Introduction
Maize (Zea mays L.) is one of the most important crops in the world (Bosque-Perez, Reference Bosque-Perez1995). Despite efforts to improve its production, poor agronomic practices, pests, and diseases cause maize yield reduction up to 31% worldwide (Oerke, Reference Oerke2006). Insect pests are a dominant component of maize production and impact negatively on yields during production and post-harvest periods. The agricultural regions of the Americas ranging from Argentina to southern Canada have hosted one of the important pests of maize called the fall armyworm (FAW), Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) (Luginbill, Reference Luginbill1928; Clark et al., Reference Clark, Molina-Ochoa, Martinelli, Skoda, Isenhour, Lee, Krumm and Foster2007; Adamczyk et al., Reference Adamczyk, Greenberg, Armstrong, Mullins, Braxton, Lassiter and Siebert2008; Farias et al., Reference Farias, Barbosa, Busoli, Overal, Miranda and Ribeiro2008). This noctuid is a destructive pest that caused important economic loss to maize in Brazil (Cruz and Turpin, Reference Cruz and Turpin1983; Cruz et al., Reference Cruz, Figueiredo, Oliveira and Vasconcelos1999; Diez-Rodrigues and Omoto, Reference Diez-Rodrigues and Omoto2001; Carvalho et al., Reference Carvalho, Omoto, Field, Williamson and Bass2013; Huang et al., Reference Huang, Qureshi, Meagher, Reisig, Head and Andow2014) and is an important yield-limiting pest of maize plants in the southern United States of America (Buntin et al., Reference Buntin, All, Lee and Wilson2004; Chilcutt et al., Reference Chilcutt, Odvody, Correa and Remmers2007; Hardke et al., Reference Hardke, Leonard, Huang and Jackson2011).
Unfortunately, Africa has been invaded by S. frugiperda (Goergen et al., Reference Goergen, Kumar, Sankung, Togola and Tamo2016; Nagoshi et al., Reference Nagoshi, Koffi, Agboka, Tounou, Banerjee, Jurat-Fuentes and Meagher2017; Reference Nagoshi, Goergen, Tounou, Agboka, Koffi and Meagher2018; Koffi et al., Reference Koffi, Agboka, Adenka, Osae, Tounou, Adjevi, Fening and Meagher2020a, Reference Koffi, Kyerematen, Eziah, Osei-Mensah, Afreh-Nuamah, Aboagye, Osae and Meagher2020b) which occur in at least 44 African countries (Prasanna et al., Reference Prasanna, Huesing, Eddy and Peschke2018; Rwomushana et al., 2018). The females of this pest lay eggs on the tender tissues of maize plants, hatch about 2.5 d later (Sharanabasappa et al., Reference Sharanabasappa, Kalleshwaraswamy, Maruthi and Pavithra2018). The newly hatched larvae disperse within the plant or migrate to adjacent plants by ballooning and then feeding on young leaves (Ali et al., Reference Ali, Luttrell, Pitre and Davis1989, Reference Ali, Luttrell and Pitre1990). The damage on maize leaves reduces the photosynthetic areas and indirectly causes yield losses (Cruz and Turpin, Reference Cruz and Turpin1983; Pitre and Hogg, Reference Pitre and Hogg1983; Buntin, Reference Buntin1986; Melo and Silva, Reference Melo and Silva1987; Capinera, Reference Capinera2000; Vilarinho et al., Reference Vilarinho, Fernandes, Hunt and Caixeta2011). Moreover, larvae attack all phenological stages of maize (Flanders et al., Reference Flanders, Ball and Cobb2007; Knutson, Reference Knutson2009), from young leaves at plant emergence to ears at harvest time. Plant injury of S. frugiperda on maize ears facilitates disease infection of grain causes direct loss of yields (Capinera, Reference Capinera2017). The documented yield losses caused by S. frugiperda was estimated up to 72% in Argentina (Murúa et al., Reference Murúa, Molina-Ochoa and Coviella2006), 40% in Honduras (Wyckhuys and O'Neil, Reference Wyckhuys and O'Neil2006), 35% in Zambia, 26.6% in Ghana (Rwomushana et al., 2018), between 21 and 53% in 12 African