The Minnesota Center for Twin and Family Research (MCTFR) comprises multiple coordinated, longitudinal (up to 9 waves over 30 years), community-representative investigations of twin and adoptive families first assessed in childhood and adolescence (see www.mctfr.psych.umn.edu for more information). Historically, the major research focus has been on the development and etiology of psychopathology, substance use, personality, neurocognitive functioning and psychosocial functioning in adolescence and early adulthood. An overview of studies and samples in the MCTFR, including the timing of assessments, is presented in Table 1 and Figure 1.
Table 1. Overview of established MCTFR cohorts
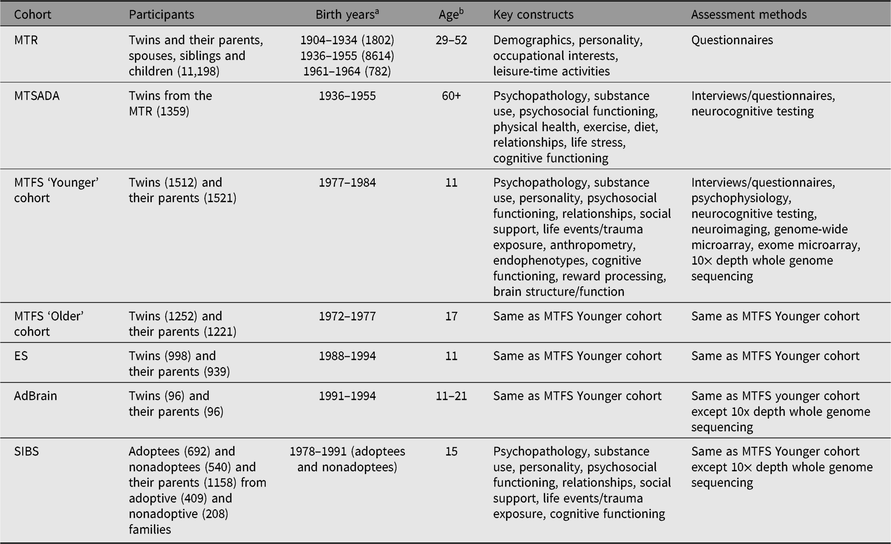
Note: MCTFR = the Minnesota Center for Twin and Family Research, MTR = Minnesota Twin Registry, MTSADA = Minnesota Twin Study of Adult Development and Aging, MTFS = Minnesota Twin Family Study, ES = Enrichment Study, AdBrain = Adolescent Brain Study, SIBS = Sibling Interaction and Behavior Study.
a Birthyears of twins or adoptees, N in parenthesis.
b Age in years at first assessment.
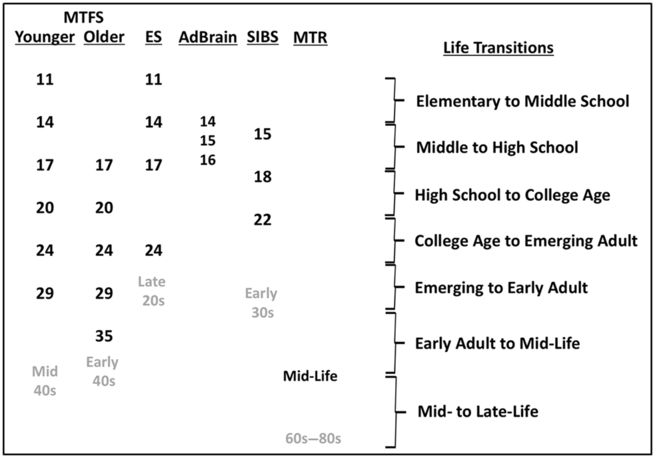
Fig. 1. Ages at assessment of established MCTFR participant cohorts.
