Introduction
To achieve successful establishment, plants have evolved several seed traits that allow them to sense environmental cues that trigger germination in specific temporal windows and particular microhabitats (Grubb, Reference Grubb1977; Ooi et al., Reference Ooi, Denham, Santana and Auld2014). This process, which defines the germination niche of a species, is driven by complex mechanisms involving the interaction of light and temperature conditions (Grubb, Reference Grubb1977; Donohue et al., Reference Donohue, Rubio de Casas, Burghardt, Kovach and Willis2010; Larson and Funk, Reference Larson and Funk2016). For instance, the occurrence of vegetation gaps generates heterogeneous environments with patches differing in physical environmental conditions such as radiation, temperature and soil moisture (Bazzaz and Pickett, Reference Bazzaz and Pickett1980; Chazdon and Fetcher, Reference Chazdon, Fetcher, Medina, Mooney and Vázquez-Yánes1984; Pearson et al., Reference Pearson, Burslem, Mullins and Dalling2002). In these variable environments, plant species usually have several seed attributes that allow them to cope with or avoid stressful environmental conditions (e.g., large seed mass, low seed moisture content and the presence of dormancy; Daws et al., Reference Daws, Crabtree, Dalling, Mullins and Burslem2008; Carta et al., Reference Carta, Skourti, Mattana, Vandelook and Thanos2017). The main drivers of plant species ecology are related to these seed functional traits (Saatkamp et al., Reference Saatkamp, Cochrane, Commander, Guja, Jimenez-Alfaro, Larson, Nicotra, Poschlod, Silveira, Cross, Dalziell, Dickie, Erickson, Fidelis, Fuchs, Golos, Hope, Lewandrowski, Merritt, Miller, Miller, Offord, Ooi, Satyanti, Sommerville, Tangney, Tomlinson, Turner and Walck2019), which characterize a particular plant species and allow us to understand their distributional range in natural and semi-natural habitats (Luna et al., Reference Luna, Moreno, Cruz and Fernandez-Gonzalez2007, Luna and Moreno, Reference Luna and Moreno2010; Cochrane et al., Reference Cochrane, Hoyle, Yates, Wood and Nicotra2014, Marques et al., Reference Marques, Atman, Silveira and de Lemos-Filho2014; Cochrane, Reference Cochrane2019; Kazancı and Tavşanoğlu, Reference Kazancı and Tavşanoğlu2019).
The genus Manihot is native to the tropics of the New World, particularly Brazil and Mexico (Nassar et al., Reference Nassar, Hashimoto and Fernandes2008). In dry environments, Manihot species grow in habitats previously disturbed by fire (Duputié et al., Reference Duputié, Salick and McKey2011). Successful regeneration of these plant species is frequently related to different seed adaptations for promoting germination and establishment in open areas created after a disturbance (Rogers and Appan, Reference Rogers and Appan1973; Pujol et al., Reference Pujol, Gigot, Laurent, Pinheiro-Kluppel, Elias, Hossaert-McKey and McKey2002; Nassar et al., Reference Nassar, Hashimoto and Fernandes2008). For instance, seeds of many Manihot species show physical and/or physiological dormancy (PY and PD, respectively), which can be broken in response to heat shocks. These environmental conditions can be generated by vegetation gaps due to canopy opening or after a fire event (Nassar and Teixeira, Reference Nassar and Teixeira1983; Drennan and van Staden, Reference Drennan and Van Staden1992; Ospina et al., Reference Ospina, Gómez and Orozco2000; Pujol et al., Reference Pujol, Gigot, Laurent, Pinheiro-Kluppel, Elias, Hossaert-McKey and McKey2002). In addition, the low seed moisture content (7.9–10% MC) recorded in some Manihot species (Ellis et al., Reference Ellis, Hong and Roberts1981; Becwar et al., Reference Becwar, Stanwood and Leonhardt1983) has been considered an adaptation to tolerate high ambient temperatures (Leopold and Vertucci, Reference Leopold and Vertucci1989; Le Fer and Parker, Reference Le Fer and Parker2005; Ruprecht et al., Reference Ruprecht, Lukács, Domokos, Kuhn and Fenesi2016). Altogether, the evidence suggests that the regeneration niche (sensu Grubb, Reference Grubb1977) of Manihot species might have evolved to promote successful establishment in environments subjected to periodic disturbances (Pujol et al., Reference Pujol, Gigot, Laurent, Pinheiro-Kluppel, Elias, Hossaert-McKey and McKey2002). Nevertheless, in some Manihot species, in the absence of a disturbance, low temperatures can also stimulate the germination behaviour that might allow a successful establishment in the understory (Nassar and Teixeira, Reference Nassar and Teixeira1983; Adjata et al., Reference Adjata, Tchaniley, Banla, Tchansi and Gumedzoe2013).