countries (Abrahams et al., Reference Abrahams, Bateman, Beale, Clottey, Cock, Colmenarez, Corniani, Day, Early, Godwin, Gomez, Moreno, Murphy, Oppong-Mensah, Phiri, Pratt, Richards, Silvestri and Witt2017), and 11.57% in Zimbabwe (Baudron et al., Reference Baudron, Zaman-Allah, Chaipa, Chari and Chinwada2019). The variations in yield losses can be due to S. frugiperda infestation levels, abiotic factors such as heavy rainfall or temperature extremes, biotic factors such as natural enemies, and agronomic and control methods used by farmers. Recent studies in Togo and Ghana from 2016 to 2018 on S. frugiperda showed differential infestation among the Agro-Ecological Zones (AEZs) that vary between the southern and northern parts of each country (Koffi et al., Reference Koffi, Agboka, Adenka, Osae, Tounou, Adjevi, Fening and Meagher2020a). Also, plant infestations from 2016 to 2018 have been reduced due to several factors such as agricultural practices that include insecticide applications, a rise of natural enemies (Koffi et al., Reference Koffi, Agboka, Adenka, Osae, Tounou, Adjevi, Fening and Meagher2020a, Reference Koffi, Kyrematen, Eziah, Agboka, Adom, Goergen and Meagher2020c).
Since 2018, infestation levels of FAW on maize in Togo are not yet investigated as well as the impact on plant phenological stages and yield losses under different infestation levels. Consequently, this study was conducted in the five AEZs during the cropping seasons of 2019 and 2020 to assess the infestation and damage levels in the countryside and FAW's impact on maize plants and yields in an on-station experiment.
Materials and methods
Study sites
Farm inspections were conducted in different localities of the five AEZs, numbered from one in the north to five in the south. AEZ1 has Sudan Savannah characteristics of tropical grassland and warm temperatures. AEZ2 is characterized by dry savannah with a mix of dry forest and savannah plants. These two AEZs have one rainy season from May to October and one dry season from November to April. AEZ3 is the largest zone in Togo and contains a mix of savannah plants and small wooded forest areas located in mid-eastern Togo. AEZ4 is characterized by a semi-deciduous mountain forest habitat located west of AEZ3. AEZ5 is a mix of small wooded forest and coastal savannah habitats in the south of Togo. The southern three AEZs have two rainy seasons from April to July and September to November, and two dry seasons in August and from December to March. The experiments were conducted at the Station d'Expérimentations Agronomiques de Lomé, Lomé, Togo located in AEZ5 (fig. 1).

Figure 1. The five Agro-Ecological Zones (AEZs 1–5) of Togo with the location of the sampling sites and Station d'Expérimentations Agronomiques de Lomé (SEAL).
Inspection of maize farms within AEZs
From each AEZ, 15 farms growing maize (variety Ikene) at vegetative stages V1 to V12 leaves were inspected each year, for a total of 150 farms in the 2019 and 2020 seasons. During the two years, farms in AEZs 3, 4 and 5 were inspected in June while those in AEZs 1 and 2 were visited in July. At each farm, all instar larvae and egg masses were collected, and the number of infested and damaged plants by S. frugiperda were recorded on inside quadrants designed in the four corners and one in the center of the inspected farm (Koffi et al., Reference Koffi, Agboka, Adenka, Osae, Tounou, Adjevi, Fening and Meagher2020a). The selected plants were distributed as 12 plants per quadrant and carefully examined using forceps and a hand magnifying glass without destroying the standing plants (non-destructive sampling). The larval and egg mass population densities, infestation level, and percentage of damaged plants were then calculated for each farm.
