Findings of foetal and childhood abnormality have been interpreted as evidence of a distinct neurodevelopmental aetiology in a large minority of patients with schizophrenia (Reference Murray and LewisMurray & Lewis, 1987). A range of childhood developmental characteristics have been shown to be abnormal, specifically in those who are diagnosed with psychosis in adulthood (Reference Jones, Rogers and MurrayJones et al, 1994a ). It remains unclear exactly how those factors that affect childhood development predispose individuals to schizophrenia. Ventricular enlargement is found in the majority of controlled neuroimaging studies in schizophrenia (Reference McCarley, Wible and FruminMcCarley et al, 1999) However, the third ventricle has been examined much less frequently in first-episode subjects. It has been suggested that third ventricular enlargement may be a more specific finding in schizophrenia than lateral ventricular abnormality (Reference Bornstein, Schwartzkopf and OlsonBornstein et al, 1992). Neuropathology in midline structures mediating attention and information processing has been implicated as the cause of schizophrenia symptomatology (Andreasen, 1994a). Imaging work has focused on morphological changes in the diencephalon and limbic system, which may be related to third ventricle enlargement.
METHOD
Thirty-seven patients (26 men and 11 women) aged between 16 and 40 years were recruited as in-patients or out-patients from catchment areas comprising East, South-east and South-west London and West Kent, UK. Patients met DSM-IV (American Psychiatric Association, 1994) criteria for schizophrenia, schizophreniform psychosis or schizoaffective disorder as assessed using the Structured Clinical Interview for DSM-IV, patient version (SCID-P; Reference Spitzer, Williams and GibbonSpitzer et al, 1990). All patients were experiencing their first episode of psychosis with no more than 12 weeks of previous exposure to neuroleptic medication. Twenty-five comparison subjects (17 men and 8 women) were recruited from the general population through local advertising in the same catchment areas and were screened using the non-patient version of the SCID. Patients and controls were matched on age (within 3 years), gender, ethnicity and parental socio-economic status. Both ethnicity and parental socio-economic status were assessed using standard classifications (Office of Population Censuses and Surveys, 1991). Handedness was determined using the Edinburgh Inventory (Reference OldfieldOldfield, 1971).
Subjects who had a DSM-IV diagnosis of substance abuse or dependence, mental retardation, neurological disorder or had a medical condition that may affect brain structure or function were excluded from the study. In addition, control subjects were excluded if there was a personal history of any Axis I or II disorder or a family history of psychosis. Symptom measures were performed using the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OpierKay et al, 1987). Detailed childhood developmental information was collected from the mothers of 21 patients (15 men and 6 women) using the schedule outlined in Table 1. Classification of developmental status was a priori. Developmental delay was deemed present if ‘developmental score’ was either 1 (‘problems reported by mothers in one or more areas but no professional advice sought’) or 2 (‘professional advice sought by mother or referral to educational psychologist or speech therapist by school for at least one of the above')’). Those patients with no developmental problems reported by mother were classified as having no developmental delay. Developmental information was not available for the remaining 16 patients, whose mothers did not consent or were not contactable. Ethical approval was obtained from the Bethlem and Maudsley Ethics Committee (Research). After a complete description of the proposed study was discussed with each participant, written informed consent was obtained.
Table 1 Rating scale for developmental problems

| Score | Speech | Motor | Encopresis | Enuresis | Reading | Developmental score |
|---|---|---|---|---|---|---|
| 0 | No problems reported by mother | No problems reported by mother | No problems reported by mother | No problems reported by mother | No problems learning to read or spell | No problems reported by mother in any area |
| 1 | No talk other than ‘mama’ or ‘dada’ by age 3 years. Speech problems still exist at school entry. Either grammar or pronunciation faulty | Could not walk unsupported before 2 years | Soiling after age 4 years at least once a week over a period of at least 6 weeks | Bed wetting or day time wetting continuously beyond the age of 5 years at least once a week | Reading or spelling difficulties reported by mother | Problems reported by mother in one or more areas, but no professional advice sought |
| 2 | As above, but professional help sought or child referred to educational psychologist or speech therapist by school | As above, but professional help sought | As above, but professional help sought and physical cause excluded | Professional help sought and physical causes excluded | Remedial teaching for reading and spelling required, but no special schooling | Professional advice sought by mother or referral to educational psychologist or speech therapist by school for at least one of the above |
Image acquisition
Magnetic resonance imaging (MRI) scanning was performed on a 1.5-T G.E. Signa system at the Maudsley Hospital, London. Initially, a series of sagittal and axial scout views were acquired to correct for head tilt and to localise imaging coordinates. Next, the whole brain was scanned with a three-dimensional inversion-recovery-prepared fast-spoiled GRASS (SPGR) T1-weighted data-set. These T1-weighted images were obtained in the axial plane with 1.5-mm contiguous sections; TR was 11.3 ms, TI was 300 ms, TE was 2.2 ms and the flip angle was 20° with one data average and a 256 × 256 × 128 pixel matrix.