One of the two species reaching the southernmost distribution of this genus is Manihot grahamii Hook., which grows mainly in vegetation gaps of disturbed shrublands and forests (Duputié et al., Reference Duputié, Salick and McKey2011). In central Argentina, the southernmost distribution of this plant species, some authors claim that M. grahamii has been introduced as an ornamental (Giorgis and Tecco, Reference Giorgis and Tecco2014). However, the expansion of the southern range of this plant species in South America could also have occurred naturally (Duputié et al., Reference Duputié, Salick and McKey2011). In this sense, although this species has been defined as a native invasive in central Argentina (Giorgis and Tecco, Reference Giorgis and Tecco2014), it could also be considered a neonative species (i.e., species whose range expansion into new regions is driven mainly by human-induced environmental changes sensu Essl et al., Reference Essl, Dullinger, Genovesi, Hulme, Jeschke, Katsanevakis, Kühn, Lenzner, Pauchard, Pyšek, Rabitsch, Richardson, Seebens, van Kleunen, van Der Putten, Vilà and Bacher2019). Thus, its range expansion may be related to some particular features of its germination niche, which could allow this plant species to adapt to the anthropogenic ecosystem transformations. In central Argentina, the high rate of forest fragmentation in Chaco and Espinal ecoregions during the 20th century (Schofield and Bucher, Reference Schofield and Bucher1986; Fehlenberg et al., Reference Fehlenberg, Baumann, Gasparri, Piquer-Rodriguez, Gavier-Pizarro and Kuemmerle2017; Muñoz Garachana et al., Reference Muñoz Garachana, Aragón and Baldi2018), and the high fire incidence recorded in the mountain Chaco Forest (Argañaraz et al., Reference Argañaraz, Radeloff, Bar-Massada, Gavier, Scavuzzo and Bellis2015; Argañaraz et al., Reference Argañaraz, Cingolani, Bellis and Giorgis2020) might have allowed M. grahamii seeds to colonize newly disturbed areas. Relying on all the evidence reported for this genus, we aimed to identify if some seed traits and germination requirements of M. grahamii (i.e., germination niche) could help to understand its successful establishment in disturbed environments commonly found in the species’ southernmost distribution. Thus, we predict that some seed traits of this species would be related to a heat-tolerant strategy, such as low seed moisture content, and that high ambient temperatures would stimulate germination and break seed dormancy. Moreover, the characterization of the germination niche can be a powerful indicator of the species sensitivity to global change (Walck et al., Reference Walck, Hidayati, Dixon, Thompson and Poschlod2011; Baskin and Baskin, Reference Baskin and Baskin2022).
The germination ecology of M. grahamii is poorly known, both in terms of the presence of dormancy and the response of seeds to different temperatures and light conditions. Thus, in this work, we evaluated the germination ecology of the seeds of M. grahamii with the aims to: (1) characterize seed traits (viability, mass and moisture content); (2) determine whether it has dormancy and if it is physical or physiological; (3) evaluate the effect of several pre-treatments (gibberellic acid, after ripening, dry prechilling and dry prechilling + warm) on seed dormancy; and (4) assess the effect of different environmental events of high-temperatures on the germination process simulating two treatments: fire intensities (with three levels of heat shock) and a gap temperature. The characterization of seed functional traits and germination requirements of M. grahamii will allow us to expand the knowledge about the germination niche and the regeneration ecology of Manihot species. In addition, this knowledge can be useful to understand and discuss if the geographical expansion of the species in disturbed environments could be associated with its germination characteristics.
Materials and methods
Study area and species description
The study area is located in the Chaco Serrano Forest in the Mountains of Córdoba province, central Argentina. The study area presents a warm temperate climate with dry winter and hot summer according to Köppen–Geiger classification (Kottek et al., Reference Kottek, Grieser, Beck, Rudolf and Rubel2006). The mean annual precipitation is 959 mm (Whitworth-Hulse et al., Reference Whitworth-Hulse, Magliano, Zeballos, Gurvich, Spalazzi and Kowaljow2020) and the mean annual temperature is 17.5 °C (De Fina, Reference De Fina1992). Rainfall is concentrated mainly in the warm season with 80–85% of the rain falling between November and March (Whitworth-Hulse et al., Reference Whitworth-Hulse, Magliano, Zeballos, Gurvich, Spalazzi and Kowaljow2020).
M. grahamii occurs naturally in southeastern Brazil, northern Argentina, Paraguay, and Uruguay. This species is a heliophile arborescent shrub with a dense crown that can reach up to 7 m in height. M. grahamii is occasionally used as an ornamental and in botanical gardens. The fruit is a subspherical three-carpellate capsule, with dehiscence septicidal (Rogers and Appan, Reference Rogers and Appan1973). Seeds have a visible raphe and a small, light, coffee-coloured caruncle located on the micropylar region (Rogers and Appan, Reference Rogers and Appan1973). This species has a two-phase dispersal process: first, their seeds are ballistically dispersed and then, they are dispersed and buried on the ground by ants (Rival and McKey, Reference Rival and McKey2008; Duputié et al., Reference Duputié, Salick and McKey2011).
Seed collection
Seeds of M. grahamii were collected from 10 plants of a population growing in Paravachasca valley (31° 43′ 04.48″S; 64° 28′49.20″W), central Argentina. Under each focal tree, the vegetation cover was removed and a fragment of sheet (2 m × 2 m) was spread under the trees. Naturally shed seeds on sheets were collected daily. Seeds were cleaned and stored dry in paper bags at room temperature, until the germination trials were conducted. Mature seeds were collected from 20 March to 15 April 2019 because fruit ripening was asynchronous, and the germination experiments started in May 2019.
Seed traits: viability, mass and moisture content
Initial viability of seeds (five replicates of 20 seeds each) was assessed using the topographical tetrazolium test (ISTA, 2014). Before the test, to allow the tetrazolium solution to easily enter the seed and stain the tissues, the seed coats were pierced using a sharp scalpel, avoiding possible damage to the micropyle zone. Then, seeds were completely immersed in a tetrazolium solution at a concentration of 1%, at 25 °C in dark conditions, during 24 h. Finally, seed coloration was assessed according to the methodology proposed by Ospina et al. (Reference Ospina, Gómez and Orozco2000).
Seed mass was determined by weighing 100 seeds without caruncle using a precision balance (0.1 mg, Shimadzu AUW220D, Shimadzu Latin America, Montevideo, Uruguay). Seed moisture content was calculated using 100 seeds of freshly harvested fruits. Seeds were initially weighed (i.e., fresh weight, FW) using a precision digital balance (0.1 mg). Then, seeds were placed on sand in an aluminium container and oven dried at 103 °C for 17 h (ISTA, 2014). After that, seeds were weighed again (i.e., dry weight, DW). Seed moisture content percentage was calculated as = ((FW – DW)/FW) * 100 (Baskin et al., Reference Baskin, Davis, Baskin, Gleason and Cordell2004).