Registry-based twin research at the University of Minnesota began with David Lykken, who believed that any psychological research is more informative if undertaken with twins, rather than singletons (or college sophomores). Lykken and his colleagues developed protocols that allowed for the successful ascertainment of large representative samples of twins from birth records (Lykken et al., Reference Lykken, Bouchard, McGue and Tellegen1990) that can be accessed for research purposes in Minnesota State. As a result of this early work, the MCTFR now includes five established twin cohorts and one adoption cohort. The Minnesota Twin Registry (MTR) includes monozygotic (MZ) and same-sex dizygotic (DZ) twins born between 1904 and 1934 (1802 twins), 1936 and 1955 (8614 twins), and 1961 and 1964 (782 twins) who have completed assessments in middle and older adulthood (Krueger & Johnson, Reference Krueger and Johnson2002). The Minnesota Twin Family Study (MTFS) is an investigation of two cohorts of 2764 MZ and same-sex DZ twins born between 1972 and 1984, who were first assessed with their parents at age 11 (the ‘Younger’ cohort) or age 17 (the ‘Older’ cohort; Iacono et al., Reference Iacono, Carlson, Taylor, Elkins and McGue1999, Reference Iacono, McGue and Krueger2006; Iacono & McGue, Reference Iacono and McGue2002). The Enrichment Study (ES) is a third cohort of 998 MZ and same-sex DZ twins born between 1988 and 1994 who were also first assessed with their parents at age 11; ES twins were oversampled for risk of developing substance-use disorders (Keyes et al., Reference Keyes, Malone, Elkins, Legrand, McGue and Iacono2009). The Adolescent Brain (AdBrain) cohort consisted of a longitudinal co-twin control neuroimaging feasibility study, and includes 96 MZ twins born between 1991 and 1994 who were assessed in adolescence with their primary caregiver (all mothers), with a 1-year follow-up assessment (Malone et al., Reference Malone, Luciana, Wilson, Sparks, Hunt, Thomas and Iacono2014). Complementing the twin design is an adoption cohort — the Siblings Interaction and Behavior Study (SIBS) — of 409 adoptive and 208 nonadoptive families (McGue et al., Reference McGue, Keyes, Sharma, Elkins, Legrand, Johnson and Iacono2007).
These studies and samples, recruitment methods and assessments have been extensively described, along with summaries of early findings, and we refer the interested reader to the above references for additional details. Here we provide a brief overview of key features of these established studies and samples, and describe several new MCTFR investigations that follow up and expand upon existing studies or recruit and assess new samples.
Overview of Established MCTFR Cohorts
The MCTFR twin samples are population-based and representative of Minnesota State in demographic characteristics (for adult twins and for families with children living at home for adolescent twins) at the targeted birth years. MTR twins were identified from Minnesota State birth certificates, which are publically available. All located twins were invited to participate. Zygosity was determined using self-report on a zygosity questionnaire and follow-up genotyping was sought in some cases (e.g., when zygosity was uncertain). MTR twins completed brief questionnaires via email when they were enrolled, and subsets of MTR twins have completed subsequent in-person assessments; parents, spouses, same-sex siblings and children of MTR twins were also recruited and completed brief questionnaires via email at the time of the initial ascertainment. The Minnesota Twin Study of Adult Development and Aging (MTSADA) includes MTR twins born between 1904 and 1934 who were assessed in later life (60 years and older) in their homes on a battery of cognitive and health-related assessments relevant to aging (Finkel & McGue, Reference Finkel and McGue1993). MTFS, ES and AdBrain twins were identified from birth records and, based on a brief interview with a parent (usually the mother), twins were invited to participate if they met minimal inclusion criteria (e.g., no physical/psychological characteristics that would preclude participation). To enrich the representation of twins at high risk for substance misuse, a subset of twins in the ES sample was recruited only if at least one member of the twin pair exceeded a predetermined threshold that maximized sensitivity for identifying disruptive behavior disorders. An additional exclusion criterion for twins in the AdBrain sample was contraindication for undergoing a neuroimaging assessment. Zygosity in MTFS, ES and AdBrain was determined using parent report on a zygosity questionnaire, staff evaluation of physical similarity, fingerprint ridge count and DNA-based confirmation for dizygotic twins. At study intake and follow-up assessments, MTFS and ES twins (and all available biological and stepparents through twin age 17 years) visited the MCTFR laboratories to complete a daylong assessment, including interviews and questionnaires assessing psychopathology, personality, relationships, social adjustment