Measurements
Brain images were reoriented parallel to the anterior commissure—posterior commissure (AC-PC) line and the interhemispheric fissure prior to measurement. Volumetric measurements were obtained by stereological assessment using the MEASURE program (Reference Barta, Dhingra and RoyallBarta et al, 1997). All ratings were performed (by A.S. and J.L.) blind to diagnosis, with high interrater and intrarater reliability. Intraclass correlations ranged from 0.95 to 0.99 for measurements of whole brain and lateral and third ventricles.
Regions of interest
The lateral ventricular volume was that bounded anteriorly by the frontal lobe, medially by the septum pellucidum, intraventricular foramen and corpus callosum and posteriorly by the occipital lobe. The third ventricular volume was that bounded by the anterior commissure, the fornix, the stria medullaris, the pineal body, the superior and inferior colliculi, the midbrain and mamillary body, the thalamus and hypothalamus. Whole brain volume was measured as the area defined as the entire brain excluding ventricles, cerebrospinal fluid, cerebellum, dura mater and brainstem.
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS). Chi-squared analyses were used to look for differences in socio-economic status, handedness and gender distribution. Analyses of variance (ANOVAs) were used to examine for differences in age, years of education and height. Comparisons of regional volumes between groups were performed using the General Linear Model in SPSS. The ANOVA used whole brain volume or ventricular volume as dependent variables and height, age, gender and diagnosis as independent variables. Age was used to control for possible age-related changes in the subjects (Reference Cowell, Turetsky and GurCowell et al, 1994). Height was used as the independent variable to control for body and head size. Covariation with height alone did not change our findings. Height, instead of total brain volume, was used because it has been shown to be a predictor of general head size (Reference Andreasen, Flashman and FlaumAndreasen et al, 1994b ). Covarying for height has been used previously in MRI studies of schizophrenia (Reference Bilder, Wu and BogertsBilder et al, 1994; Reference Tibbo, Nopoulos and ArndtTibbo et al, 1998) and depression (Reference Sheline, Sanghavi and MintunSheline et al, 1999). Spearman's correlations were used to test for relationships between ventricular volumes and developmental scores. All analyses used a two-tailed estimation of significance, P<0.05 was regarded as significant.
RESULTS
Subject characteristics are outlined in Table 2. Patients and controls were well matched on all variables, with the exception of years of education (ANOVA, F=20, P<0.001). Patients had significantly less whole brain volume (F=5.9, P<0.02) and significantly larger third ventricle (F=5.9, P<0.02) and lateral ventricles (F=4.8, P<0.03) than controls. No group by gender interactions were found. Spearman's correlation coefficient showed a significant relationship between developmental score and third ventricle (ρ=0.48, P<0.03) and lateral ventricle (ρ=0.65, P<0.01) volumes. Third and lateral ventricular volumes were correlated significantly with each other (ρ=0.44, P<0.05). Years of education did not correlate with lateral or third ventricle volume in either group (all r>-0.2, P>0.4).
Table 2 Characteristics of study sample
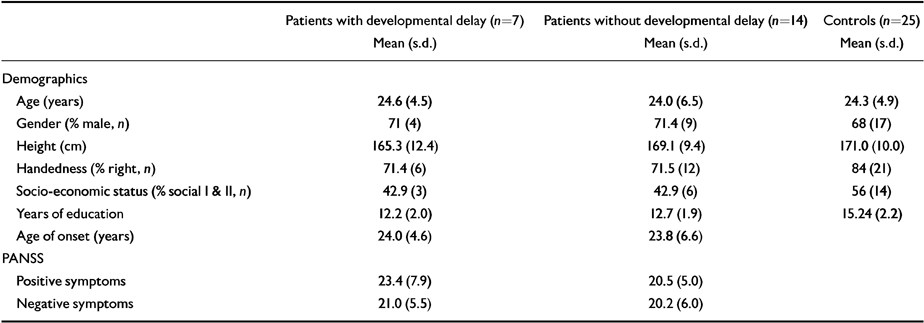
| Patients with developmental delay (n=7) Mean (s.d.) | Patients without developmental delay (n=14) Mean (s.d.) | Controls (n=25) Mean (s.d.) | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 24.6 (4.5) | 24.0 (6.5) | 24.3 (4.9) |
| Gender (% male, n) | 71 (4) | 71.4 (9) | 68 (17) |
| Height (cm) | 165.3 (12.4) | 169.1 (9.4) | 171.0 (10.0) |
| Handedness (% right, n) | 71.4 (6) | 71.5 (12) | 84 (21) |
| Socio-economic status (% social I & II, n) | 42.9 (3) | 42.9 (6) | 56 (14) |
| Years of education | 12.2 (2.0) | 12.7 (1.9) | 15.24 (2.2) |
| Age of onset (years) | 24.0 (4.6) | 23.8 (6.6) | |
| PANSS | |||
| Positive symptoms | 23.4 (7.9) | 20.5 (5.0) | |
| Negative symptoms | 21.0 (5.5) | 20.2 (6.0) | |
Follow-up comparisons between patients with and without developmental delay showed significantly greater third ventricular (F=5.3, P<0.03) and lateral ventricular (F=11.9, P<0.01) enlargement in those with a history of childhood developmental delay (see Table 3). There were no group differences in positive (F=0.05, P<0.32) or negative (F=0.08, P<0.78) symptom scores or age of onset (F=0.48, P<0.50).