Seed water imbibition test
A water imbibition experiment was performed to assess seed coat permeability to water (i.e., physical dormancy; PY). Three treatments were considered: non-scarified seeds with caruncle (control), non-scarified seeds without caruncle and scarified seeds with caruncle. Treatments with and without caruncle were performed to explore if the caruncle affects the seed imbibition process. In some species of Euphorbiaceae, the caruncle has the capacity to absorb and temporarily retain water, then pass it to the rest of the seed (Lagoa and Pereira, Reference Lagoa and Pereira1987; Pacini, Reference Pacini1990). Seeds were scarified smoothly with sandpaper on the opposite side of the caruncle until the endosperm was observed. Four replicates of 20 seeds each were placed in Petri dishes on filter paper moistened with distilled water and then incubated in a chamber at 25 °C under constant light. After 0 (i.e., initial seed weight), 1, 2, 4, 24, 48 and 72 h, the surface of the seeds was blotted dry; the seeds were weighed with a precision balance (0.1 mg) and then returned to the moist filter paper in the Petri dishes. The amount of water uptake was determined as the actual increase in seed weight and converted to percentage (Baskin et al., Reference Baskin, Davis, Baskin, Gleason and Cordell2004).
Germination experiments
After seed collection, from the main seed lot, seven seed subsamples were fractionated to carry out the different germination tests to evaluate: (1) the germination of freshly collected seeds, (2) the effect of pre-treatments on seed dormancy (i.e., gibberellic acid, after ripening, dry prechilling, dry prechilling + warm) and (3) assess the effect of different environmental events of high temperatures on the germination process simulating two treatments: fire intensities (with three levels of heat shock) and a gap temperature.
First, the germination of freshly collected seeds was tested (hereafter ‘control’). Seeds were set to germinate at three alternating temperatures (15/5 °C, 25/15 °C and 35/20 °C) and a 12/12 h daily photoperiod and under continuous darkness for 30 days. These temperatures approximate those that could occur at Córdoba in winter - autumn, spring and summer, respectively (De Fina, Reference De Fina1992). Germination chambers were equipped with fluorescent tubes of 20 W cool white lights with a photon flux density (400–700 nm) of about 38 μm m−2 s −1. Seeds were placed in Petri dishes on filter paper. Four Petri dishes, with 20 seeds each, were used for each germination assay (but see also after ripening). Filter papers were kept moist during the experiment by adding distilled water as required. Germination was recorded every 2 days. We evaluated the effect of permanent dark on the germination process and its interaction with alternating temperatures on each treatment because in this genus the secondary dispersal of seeds by ants is quite common (Duputié et al., Reference Duputié, Salick and McKey2011). Darkness was achieved by wrapping Petri dishes with two layers of aluminium foil. Once a week, germination was recorded using a green safelight (Baskin et al., Reference Baskin, Thompson and Baskin2006). Radicle emergence was the criterion used to consider a seed has germinated; after that, the seed was removed from the Petri dish. This methodology was also followed after applying each pre-treatment.
Seed dormancy-breaking pre-treatments
Despite M. grahamii is a heliophile species, it has been observed growing in the forest and shrublands understory in the absence of any disturbance (Giorgis and Tecco, Reference Giorgis and Tecco2014) as other species of this genus (Nassar and Teixeira, Reference Nassar and Teixeira1983; Adjata et al., Reference Adjata, Tchaniley, Banla, Tchansi and Gumedzoe2013). Hence, we decided to test the effect of winter (dry prechilling) and winter plus spring temperatures (dry prechilling + warm) on germination. Both treatments were performed at dry conditions because in our study area rainfall is largely concentrated in the warm season, from November to March, and winter and at least half of spring are dry (De Fina, Reference De Fina1992; Whitworth-Hulse et al., Reference Whitworth-Hulse, Magliano, Zeballos, Gurvich, Spalazzi and Kowaljow2020). Furthermore, the gibberellic acid treatment was selected to test how deep could be the PD, while the after ripening treatment was performed because it is well known to alleviate physiological dormancy (Baskin and Baskin, Reference Baskin and Baskin2004) and also it promotes germination of many species from dry regions.
The effect of GA3 on germination was determined by imbibing seeds in a solution of GA3 at a concentration of 200 ppm at 25 °C in darkness, and for a 48 h period. Then, seeds were washed with distilled water before the start of germination experiments.
During 3 months, one seed subsample was stored dry in a paper bag at constant room temperature (ca. 25 °C) (i.e., after ripening), while two other seed subsamples were dry stored in a refrigerator at 5 °C in darkness (i.e., dry prechilling). After this period, one of the seed subsamples was taken to an incubation chamber at 25/15 °C in darkness for an additional month at dry conditions (i.e., dry prechilling + warm treatment), while the other two seed subsamples (i.e., after ripening and dry prechilling) were placed to germinate in the alternating temperatures. Regarding the after ripening pre-treatment, germination was assessed only under light conditions and on three replicates with 20 seeds each, due to a lack of seeds. Moreover, this experiment was performed under light conditions because of the results obtained in the germination test of freshly collected seeds (see below).
Heat-shock and gap temperature pre-treatments
These pre-treatments were selected according to the information available on the germination and population dynamics of the species of the genus Manihot. In this sense, ecological conditions of extreme temperature events such as those generated by fire (i.e., heat-shock pre-treatment) and vegetation gaps (i.e., gap temperature pre-treatment), would influence the germination of M. grahamii. For these pre-treatments, seeds were placed in an aluminium container with sand in a forced-air oven. The heat-shock experiment consisted of three pre-treatment levels to simulate different fire intensities: low (90 °C during 5 min), medium (120 °C during 5 min) and high (120 °C during 20 min). These pre-treatment levels represent a mixture of potential exposure times and temperatures that seeds might experience on the soil surface during fires, according to previous evidence (Ferreras et al., Reference Ferreras, Funes and Galetto2015; Jaureguiberry and Díaz, Reference Jaureguiberry and Díaz2015; Venier et al., Reference Venier, Cabido and Funes2017; Moreschi et al., Reference Moreschi, Funes, Zeballos and Tecco2019). For each of the different pre-treatment levels we calculated the heat index (H) proposed by Paula and Pausas (Reference Paula and Pausas2008), as follows: H = T * ln (t + 1), where T is the temperature (°C) to which the seeds were exposed, ln is the natural logarithm and t is the exposure time in minutes. In our study, the H takes the values of 161.2 (low fire intensity), 215.0 (medium) and 365.3 (high).