and other characteristics. Twins also participated in psychophysiological assessments designed to identify and evaluate electrophysiological endophenotypes (Gottesman & Gould, Reference Gottesman and Gould2003; Iacono et al., Reference Iacono, Carlson and Malone2000), neurocognitive testing and, more recently, neuroimaging. At study intake and the follow-up assessment, AdBrain twins (and their primary caregivers, all mothers) visited the MCTFR laboratories to complete a daylong assessment comparable to that completed by MTFS and ES twins except with expanded neurocognitive assessment and neuroimaging, which was subsequently adopted in MTFS and ES assessments. Adoptive and nonadoptive families in SIBS were recruited through private adoption agencies in Minnesota and birth records, respectively. At intake, SIBS families visited the MCTFR laboratories to complete a half-day assessment that included interviews and questionnaires, neurocognitive testing and videotaped family interaction tasks. The SIBS sample has been followed up multiple times, with both in-person and telephone-based assessments. Blood, saliva or buccal samples were also collected from children and their parents, allowing for subsequent genome-wide and rare variant exome genotyping, and moderate-depth whole genome sequencing on a large subset of children and their parents in MTFS, ES and SIBS cohorts (Miller et al., Reference Miller, Basu, Cunningham, Eskin, Malone, Oetting and McGue2012; Vrieze et al., Reference Vrieze, Feng, Miller, Hicks, Pankratz, Abecasis and McGue2014).
MTFS, ES, AdBrain and SIBS assessments were carefully coordinated across MCTFR studies and samples such that most analyses can be conducted using the combined twin cohorts and the adoption sample; MTR assessments were also coordinated with other MCTFR studies and samples, as well as the Minnesota Study of Twins Reared Apart (MISTRA; Bouchard et al., Reference Bouchard, Heston, Eckert, Keyes and Resnick1981), so data may be combined and/or compared with these samples. Overall, we have had consistently high rates of participation in our longitudinal studies, generally between 85% and 90%.
Overview of Current MCTFR Investigations
The genetically informative study designs that comprise the MCTFR — especially the integration of twins, adoptive and nonadoptive siblings, and their parents — makes it possible to investigate the separate, as well as combined, influence of genes and environments on adaptive and maladaptive outcomes. Assessments are multimodal and relatively comprehensive across varied forms of psychopathology, as well as other indicators of psychosocial functioning, individual difference factors, and familial and environmental risk and protective factors. The coordinated nature of assessments across studies yields moderately sized samples with longitudinal assessments spanning decades. Psychophysiology is a longstanding strength of the MCTFR, and we now expand upon brain-based assessment with neuroimaging assessments in our twin samples. Combining longitudinal assessments across key developmental periods with twin discordance and difference methods (e.g., the co-twin control method; Lee, Reference Lee2012; McGue et al., Reference McGue, Osler and Christensen2010; Rutter, Reference Rutter2007) increases the quality of causal inferences.
All of this has allowed for impactful research on the nature of genetic and environmental etiology on behavior and the brain. To give just a few examples, the MCTFR has produced important work on the genetic architecture of externalizing psychopathology and substance misuse (Hicks et al., Reference Hicks, Krueger, Iacono, McGue and Patrick2004; Krueger et al., Reference Krueger, Hicks, Patrick, Carlson, Iacono and McGue2002); the development and longitudinal outcomes of substance misuse and related psychopathology (Elkins et al., Reference Elkins, McGue and Iacono2007; Iacono et al., Reference Iacono, Carlson, Malone and McGue2002; Irons et al., Reference Irons, McGue, Iacono and Oetting2007; Keyes et al., Reference Keyes, Legrand, Iacono and McGue2008; King et al., Reference King, Iacono and McGue2004; Vrieze et al., Reference Vrieze, Hicks, Iacono and McGue2012); the familial transmission of psychological, psychophysiological and psychosocial characteristics (Hicks et al., Reference Hicks, Foster, Iacono and McGue2013, Reference Hicks, Krueger, Iacono, McGue and Patrick2004; Tully et al., Reference Tully, Iacono and McGue2008); rigorous tests of candidate endophenotypes (Iacono et al., Reference Iacono, Carlson, Malone and McGue2002, Reference Iacono, Vaidyanathan, Vrieze and Malone2014, Reference Iacono, Malone and Vrieze2017); and the causal nature of substance exposure on cognitive functioning and brain structure and functioning (Harper et al., Reference Harper, Malone and Iacono2018; Jackson et al., Reference Jackson, Isen, Khoddam, Irons, Tuvblad, Iacono and Baker2016; Malone et al., Reference Malone, Luciana, Wilson, Sparks, Hunt, Thomas and Iacono2014; Wilson et al., Reference Wilson, Malone, Thomas and Iacono2015).