Design and data collection of on-station experiments
The on-station experiments were conducted using eight treatments of five replications on ‘Ikene’ maize. The treatments included netting artificially infested plots at different levels – 25, 30, 50, 75, and 100%, netting insecticide-treated plots, netting plots without any treatment, and open plots to natural infestation. The 100D, 24 holes per cm2 nets of 100% polyester (L3.0 × W3.0 × H2.5 m) (Vestergaard Group SA, Vietnam) were locally manufactured for this study. The mesh sizes were small enough that neonate larvae could not pass through and were set with the bottom sealed in the soil before the emergence of maize. At ten days old, plants were artificially infested with 5-day-old third instar larvae by placing them in the whorl of each selected plant. These larvae were previously fed under laboratory conditions with tender maize leaves. The insecticide plots were sprayed with emamectin benzoate (Emacot 019EC™) at 1.5 ml in 1 liter of water at ten days old plants. Treatment plots were arranged in a Latin square design and plots were distanced 2 m apart (fig. 2). The numbers of larvae, egg masses, and damaged plants, leaves and ears were recorded weekly from in situ 10 plants per plot to calculate the percent damaged plants, leaves and ears. After harvesting, 10 healthy ears and 10 damaged ears by S. frugiperda were selected from each plot and grain (kernels) were separately weighted to calculate losses from direct feeding on ears. Total grain in each plot was weighted to determine the yield per treatment, which was extrapolated into area (hectare) based on the density of maize plants within plots.

Figure 2. On-station experiment design of netted plots infested to 25, 30, 50, 75, 100% with third instar FAW larvae, netted plot sprayed with emamectin benzoate (N + Eb), simple netted plots (SN), and open plots.
The densities of larvae and egg masses were calculated by dividing the number of collected larvae or egg masses by the total number of selected plants. Infestation levels were calculated by dividing the number of infested plants by the total number of sampled plants. The percent damaged plants or ears were calculated by dividing the number of damaged plants or ears by the total number of sampled plants or ears. Direct grain losses were determined by subtracting the grain weight from damaged ears from the grain weight from undamaged ears per plot.
Data analysis
For the whole country study, densities of larvae and egg masses and percent damaged plants were calculated for each inspected farm before being grouped into AEZs and years. While calculations of larval and egg masses densities, grain losses, and percent damaged plants were determined for each plot, damage to leaves and ears were calculated for each on-station experiment plot and grouped by infestation treatment. The calculated percentages and infestations were arcsine square root transformed prior to analysis. All calculations and transformations were carried out in Excel. All data were submitted to a Shapiro test in GenStat Twelfth Edition GenStat Procedure Library Release PL20.1 to test for normality. A non-parametric test (Kruskal-Wallis) was performed at the 5% significance level for non-normal data while the normal data were submitted to ANOVA. Means were determined from data subjected to one-way analysis of variance at 95% confident interval. Multiple mean comparisons were separated using Tukey tests while t-tests were used to separate two means in the GenStat software.
Results
Infestation and damage levels across AEZs
The larval and egg mass densities of S. frugiperda on maize plants in Togo were similar between 2019 and 2020. The infestations (18.7 ± 1.1% and 17.1 ± 0.87% in 2019 and 2020, respectively; t 149 = 0.44, P = 0.507) and percent damaged plants (16.3 ± 1.8% and 18.1 ± 1.7% in 2019 and 2020, respectively; t 149 = 1.49, P = 0.224) were also similar between years. Collected larvae from plants were from third instar or older.
Generally, larval densities, infestation levels, and percent damaged plants were higher in AEZ5 than the other AEZs during the cropping seasons of 2019 and 2020. Egg masses were rarely found, leading to similarities among AEZs (Table 1). No egg masses were found in AEZ1-3; only one egg mass was found in AEZ4, and two masses in AEZ5. The mean numbers of larvae on 60 maize plants within AEZ1 were nine in 2019 and 10 in 2020, AEZ2 were 12 in the two years, AEZ3 were 13 in 2019 and 14 in 2020, AEZ4 were 22 in 2019 and 25 in 2020, AEZ5 were 22 in 2019 and 30 in 2020. The infestation levels were low in AEZ1 to AEZ4 during the two years, ranging between 9 and 17%, while the infestations were higher in AEZ5 (24%). Percent damaged plants from 9 to 17% were recorded in AEZ1-4, and increased to 35% in AEZ5 (Table 1).