The gap temperature pre-treatment was performed to simulate the temperature on the soil surface during gap formation (Pujol et al., Reference Pujol, Gigot, Laurent, Pinheiro-Kluppel, Elias, Hossaert-McKey and McKey2002). For this treatment, seeds were dry stratified, for 10 days, in a forced-air oven at 40 °C.
Data analysis
For germination of freshly collected seeds (i.e., control) and all the germination tests performed after the pre-treatments, the germination process was characterized by measuring the germination percentage, time to the onset of germination (T on), mean germination time (MGT) and relative light germination index (RLG; Milberg et al., Reference Milberg, Andersson and Thompson2000). RLG index ranges from zero to one for germination occurring only in dark or only in light conditions, respectively. Values lower than 0.25 indicate that seeds are negatively photoblastic, values between 0.25 and 0.75 indicate that seeds are light insensitive, while values higher than 0.75 indicate that seeds are positively photoblastic. All of these seed parameters were calculated for each alternating temperature and light condition whenever possible. T on and MGT was calculated only for light conditions because germination was recorded only once a week in darkness (Bewley et al., Reference Bewley, Bradford and Hilhorst2013; Baskin and Baskin, Reference Baskin and Baskin2014), whereas the RLG index was calculated only in those germination assays that showed a germination percentage higher than 50% on any of lights conditions (Ranal and Santana, Reference Ranal and Santana2006; D'Agostino et al., Reference D'Agostino, Gurvich, Ferrero, Zeballos and Funes2012; Bewley et al., Reference Bewley, Bradford and Hilhorst2013).
Generalized linear models (GLMs) with a quasibinomial error structure and logit link were performed to analyse the differences in water imbibition percentage between scarified and non-scarified seeds. GLMs were also performed to analyse the effect of pre-treatments, alternating temperature, light conditions and their interactions (i.e., fixed factors). Independent analyses were performed for control and for each pre-treatment, but control was included as a level factor in GLMs for each pre-treatment. A quasibinomial distribution for fitting under-dispersed data was used. When significant differences were observed among pre-treatments, a post-hoc Tukey test was performed for multiple comparisons using the ‘emmeans’ package and ‘lsmeans’ function. All analyses were conducted using the open-source software R 4.1.1 (R Development Core Team, 2022).
Results
Seed traits: viability, mass and moisture content
Seeds of M. grahamii showed 100 ± 0.00% (mean ± standard deviation) of viability in the five replicates. Seed mass was 0.24 ± 0.04 g, and seed moisture content was 8.06 ± 0.06%.
Water uptake of non-scarified and scarified seeds was similar (Fig. 1). Regardless of the treatment considered, seed mass percentage increased, suggesting that the seed coat of M. grahamii is permeable to water. However, significant differences among treatments were observed (χ 2 = 62.65; p < 0.001), which could be explained by the absence of the caruncle in one of the treatments (Fig. 1).

Figure 1. Imbibition curves for different treatments of M. grahamii seeds: non-scarified seeds with caruncle, non-scarified seeds without caruncle and scarified seeds with caruncle. Error bars indicate standard deviation.
Germination of freshly collected seeds
Germination was higher under dark than under light conditions. In fact, under light conditions, germination was observed in a low percentage (5%) and only at 35/20 °C (Fig. 2A). The highest germination was observed at 35/20 °C (ca. 60%) under darkness, with significant differences from the other two alternating temperatures (Fig. 2A; Supplementary Table S1). Furthermore, no significant term was observed for the interaction between alternating temperatures and light conditions (Supplementary Table S1). T on, MGT and RLG were calculated only for the highest alternating temperature (Table 1). The RLG values observed in freshly collected seeds were related to a negatively photoblastic behaviour of seeds (Table 1).

Figure 2. (A) Effect of gibberellic acid (GA3); (B) after ripening (AR); (C) dry prechilling (DPC); and (D) dry prechilling + warm (DPC + W) pre-treatments on germination of M. grahamii seeds under different alternating temperatures (15/5 °C, 25/15 °C and 35/20 °C) and light conditions (light and darkness). Error bars indicate the standard deviation. For GA3 pre-treatment (A) the same letters on the bars indicate non-significant differences (p = 0.05) between treatments (GA3 and control). For after ripening pre-treatment (B), the same letters on the bars indicate non-significant differences (p = 0.05) between alternating temperatures. For dry prechilling pre-treatment (C), the same letters on the bars indicate non-significant differences (p = 0.05) in the interaction between treatments (DPC and control) and light conditions. For dry prechilling + warm pre-treatment (D), the same letters on the bars indicate non-significant differences (p = 0.05) in the interaction between treatments (DPC + W and control) and alternating temperatures.
Table 1. Mean (± standard deviation) of time to the onset of germination (T on), mean germination time (MGT), and relative light germination index (RLG) for freshly collected seeds (i.e., control) and all the applied seed pre-treatments. The results are presented only for 35/20 °C and 25/15 °C alternating temperatures (T), due to the null germination values at 15/5 °C. High heat intensity was not included because none seed germination was scored.

Seed dormancy-breaking pre-treatments
All the dormancy-breaking pre-treatments showed a significant effect (see Supplementary Table S2–S5) on germination percentages at the different alternating temperatures and light conditions considered (Fig. 2A–D). Furthermore, no germination was observed at 15/5 °C for any of the dormancy-breaking pre-treatments (Fig. 2A–D).
