live births occurring in each season. For each birth cohort, the risk of rotavirus diarrhoea at age m months was calculated by dividing the number of rotavirus reports in children of age m born in a given season and year, by the number of live births in the corresponding birth cohort. Cumulative risks for each birth cohort up to age 5 years were calculated. Poisson regression models were fitted to estimate the effect of birth season on risk of rotavirus diarrhoea. The measure of effect used was the relative risk (RR). The population attributable risk fraction (PAF) was calculated to estimate the proportion of all cases in the study population that could be attributed to being born in the summer months.
The formula used was:
where p is the proportion of reported cases that occurred in children born in the summer months and RR is the relative risk.
There were 95 837 reports of laboratory-confirmed rotavirus infections in children aged 0–4 years born between 1998 and 2007. Of these cases 55% were male, and the highest rate of laboratory reported disease was in children aged <1 year (9·2/1000 person-years) (Table 1). Rotavirus reports showed marked seasonality during the study period. The annual rotavirus season began in November, peaked between February and April and returned to baseline by June. Sixty-five to 70% of all reports occurred between February and April each year.
Table 1. Gender, age and season of birth distribution of reports of laboratory-confirmed rotavirus infections in children aged 0–4 years in England and Wales (1998–2007)
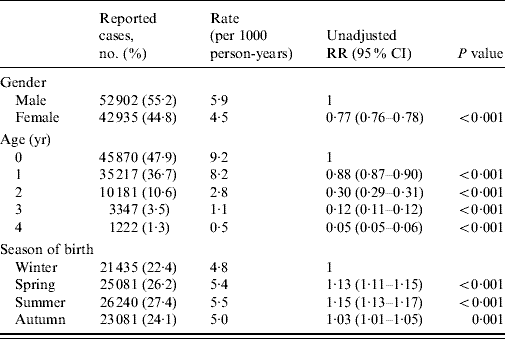
RR, Relative risk; CI, confidence interval.
Male gender, age and season of birth were associated with an increased risk of reported rotavirus diarrhoea (Table 1). In their first year of life, children born in summer had the highest risk of reported disease (12·0/1000 live births), which was more than double that observed for winter births (RR 2·13, 95% CI 2·07–2·19) (Table 2). However, in the second and subsequent years, summer-born children had a lower risk than those born in winter (Table 2). Despite the lower risk of disease from the second to fifth year of life, children born in summer had a higher cumulative risk of disease by age 5 years (RR 1·15, 95% CI 1·13–1·17) compared with winter births (Table 2). All the excess relative risk for summer births occurred during the first year of life. Similar observations were seen for children born in spring and autumn compared with winter births, but with more moderate effects.
Table 2. Age-stratified season of birth-specific risk of reported laboratory-confirmed rotavirus infections in children aged 0–4 years in England and Wales (1998–2007)
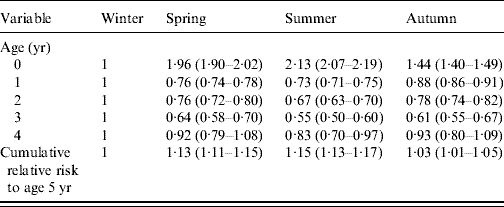
Values given are relative risk (95% confidence interval).
We have found evidence that season of birth partly determines risk of rotavirus disease, particularly in the first year of life. These findings are probably explained by an interaction between the seasonality of the disease in England and Wales and the immunological factors that determine susceptibility and protection to infection.
Children born in winter encounter their first rotavirus season aged 0–3 months. At this age, they are at low risk of developing disease because of high levels of maternal antibody, breastfeeding and limited contact with infectious individuals. Further, if infection does occur at this early age, it is often asymptomatic but still confers some protection against subsequent rotavirus infection and disease [Reference Offit6]. Those born in spring and summer encounter their first rotavirus season aged about 6–11 months, at around the age when maternal antibody levels wane, breastfeeding stops and the potential for exposure to rotavirus is greater due to increased social interaction and increased oral–faecal contact. Thus, in their first year of life, spring and summer births reach the period in the year of highest virus circulation at an age of high exposure, and low maternal antibody protection. The finding that spring and summer births have a lower risk of clinical disease in subsequent years compared with winter births supports previous observations of the protection offered by natural rotavirus infection against subsequent severe disease [Reference Velazquez9]. Winter-born children have the highest risk of clinical disease in the second year of life because the protection afforded by maternal antibody during their first rotavirus season delays the average age of disease in this group.
Summer birth accounted for 3·6% (PAF 3·6%, 95% CI 3·2–4·0) of reported laboratory-confirmed rotavirus infections in our study population. During the study period, the average annual number of reports of laboratory-confirmed rotavirus diarrhoea in <5-year-olds in England and Wales was 13 431. Thus, if the risk of reported rotavirus disease in summer-born infants was the same as that in winter-born infants, an average of 484 reported cases would be prevented.
This analysis is predicated on the assumption that seasonal patterns in incidence of reported, laboratory-confirmed rotavirus infections are representative of seasonal patterns of rotavirus diarrhoea in the population. Reports of laboratory-confirmed rotavirus infections represent only a fraction of cases occurring in the community as only a proportion seek medical attention and, of these, stool specimens are investigated for only a fraction [Reference Wheeler1]. It has been estimated that for every rotavirus case reported to national surveillance there are 1·5 laboratory investigations, 11·3 cases who present to general practice, and 35 community cases [Reference Wheeler1]. If rotavirus testing is less likely to be offered by laboratories outside of the winter months, and summer births are more likely to be infected in winter months during their first year of life, then our analysis could overestimate the frequency of reported disease in summerborn relative to winter-born infants. Further, disease severity is a strong determinant of seeking medical attention [Reference Tam, Rodrigues and O'Brien10]. As a result these cases are more likely to have stool specimens sent and rotavirus-positive specimens reported. If severe disease is more common in children aged <12 months and disease is more common in summer births only in this age group, the season of birth difference in cumulative risk may partly be an artefact of higher reporting of cases in the <1-year group. However, even if winter births do not have overall lower rates of disease, they would tend to have less severe disease, since infections would occur at an older age when risk of severe disease is lower.
The association between season of birth and risk of rotavirus diarrhoea is an important finding. In England and Wales, a successful rotavirus vaccination programme aimed at reducing transmission and disease incidence would need to ensure high coverage rates of spring and summer births. This is because those born in spring and summer are likely to have an important role in transmission of the virus, as they are at higher risk of diarrhoea during their first year of life, and as a consequence are more likely to be infectious to others. Protecting all infants should be the ultimate goal of a vaccination programme, but season of birth effects should be considered. If good coverage can be rapidly achieved in spring and summer births prior to exposure to their first rotavirus season, the programme may have a more immediate impact and a higher initial effectiveness.
These results are likely to be applicable to other temperate countries where rotavirus gastroenteritis is highly seasonal. To date, no other studies have directly addressed this question. Similar studies in other settings, and in settings with different seasonal patterns of rotavirus diarrhoea, would help better define the role of season of birth in determining risk of rotavirus disease.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Medical Research Council to Dr C. J. Atchison. The views and opinions expressed in this paper do not necessarily reflect those of the funding body.
DECLARATION OF INTEREST
None.