New initiatives will continue to refine and extend these discoveries. Twins in the MTR are now in older adulthood, and twins and siblings in the original MCTFR samples are in early and middle adulthood. In addition to continuing and expanding upon regular assessments of the existing MCTFR studies and samples described here to ask new questions about adult development and functioning, we are also engaged in a number of new investigations, including new recruitments of twins and their children (see Table 2).
Table 2. Overview of current MCTFR investigations
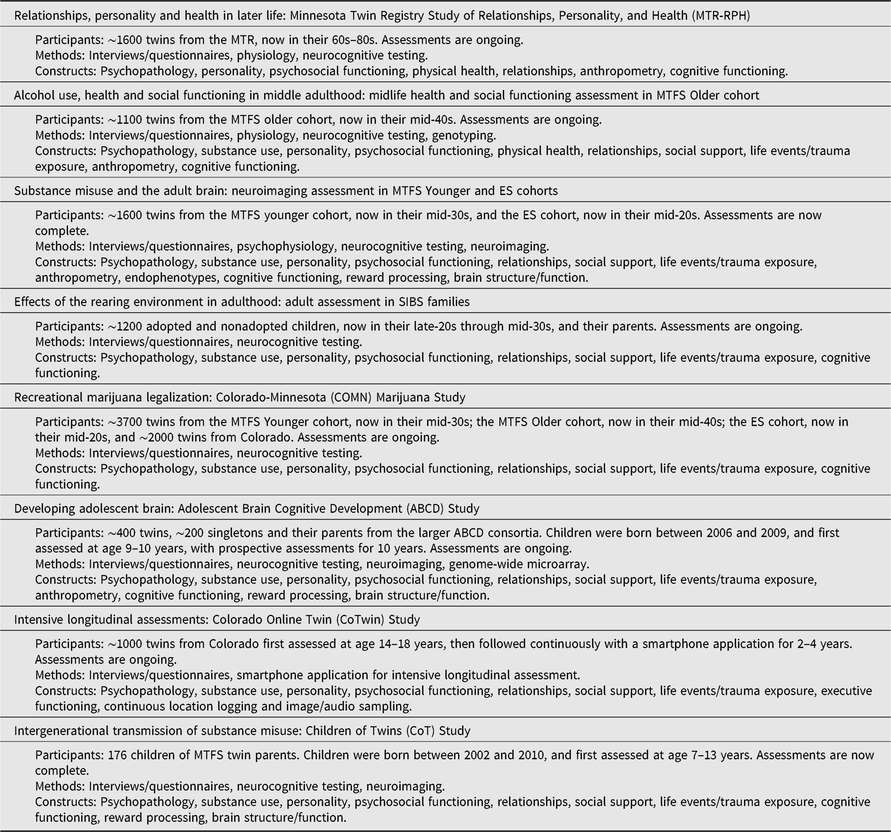
Note: Several current investigations are ongoing follow-up assessments or extensions of the established MCTFR participant cohorts described in Table 1.
Effects of Relationships and Personality on Health in Later Life
The MTR Study of Relationships, Personality, and Health (MTR-RPH) is a classical twin study of relationships, personality and both physical and cognitive health in later life in the MTR cohort. We are now recruiting ∼400 MZ and ∼400 same-sex DZ twin pairs for a follow-up assessment of relationships, personality and physical health, including blood-based biomarkers and anthropometric markers. We are using the co-twin control design to disambiguate the etiology of well-documented phenotypic associations among relationship quality, personality and both physical and cognitive health outcomes in later life.