Germination following seed pre-treatment with GA3 differed significantly from control (Supplementary Table S2). The GA3 pre-treatment showed a reduction in germination percentages under all light conditions and alternating temperatures compared to the control, but this effect seemed higher under dark conditions (Fig. 2A). After ripening showed a slightly higher germination percentage than control at high and medium alternating temperatures (Fig. 2B), but this effect was not significant (Supplementary Table S3).
The dry prechilling pre-treatment showed a significant interaction term with light condition (Supplementary Table S4), with an increase in germination percentages under light condition, but without significant differences from control (Fig. 2C). The dry prechilling + warm pre-treatment showed a significant interaction with alternating temperatures and light conditions (Supplementary Table S5). This seed pre-treatment promoted a significantly higher germination percentage than control under light and dark conditions at medium and high alternating temperatures (Fig. 2D).
The measurements related to germination time (i.e., T on and MGT) were affected in different degrees according to the seed dormancy-breaking pre-treatment applied (Table 1). In general, these pre-treatments caused a slight delay in the T on and an increase in MGT compared with control (Table 1). However, the dry prechilling + warm accelerated the T on (Table 1). The RLG index was calculated only for the dry prechilling and dry prechilling + warm pre-treatments because the other dormancy-breaking pre-treatments showed germination percentages lower than 50%. At the highest alternating temperature, the dry prechilling and dry prechilling + warm pre-treatments showed germination values corresponding to light indifferent germination behaviour, whereas at the medium alternating temperature, seeds were still negatively photoblastic (Table 1).
Heat-shock and gap temperature pre-treatments
Heat-shock and gap temperature pre-treatments showed a significant triple interaction term with alternating temperatures and light conditions (Supplementary Table S6 and S7, respectively). No germination was recorded at 15/5 °C in either heat-shock and gap temperature pre-treatments (Fig. 3A, B). Moreover, regarding the highest heat intensity treatment (Fig. 3A) we detected no germination and all the seeds were dead at the end of the experiment. At the highest alternating temperature (i.e., 35/20 °C), the medium and low heat intensity pre-treatments showed a significant and higher germination percentage than control under light conditions. Nonetheless, similar values were recorded under dark conditions. At the medium alternating temperature (i.e., 25/15 °C), no germination was observed in any of the simulated heat intensity pre-treatments under light conditions (Fig. 3A). However, after the low heat-shock pre-treatment, the germination was significantly higher than control at the medium alternating temperature in darkness (Fig. 3A).
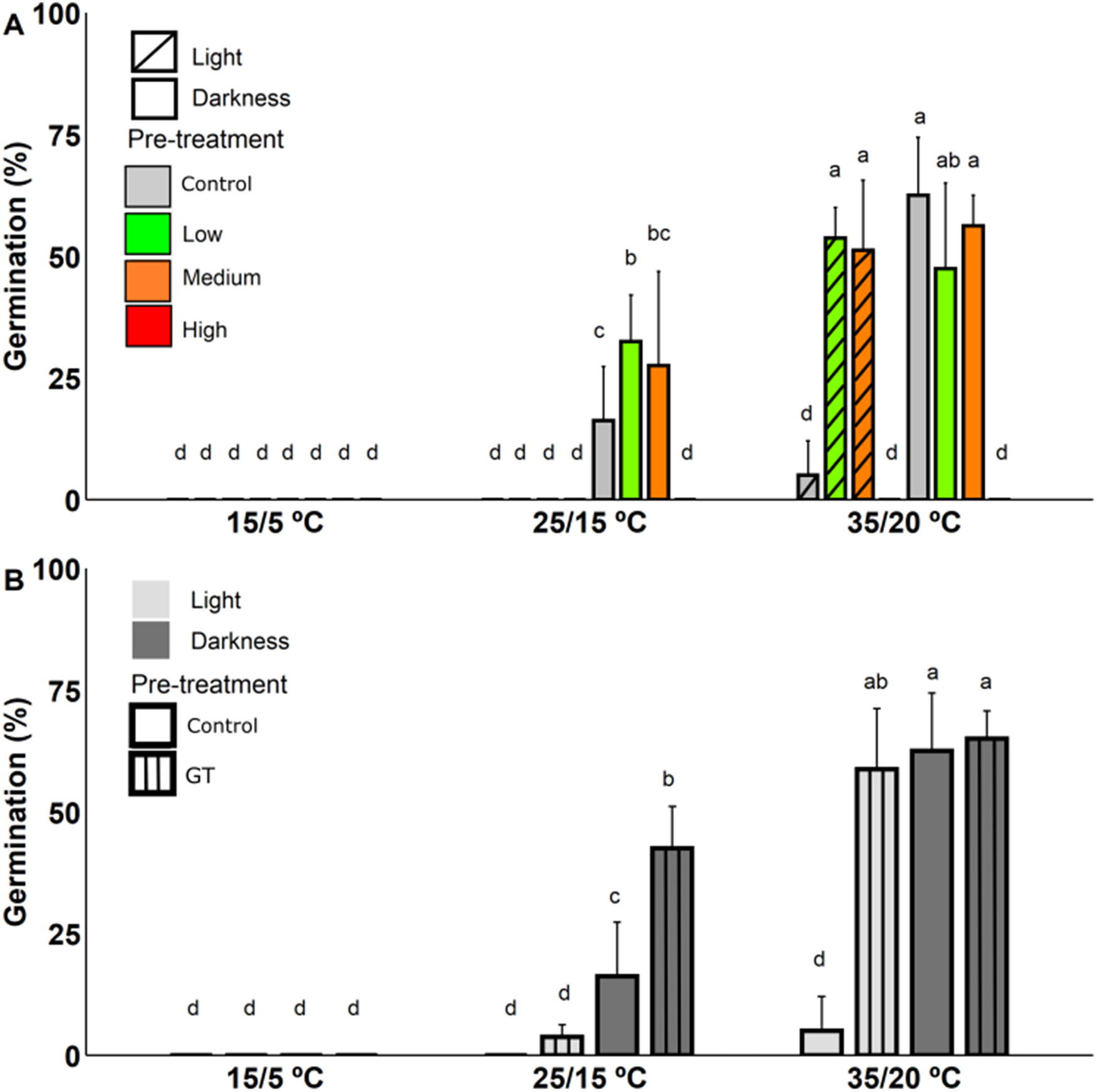
Figure 3. Effect of (A) heat-shock (low, medium and high); and (B) gap temperature (GT) on M. grahamii seed germination under different alternating temperatures (15/5 °C, 25/15 °C and 35/20 °C) and light conditions (light and darkness). Error bars indicate the standard deviation. For the heat-shock and gap temperature seed pre-treatments (A, B, respectively), the same letters on the bars indicate non-significant differences (p = 0.05) in the interaction between treatments (heat-shock and control; gap temperature and control), alternating temperatures and light conditions.