Table 1. Larvae and egg densities, infestation levels, and percent of damaged plants on 60 maize plants selected per farm in the five AEZs of Togo during the cropping seasons of 2019 (A) and 2020 (B)

Means within each variable followed by the same letter are not significantly different (P > 0.05). Z-Agro-Ecological Zone followed by the mean number attributed to the zone in Togo.
Impacts on maize plants and yields
A success artificial infestation was observed during this study with larval densities in plots following the infestation patterns. High densities were recorded in high infested plots and low densities in low infested plots (Table 2). The netted plots which were sprayed with Emamectin benzoate and simple netting plots surprisingly hosted larvae but with different densities. Larval density was slightly lower in treated plots than the simple netted plots (Table 2), whereas larval density in open plots was similar to densities from plots artificially infested at 30 and 50%. Egg masses were rarely found, therefore, egg mass densities were similar among all eight treatments. The percent damaged plants and leaves followed the infestation pattern. Unless in plots infested at 30 and 50% where similarities were observed, the percent damaged plants and leaves were high in high infested plots and low in low infested plots. However, the open plots which recorded similar larval density to the 30 and 50% infested plots recorded percent damaged plants similar to the 75% infested plots, and percent damaged leaves slightly higher than the 30 to 50% artificial infestations. The percent damaged plants in the simply netted plots were similar to those in the 30 and 50% infestation plots. The insecticide-treated plots recorded low percent damaged plants.
Table 2. Means of larval and egg mass densities on plants and ears, percent of damaged plants, leaves and ears, yields, grain losses caused by S. frugiperda feeding on ears, total yield losses, and weights of 1000 grains recorded from 60 maize plants and ears submitted to different infestation levels and controls
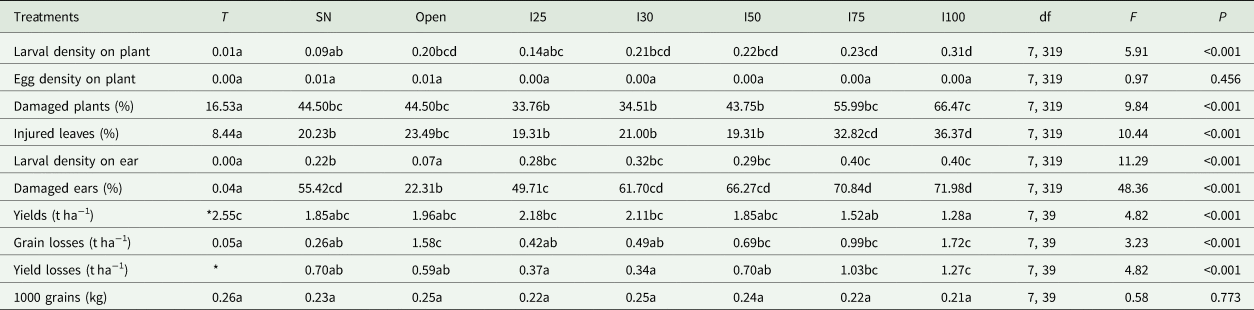
Treatment means within each variable followed by the same letter are not significantly different (P > 0.05).
*Baseline yield used to calculate yield losses. Treatments: T – treated, SN – simple netted, I25 – 25% infestation, I30 – 30% infestation, I50 – 50% infestation, I75 – 75% infestation, and I100 – 100% infestation.
Larval densities recorded on ears were similar among the artificially infested plots. Densities were low in the simply netted plots, very low in open plots, and were rare in the netting insecticide plots. In contrast, the percent damaged ears were similar between plots of simply netted, 30 and 50% infestations, then became high in plots of 75 and 100% infestations which were similar. Except from the insecticide-treated plots, the open plots recorded lower percent damaged ears than other netted plots.