Role of Adolescent Alcohol Use on Health and Social Functioning in Middle Adulthood
The effects of alcohol use are heavily studied in adolescence and young adulthood. Much less is known about the effects of alcohol use during middle age. In the MTFS Older cohort, we are evaluating how adolescent alcohol use is related to physical and mental health, as well as social (e.g., educational and occupational attainment) outcomes in participants’ 40s and 50s. Once again using a co-twin control design, this research will allow relatively stringent tests of the environmentally mediated effect of adolescent and adult alcohol exposure on midlife functioning.
Effects of Substance Misuse on the Adult Brain
Most models of addiction implicitly or explicitly attribute deviations to the neurotoxic effect of substances and their sequelae on the brain (Jacobus & Tapert, Reference Jacobus and Tapert2013; Volkow et al., Reference Volkow, Wang, Fowler and Tomasi2012). We have recently completed neuroimaging assessments in approximately half of the MTFS Younger cohort in their mid-30s and the ES cohort in their mid-20s. We are now using the co-twin control method to evaluate the causal effect of adolescent substance use and misuse, as well as persistence and desistence in adulthood, on the adult brain (Wilson et al., Reference Wilson, Malone, Hunt, Thomas and Iacono2018).
Effects of the Rearing Environment in Adulthood
Adoption designs allow effective controls for passive gene–environment correlation, but few have prospectively evaluated the effect of the rearing environment in early to mid-adulthood. We are conducting a follow-up assessment of the SIBS adoption sample (parents and children) in a study evaluating the long-term effects of the rearing environment on behavioral outcomes in participants’ late 20s and early 30s — typically well after they have moved out of their parents’ home. The study will also help to address how individuals understand heritability and genetic risk among individuals where genetic influences might have special significance due to their involvement in adoption (Willoughby, Love et al., Reference Willoughby, Love, McGue, Iacono, Quigley and Lee2019).
Effects of Recreational Marijuana Legalization on Adults
Many states in the United States are legalizing the recreational use of marijuana, with little known about the possible effects of such changes. In collaboration with the Institute for Behavioral Genetics at the University of Colorado Boulder on the Colorado-Minnesota (COMN) Marijuana Study, we are conducting assessments with all MTFS and ES twins in a total sample of more than 5500 twins across both institutions. We will evaluate the effect of recreational marijuana legalization on drug use, psychopathology, cognitive ability, personality and psychosocial functioning trajectories. The legalization event in Colorado will be complemented with a co-twin control analysis to better evaluate the causal impact of marijuana use on these outcomes.
The Developing Brain in Adolescence
The Adolescent Brain Cognitive Development (ABCD) study is a 21-site longitudinal Consortium study of over 11,875 children first assessed with their parents at age 9 or 10, who are being prospectively followed for a 10-year span into adolescence and early adulthood. The ABCD study examines neurobehavioral development through adolescence and into early adulthood using behavioral, genetic and neuroimaging methods, with the goal of identifying neurobiological, psychological, familial and environmental risk and protective factors for substance initiation and misuse, and mental, physical and psychosocial functioning (see www.abcdstudy.org for more information). The MCTFR is the leader of the four-site Twin Hub of the ABCD study, which has recruited over 1600 MZ and same-sex DZ twins (from over 800 twin pairs); 400 twins and 200 singletons were recruited in Minnesota. The inclusion of twins within the ABCD study greatly increases the conclusions that can be drawn regarding causal impacts of substance use on developmental trajectories (Iacono et al., Reference Iacono, Heath, Hewitt, Neale, Banich, Luciana and Bjork2018). Using ABCD data, we will evaluate the relative influence of genetic and environmental factors for a range of phenotypes, as well as use the co-twin control design to address critical questions about the causal relationships among substance use and other environmental exposures for the developing brain and related functioning.
Intensively Longitudinal Assessments With Wireless Technology
New technologies create new avenues to measure behavior. In collaboration with the Institute for Behavioral Genetics at the University of Colorado Boulder in the Colorado Online Twins (CoTwins) study, we have begun a new recruitment of ∼1000 adolescent MZ and same- and opposite-sex DZ twins from Colorado. In addition to in-person assessments, twins are followed continuously for 2–4 years with a smartphone application. We will evaluate psychological change (e.g., mood, substance use, cognitive ability, personality) with weekly longitudinal assessments. The application also logs location information that can be referenced against geographical information systems to extract location-based environmental context. This information will be used to systematically measure and evaluate environmental exposures.