The gap temperature and the heat-shock (i.e., medium and low heat intensity) pre-treatments had a similar effect on germination percentage. Thus, for the gap temperature pre-treatment germination values showed a significant increase compared to the control at the highest alternating temperature under light conditions, and at the medium alternating temperature under darkness (Fig. 3B).
Both heat-shock and gap temperature seed pre-treatments showed an effect on germination time (i.e., T on and MGT). The heat-shock pre-treatment caused a slight delay in T on and an increase in MGT compared with control (Table 1). On the contrary, gap temperature pre-treatment accelerated T on at the highest alternating temperature (Table 1). This effect was stronger than the effects of any of the seed pre-treatments performed. The RLG showed values related to a light indifferent germination behaviour at the highest alternating temperature, while seeds were still negatively photoblastic at medium alternating temperature (Table 1).
Discussion
The high seed viability percentage observed and the almost exclusive germination recorded under darkness for freshly collected seeds suggest that the pioneer woody species M. grahamii has seeds with a negatively photoblastic behaviour. Furthermore, the germination of freshly collected seeds showed a narrow temperature range, i.e., germination occurred almost exclusively at 35/20 °C and with values that did not exceed 60%. In addition, the seed light-dark requirement for germination changed after the application of seed pre-treatments (Moyo et al., Reference Moyo, Kulkarni, Finnie and Van Staden2009; Baskin and Baskin, Reference Baskin and Baskin2014). These results suggest that this species might have physiological dormancy (i.e., non-deep simple physiological dormancy Type 2; Baskin and Baskin, Reference Baskin and Baskin2014). Regarding the different treatments considered to alleviate seed dormancy used in this study, we observed that mostly dry prechilling + warm, as well as the heat-shock and gap temperature seed pre-treatments, were involved in the physiological dormancy-breaking process of M. grahamii seeds. The effect of these pre-treatments widened the temperature range and light conditions under which the seeds can germinate, i.e., expanded its germination niche (Soltani et al., Reference Soltani, Baskin and Baskin2017; Cochrane, Reference Cochrane2019). In particular, the gap temperature pre-treatment had the strongest effect on the onset and speed of germination, since seeds germinated earlier and faster than seeds under the effect of the other pre-treatments performed. Moreover, the low seed moisture content (ca. 8%) and large seed mass (ca. 0.24 g) of M. grahamii observed in this study might be related to heat tolerance and seed burial (see below). Altogether, the germination response observed in M. grahamii seeds that are exposed to cold plus warm environmental conditions or to a sudden event of high temperatures, would promote secure germination timing into the rainy season on undisturbed habitats. Moreover, this response would be related with a cue for competition-released gaps, which in turn could favour seedling recruitment in open, disturbed and dry habitats, respectively (Thanos et al., Reference Thanos, Georghiou, Douma and Marangaki1991; Bell et al., Reference Bell, Plummer and Taylor1993; Daws et al., Reference Daws, Crabtree, Dalling, Mullins and Burslem2008; Tangney et al., Reference Tangney, Merritt, Fontaine and Miller2019, Reference Tangney, Merritt, Callow, Fontaine and Miller2020), as occurs in other species of Manihot (Pujol et al., Reference Pujol, Gigot, Laurent, Pinheiro-Kluppel, Elias, Hossaert-McKey and McKey2002; Duputié et al., Reference Duputié, Salick and McKey2011; Carta et al., Reference Carta, Skourti, Mattana, Vandelook and Thanos2017).
As it has been described for other Manihot species (Ospina et al., Reference Ospina, Gómez and Orozco2000; Pujol et al., Reference Pujol, Gigot, Laurent, Pinheiro-Kluppel, Elias, Hossaert-McKey and McKey2002), seeds of M. grahamii did not show PY. Seeds of this species imbibed both in scarified and non-scarified treatments. However, when seeds imbibed, their mass increased in a low percentage after 72 h (less than 20%). This low seed water uptake could be related to lipids as one of the major storage reserves in seeds of Manihot species and in the Euphorbiaceae family (Nartey et al., Reference Nartey, Møller and Andersen1974; Suda and Giorgini, Reference Suda and Giorgini2000; Can and Küçüker, Reference Can and Küçüker2015). Therefore, since lipids have a low osmolality compared to oligosaccharides, the high seed matric potential component could limit seed water uptake (Bewley et al., Reference Bewley, Bradford and Hilhorst2013). On the other hand, this mechanism could constrain the diffusion of GA3 solution into the seed, explaining the lack of positive effects of the GA3 pre-treatment on physiological dormancy breaking. Similar results were found in other studies performed with Manihot species (Ospina et al., Reference Ospina, Gómez and Orozco2000). Nonetheless, as it was suggested in Ospina et al. (Reference Ospina, Gómez and Orozco2000), the GA3 concentration used might not have been enough to alleviate seed dormancy.