The higher the infestations, the lower were the yields, with the yield from open plots similar to yields from the simply netted and 50% infested plots. A slightly higher yield was observed from the treated plots than other plots. Direct grain losses due to FAW feeding on ears were high in open plots and in plots with more than 30% infestations. But the lowest grain loss was recorded from the insecticide-treated plots. Generally, yield losses due to FAW larval feeding on plants and ears were high in plots infested at 75 and 100%, similar between open plots, simply netted plots and 50% infested plots, which were all slightly higher than recorded in 25 and 30% infested plots. To verify if the infestation levels of plants can affect the size or weight of single maize grain, similarities were observed between weights of 1000 grains sampled from the eight treatments (Table 2).
Severities across maize plant phenology
At the emergence stage of maize plants, with totally plants checked, larvae were rarely collected and observed larvae were younger than the third instar. Ten days post emergence, plants were at V4–6 leaves and were artificially infested by 5-day-old larvae. The first adults were observed in netted plots at the beginning of the tasseling stage when plants were at 42 days old. This marked a new generation of FAW under nets. However, egg masses were not found on the nets. The objective of treating the netted plot with emamectin benzoate was to exempt these plots of FAW infestation. However, a few larvae were observed in treated plots during the V6–8 stage with slight feeding damage to plants. About 25% of plants were damaged, up to 4 and 15% of leaves were chewed at emergence and at the flowering stages, respectively (Table 3). There were low numbers of larvae in the simply netted plots during the vegetative stages and became rare when plants were at the V8–10 stage. But larval density and percent chewed leaves were similar among plant phenological stages in the simple netted plots. However, half of the ears were damaged in these plots (Table 3). In the open plots, larval densities and percent damaged plants were different among phenological stages. About one larva was observed on four examined plants from emergence to V8 stages. About 30% of plants were damaged during the emergence, flowering and ear stages, and higher than 50% during vegetative stages. Between 20 and 30% of leaves were injured during the vegetative stages, this decreased to 12% when plants aged during the flowering stage in the open plots (Table 3).
Table 3. Means (±SE) of larval densities, percent damage plants and leaves on maize plants from emergence to ears in plots treated with Emamectin benzoate, simple netting, control or open for natural infestation, and artificial infestation plants by third instar larvae of S. frugiperda at 25, 30, 50, 75 and 100% levels

Means in the same row followed by the same letter are not significantly different (P > 0.05).
*No data, % Percentage, V-vegetative stage indexed by the numbers of maize leaves.
Before artificial infestation of plots, larvae were rarely found in the plots until plants reached 10 days old, which resulted in very low larval density during maize plant emergence. After artificial infestation, larval densities followed the expected increasing trends, although plants that had been naturally infested resulted in higher than expected densities from the AEZ5. Plots of 25, 30 and 50% infested plants resulted in slightly higher larval densities (0.31, 0.44 and 0.58, respectively) of V4–6 leaves than the expected densities (0.25, 0.3 and 0.5, respectively). Whereas, plots of 75 and 100% infested plants resulted in lower densities (0.63 and 0.74, respectively) during the V4–6 stage than expected (0.75 and 1.00, respectively). The highest percent of damaged plants and chewed leaves was recorded during the vegetative stages in the artificially infested plots.
Discussion
Infestations of S. frugiperda on maize in Togo observed during this study suggest population stability across the country. The larval and egg mass densities, infestations, and percent damage plants were low and similar between 2019 and 2020 as observed in 2018 (Koffi et al., Reference Koffi, Agboka, Adenka, Osae, Tounou, Adjevi, Fening and Meagher2020a). The infestations 14.7% (2019) and 17.1% (2020), are closer to the 15.7% infestation reported in 2018 by Koffi et al. (Reference Koffi, Agboka, Adenka, Osae, Tounou, Adjevi, Fening and Meagher2020a). They were four times lower than the 70.8 and 67.8% infestations recorded early years after the invasion in 2016 and 2017, respectively (Koffi et al., Reference Koffi, Agboka, Adenka, Osae, Tounou, Adjevi, Fening and Meagher2020a). This suggests that the invasion of S. frugiperda in Togo has stabilized since 2018. The stabilization of densities observed in Togo does not completely explain the economic impact of the infestation. Thus, the importance of assessing different artificial infestations under human control can clarify the impacts on maize plants and yields.