Intergenerational Transmission of Substance Misuse in Children of Twins
A longstanding question in developmental psychology is how traits are transmitted from one generation to the next. The MCTFR has been answering such questions for years using a nuclear twin family design (i.e., twins and their parents). Because both parents and their children have been densely genotyped, we can further triangulate parenting effects by testing the association of nontransmitted parental alleles with functioning in children (i.e., the so-called nature-nurture effect; Willoughby, McGue et al., Reference Willoughby, McGue, Iacono, Rustichini and Lee2019). We are now extending the nuclear twin family model through our pilot Children of Twins (CoT) study, which yields three-generation pedigrees. Like an adoption study, the children-of-twins design can control for passive gene–environment correlation to distinguish genetic from environmental mediation of parent–child transmission (McAdams et al., Reference McAdams, Neiderhiser, Rijsdijk, Narusyte, Lichtenstein and Eley2014). The children-of-twins recruitment focuses on the development and familial transmission of substance misuse, psychopathology and relevant aspects of brain structure and function that may contribute to these conditions.
Extensive Collaborations
The MCTFR is highly collaborative. In addition to coordinated data collection efforts with investigators in Colorado-Minnesota projects and in ABCD, our investigators routinely contribute data and expertise to local and international collaborations. These include the Consortium on Interplay of Genes and Environment across Multiple Studies (IGEMS; Pedersen et al. (Reference Pedersen, Gatz, Finch, Finkel, Butler, Dahl Aslan and Whitfield2019) this issue) and the Collaborative Project of Development of Anthropometrical Measures in Twins (CODATwins) consortium (Jelenkovic et al., Reference Jelenkovic, Sund, Hur, Yokoyama, Hjelmborg, Möller and Ooki2016), a brain electrophysiology consortium (Smit et al., Reference Smit, Wright, Meyers, Martin, Ho, Malone and de Geus2018) and genetic association meta-analysis consortia on traits related to MCTFR goals (Lee et al., Reference Lee, Wedow, Okbay, Kong, Maghzian, Zacher and Linnér2018; Liu et al., Reference Liu, Jiang, Wedow, Li, Brazel, Chen and Tian2019; McCarthy et al., Reference McCarthy, Das, Kretzschmar, Delaneau, Wood, Teumer and Sharp2016). We currently collaborate with dozens of investigators outside of the University of Minnesota who take advantage of the archived dataset, and we welcome new collaborations. Collaborative team science is an increasingly common way to produce ever more definitive research, and the MCTFR works within such a framework while maintaining datasets and investigations that can also stand firmly on their own.
Financial Support
Research reported in this article was supported by the National Institute of Aging of the National Institutes of Health under award numbers R01AG06886 (MTSADA; PI: M. M.) and R01AG053217 (MTR-RPH; PIs: R. F. K., G. I. R.); National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award numbers R37AA09367 (MTFS; PI: M. M.), R21AA017314 (AdBrain; PI: S. M. M.) and R01AA11886 (SIBS; PI: M. M.); National Institute on Drug Abuse of the National Institutes of Health under award numbers R37DA05147 (MTFS; PI: W. G. I.), R01DA042755 (COMN; PIs: J. K. H., C. H., M. M., S. V.), U01DA041120 (ABCD; PIs: M. M. L., W. G. I.), U01DA046413 (CoTwins; PIs: S. V., N. P. F.), R01DA037904 (PI: S. V.), R01DA044283 (PI: S. V.), U01DA024417 (PI: W. G. I.), R01DA024417 (PI: W. G. I.) and K01DA037280 (PI: S. W.); National Institute of Mental Health of the National Institutes of Health under award numbers R01MH37860 (MTR; PI: D. T. L.) and R01MH066140 (SIBS; PI: M. M.); and Grant Number 60780 from the John Templeton Foundation as part of their Genetics and Human Agency initiative (SIBS; PIs: J. J. L., M. M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the John Templeton Foundation. The research was also supported by resources from the University of Minnesota’s Center for Magnetic Resonance Research (P41-RR008079, P41-EB015894, P30-NS076408), and the Minnesota Supercomputing Institute.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