Other tropical and subtropical species that have the same type of PD as we found in seeds of M. grahamii (non-deep PD) come out of dormancy when they are exposed to an event of high temperature typically found in gaps or after a fire event (Pujol et al., Reference Pujol, Gigot, Laurent, Pinheiro-Kluppel, Elias, Hossaert-McKey and McKey2002; Rival and McKey, Reference Rival and McKey2008). However, in the absence of a disturbance, germination of some Manihot species seems to be triggered by a period of cold and/or daily temperatures higher than 25 °C (Nassar and Teixeira, Reference Nassar and Teixeira1983; Adjata et al., Reference Adjata, Tchaniley, Banla, Tchansi and Gumedzoe2013). In this sense and according to our results, if seeds of M. grahamii are on the soil surface on undisturbed sites, their germination percentages would increase when seeds are exposed to a period of dry prechilling (not significant effect). But, if seeds were exposed to a period of mild temperatures after the dry prechilling, as those recorded in spring, germination percentages would increase notably, especially under light conditions. Furthermore, after ripening seemed to have a limited effect on the germination processes of M. grahamii, which highlights the role of cold plus warm temperatures in alleviating dormancy. When seeds are buried, the effect of dry prechilling would not increase germination, since our results showed no significant differences in germination from seeds freshly collected that were incubated under darkness. Conversely, dry prechilling + warm increased germination percentages significantly in medium and high alternating temperatures under dark conditions. These results highlight the role of warm temperatures (i.e., spring temperatures) in combination with cold temperatures to alleviate seed dormancy, allowing this species to germinate in the absence of any disturbance (e.g., under closed canopy). Moreover, although the initial seed viability was 100%, germination was never higher than ca. 80% in any of the germination experiments performed. Thus, it would be necessary to explore the effect of cold or warm stratification on seed germination as other mechanisms to alleviate seed dormancy in M. grahamii. In this sense, cold or warm stratification (i.e., wet storage) could play a more important role in stimulating germination than dry storage (Baskin and Baskin, Reference Baskin and Baskin2014; Rezvani et al., Reference Rezvani, Zaefarian and Amini2014), considering that the distribution range of M. grahamii includes wetter habitats than those in the southernmost distributional range (see DryFlor database), where the present study was performed. Furthermore, in future studies the effects of dry storage (i.e., after ripening) under dark conditions must be explored.
The results of the treatments that simulated an event of high temperature suggest that seeds of M. grahamii are heat tolerant, as many plant species of the Espinal and Chaco ecoregions of central Argentina (Jaureguiberry and Díaz, Reference Jaureguiberry and Díaz2015; Arcamone and Jaureguiberry, Reference Arcamone and Jaureguiberry2018; Roca et al., Reference Roca, Jaureguiberry and Gurvich2021). Nevertheless, although all seeds died in response to the seed pre-treatment simulating the highest fire intensity, seeds were able to tolerate simulated fires of low to moderate temperatures. Since this range of fire temperatures is common in nature, fire severity commonly varies during a fire (Gómez-González and Cavieres, Reference Gómez-González and Cavieres2009; Loudermilk et al., Reference Loudermilk, O'Brien, Mitchell, Cropper, Hiers, Grunwald, Grego and Fernandez-Diaz2012). Thus, different microsites can coexist in which some seeds might remain viable and germinate after a fire (Lipoma et al., Reference Lipoma, Funes and Díaz2018). Moreover, the gap temperature pre-treatment, which simulates the effect of summer soil temperature when the vegetation is opened in Chaco Forests, showed the highest germination velocity. In fact, the MGT values in this pre-treatment were 50% faster than those obtained in the heat-shock seed pre-treatments. These differential seed germination responses to high-temperature pre-treatments suggest that the seed heat tolerance of this species is not necessarily linked to fire (Jaureguiberry and Díaz, Reference Jaureguiberry and Díaz2015). Furthermore, the low seed moisture content (ca. 8%) not only increases the ability of seeds to survive the high temperatures recorded in soils during fire (Tangney et al., Reference Tangney, Merritt, Fontaine and Miller2019), but also has been associated with a seed desiccation-tolerant (orthodox) strategy that allows seeds to survive desiccation in arid and semiarid ecosystems like Espinal and Chaco Forest (Tweddle et al., Reference Tweddle, Dickie, Baskin and Baskin2003; Lan et al., Reference Lan, Xia, Wang, Liu, Zhao and Tan2014). These germination traits observed for M. grahamii would allow this plant species to have a rapid response to cope with high temperatures such as those recorded in gaps.