The artificially infested plots compared with the open plots show some concordance between the open plots and 50% infested plots. They recorded similarities in larval density (open = 0.20; 50% infestation = 0.22), percent damaged plants (open = 44.50%; 50% infestation = 43.75%), percent damaged leaves (open = 23.49; 50% infestation = 19.31%), and yields (open = 1.96 t ha−1; 50% infestation = 1.85 t ha−1). However, during 2019 and 2020 the national infestation was about three times lower than 50% infestation, which shared similarities with the open plots at the station. This suggests that other factors such as other insect species, free movement and migration may impact our parameters for the open plots. The higher densities than the expected observed in some infested plots during the early stages of maize plants should due to possible accidental introduction into netted plots or emergence of adults from the soil. From the V6–8 stage of maize plants, the larvae used to infest plots began to pupate reducing then larval densities plant damage in the next stages (V8–12). From the V8–10 stage, plant tissues become hard and therefore unsuitable for larvae which limited food for newly hatched larvae and favored higher mortality (Williams et al., Reference Williams, Davis, Buckley, Hedin, Baker and Luthe1998). During the tasseling stage, the new generation of S. frugiperda emerged in the netted plots. This coincided with the emergence of tassels and ears which maintained a low larval population. Even though females have a high oviposition capacity and high hatching rate of eggs (Sparks, Reference Sparks1979), these poor nutritional conditions reduced larval density and kept the population at a lower level compared to the initial larval density used to infest plots. However, due to the hardness of tissues, the few surviving larvae moved from plant to plant and induced higher percent damaged ears in netted plots than the open plots, where adults were able to locate other suitable plants for their neonates. However, the ears in the open plots were exposed to other biotic factors such as ear borers (Pyralidae) and insect and avian predators, and the direct grain losses became higher as they were in the 100% infested plots. This decreased yield in the open plots was similar to the level found with the 50% infestation plots. The infestation in the open plots were approximatively 20%, and it was expected to yield higher grains than the plots from 25 and 30%. Unfortunately, a lower yield was recorded and these losses can be attributed to other unmeasurable factors. This suggests that yield loss of maize in Togo is not only due to S. frugiperda but also other factors that need to be determined. On other hand, yield losses that were much similar to the yield obtained from sprayed plots were found in the 25 and 30% infested plots, which suggests that the economic threshold is reached above 30% infestation.
Practically, farmers need careful observation of maize farms from the emergence of plants to start control measures once infestation reaches 30% to save economic losses. This must be combined with the assumption that 10 day-old larvae generally are fourth instars or less and are susceptible to insecticides and natural enemies (Cruz, Reference Cruz1995; Cruz et al., Reference Cruz, Figueiredo, da Silva, da Silva, de Souza Paula and Foster2012). One management tactic is the use of sex pheromone trapping where insecticides are applied after the collection of 3 males moths and the larval population is less than 10 d old (Cruz, Reference Cruz, Cruz, Karam, Monteiro and Magalhães2008). In absence of control methods, high infestations cause serious damage to leaves (Cruz and Turpin, Reference Cruz and Turpin1983; Harrison, Reference Harrison1986; Melo and Silva, Reference Melo and Silva1987; Bokonon-Ganta et al., Reference Bokonon-Ganta, Bernal, Pietrantonio and Setamou2003; Siebert et al., Reference Siebert, Tindall, Leonard, Van Duyn and Babcock2008). This reduction in leaf area affects photosynthesis (Cruz and Turpin, Reference Cruz and Turpin1983; Pitre and Hogg, Reference Pitre and Hogg1983; Buntin, Reference Buntin1986; Melo and Silva, Reference Melo and Silva1987; Capinera, Reference Capinera2000; Vilarinho et al., Reference Vilarinho, Fernandes, Hunt and Caixeta2011), and favors yield reduction.
Acknowledgements
The data collections within AEZs were conducted under the International Foundation of Sciences (IFS) project (C/6255-1), we appreciate and acknowledge IFS for its funding.
Conflict of interest
The authors declare none.