The photoinhibition of seed germination has been related to a physiological adaptation to avoid germination on the soil surface, with the consequent formation of a soil surface seed bank (Carta et al., Reference Carta, Skourti, Mattana, Vandelook and Thanos2017). In fact, the negative response of germination to light observed in some Manihot species was related to the importance of seed burial in seed germination ecology (Pujol et al., Reference Pujol, Gigot, Laurent, Pinheiro-Kluppel, Elias, Hossaert-McKey and McKey2002; Duputié et al., Reference Duputié, Salick and McKey2011). As many species of the Euphorbiaceae family, M. grahamii seeds reach short distances through ballistic dispersal and then they are secondarily dispersed by ants (Elias and McKey, Reference Elias and McKey2000; Vander Wall and Longland, Reference Vander Wall and Longland2004). Photoinhibition seems to avoid seed germination at or near the soil surface until seeds are buried in the soil by ants. In this sense, M. grahamii has a caruncle, a small seed appendix that could be related to ant-mediated secondary dispersal (i.e., caruncle; Rogers and Appan, Reference Rogers and Appan1973). In our study area, the seeds of M. grahamii were carried by Amoimyrmex striatus (Mariana Pereyra personal observation) (Fig. 4A,B). This interaction could promote seed burial, which would protect the seeds not only from surface foraging predators, but also from lethal temperatures that may occur on the soil surface during fire (Espadaler and Gómez, Reference Espadaler and Gómez1996; Renard et al., Reference Renard, Schatz and McKey2010; Baskin and Baskin, Reference Baskin and Baskin2014). Furthermore, M. grahamii has a large seed mass compared not only with the seed mass of most plant species of the plant communities of the study region (Funes et al., Reference Funes, Díaz and Venier2009) but also with the seed mass of plants worldwide (Carta et al., Reference Carta, Skourti, Mattana, Vandelook and Thanos2017). This seed trait (i.e., seed mass) has been related to maximum emergence depth, with heavier seeds being able to emerge from a significantly greater depth and showing faster radicle growth rates than lighter seeds (Daws et al., Reference Daws, Crabtree, Dalling, Mullins and Burslem2008; Long et al., Reference Long, Gorecki, Renton, Scott, Colville, Goggin, Commander, Westcott, Cherry and Finch-Savage2015; Tangney et al., Reference Tangney, Merritt, Callow, Fontaine and Miller2020). However, different studies have shown that small seeds are buried easily and are incorporated into the soil more quickly than larger ones (van Tooren, Reference van Tooren1988; Chambers et al., Reference Chambers, MacMahon and Haefner1991). Therefore, as M. grahamii seeds do not seem to be easily buried in the soil without ants’ help, their heat tolerance and dormancy breaking after a high thermal shock, changes its photoblastic behaviour allowing unburied seeds to germinate in gaps. High ambient temperatures seem to affect the seed phytochrome photoequilibrium, allowing the manifestation of germination under light conditions (Takaki, Reference Takaki2001; Bewley et al., Reference Bewley, Bradford and Hilhorst2013).

Figure 4. (A) Image showing a nest entrance of Amoimyrmex striatus with several buried M. grahamii seeds; and (B) an A. striatus ant probably feeding on the caruncle of M. grahamii seed in Paravachasca valley, central Argentina. Photo credit: Mariana Pereyra.
Final considerations
Our results suggest that the germination niche of M. grahamii appears to be particularly well suited to disturbed habitats as that of other species of this genus (Ospina et al., Reference Ospina, Gómez and Orozco2000; Pujol et al., Reference Pujol, Gigot, Laurent, Pinheiro-Kluppel, Elias, Hossaert-McKey and McKey2002; Duputié et al., Reference Duputié, Salick and McKey2011). Although M. grahamii freshly matured seeds have a narrow temperature and light window of opportunities for germination, which probably allows germination only in the dark and constraints seedling recruitment to a particular season (i.e., summer), a considerable portion of the seed cohort might widen their light and temperature germination requirements after a period of cold plus warm or a sudden event of high temperatures, which both alleviate non-deep physiological dormancy. Thus, unburied seeds demonstrate an altered light sensitivity and may germinate in summer (35/20 °C) under light conditions. Also, the germination niche for at least half of the buried seed population is expanded and seeds can germinate at other seasons of the year, when the temperature regime is about 25/15 °C like in spring (Cochrane, Reference Cochrane2019; Kazancı and Tavşanoğlu, Reference Kazancı and Tavşanoğlu2019). What is more, the high temperatures treatments results suggest that seeds of this plant species show a heat tolerant strategy and a possible positive feedback between seed dormancy alleviation and high temperatures; this seems to underlie the expansion dynamics of this species in central Argentina (Kazancı and Tavşanoğlu, Reference Kazancı and Tavşanoğlu2019). From this perspective, a heat shock as those recorded in a fire event as well as the high temperatures observed in gap environments could explain the presence of M. grahamii in its southernmost distribution range. In the present context of global change (Sala et al., Reference Sala, Stuart Chapin, Armesto, Berlow, Bloomfield, Dirzo, Huber-Sanwald, Huenneke, Jackson, Kinzig, Leemans, Lodge, Mooney, Oesterheld, Poff, Sykes, Walker, Walker and Wall2000), with an increasing habitat fragmentation and fire frequency (Fehlenberg et al., Reference Fehlenberg, Baumann, Gasparri, Piquer-Rodriguez, Gavier-Pizarro and Kuemmerle2017; Muñoz Garachana et al., Reference Muñoz Garachana, Aragón and Baldi2018; Argañaraz et al., Reference Argañaraz, Cingolani, Bellis and Giorgis2020; Zeballos et al., Reference Zeballos, Giorgis, Cabido, Acosta, Iglesias and Cantero2020), our results suggest that this species may become more abundant in this kind of environments through a gap-recruiting ability strategy, widening its geographic distributional range. However, other factors conditioning seedling establishment should be studied; in addition, the role of ants in the dispersal ecology and how this process interacts with seed germination traits should be explored. Finally, these kinds of experiments are highly relevant to provide insights into dimensions of the germination niche that could be also useful to predict germination success, species abundance and geographic distribution of plant species (Cochrane et al., Reference Cochrane, Hoyle, Yates, Wood and Nicotra2014).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S096025852300020X.
Acknowledgments
The authors wish to acknowledge the assistance of the following institutions: the National Scientific & Technological Research Council (CONICET) and the National University of Córdoba (SECyT – UNC). Furthermore, the authors would like to thank Dra. Ana Ferreras and Dra. Paula Marcora for reading and performing very helpful comments on the first version of the manuscript. We thank the editor and three anonymous reviewers who made valuable suggestions and commented on the first and second versions of the manuscript. We thank Jorgelina Brasca for improving the English style.
Author contributions
S.R.Z. and G.F. conceived the project and designed the experiments; S.R.Z., P.V., D.S., M.P. and G.F. performed all experiments and tests; S.R.Z. analysed the data; S.R.Z. and M.P. performed the graphs; S.R.Z., P.V. and M.P. wrote the draft manuscript; D.S., and G.F. contributed to drafts and all authors gave final approval for publication.
Competing interest
The authors declare none.