Table 3 Volumetric measurements
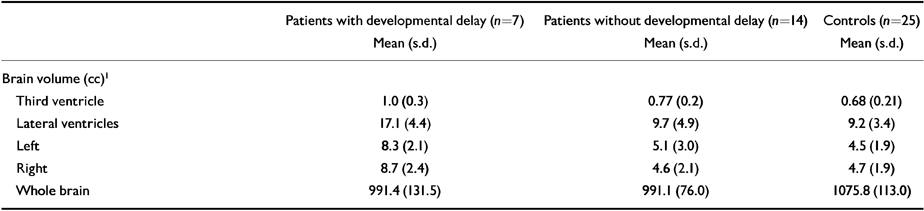
| Patients with developmental delay (n=7) Mean (s.d.) | Patients without developmental delay (n=14) Mean (s.d.) | Controls (n=25) Mean (s.d.) | |
|---|---|---|---|
| Brain volume (cc)1 | |||
| Third ventricle | 1.0 (0.3) | 0.77 (0.2) | 0.68 (0.21) |
| Lateral ventricles | 17.1 (4.4) | 9.7 (4.9) | 9.2 (3.4) |
| Left | 8.3 (2.1) | 5.1 (3.0) | 4.5 (1.9) |
| Right | 8.7 (2.4) | 4.6 (2.1) | 4.7 (1.9) |
| Whole brain | 991.4 (131.5) | 991.1 (76.0) | 1075.8 (113.0) |
DISCUSSION
This study showed significant enlargement of the third and lateral ventricles and reduced whole brain volume in patients experiencing a first episode of psychosis compared to healthy controls. The degree of ventricular enlargement, but not whole brain volume reduction, was greatest in those patients with a history of developmental disturbance in childhood. Positive relationships were found between enlargement of the third ventricle and lateral ventricle volumes and the presence of developmental delay. Specifically, enlargement of the third and lateral ventricles was related to developmental delay in childhood. Although the patient group as a whole showed larger ventricular volumes compared to controls, those patients without a history of delay were similar in terms of these volumes to normal control subjects (Table 3). In contrast to this, those patients with a history of developmental delay showed significantly larger ventricular volumes.
We set out to test the hypothesis that MRI-detected structural abnormality in schizophrenia is related to the preclinical course of the illness by investigating the developmental correlates of ventricular enlargement. We examined a group of subjects with adult-onset psychosis early in their first episode on whom information regarding childhood development was available. The neurodevelopmental model of schizophrenia attributes its cause to early disturbance of brain development before the presentation of psychotic symptoms. Therefore, the patient group was examined for differences in maternally recalled delay in developmental milestones. Those with a history of delayed development were compared with those without in terms of volumes of lateral and third ventricles. Ventricular volumes were used because they provide the most consistent evidence of structural abnormality in schizophrenia. The analysis reported here included only three brain regions because developmental information was not available on all subjects and the smallest possible number of regions of interest was chosen for analysis. The larger patient group had, in addition, significant deficits in cortical grey matter, temporal lobe grey matter and reduced thalamic volume. Future publications will report on these findings in more detail. Ventricular abnormality is the most robust structural finding in schizophrenia, although the third ventricle has been studied to a lesser extent. Therefore, investigation of the structural correlates of developmental delay was conducted with reference to ventricular volumes alone.
The results of our study indicate that enlargement of the third and lateral ventricles is present close to the onset of schizophrenia and is not attributable to the effects of the illness itself or to treatment. Our findings lend support to the view that schizophrenia involves disturbance of neurodevelopmental processes in some patients. The integration of MRI findings and premorbid findings may thus provide a greater understanding of the aetiology and pathogenesis of schizophrenia. Essentially, our findings are consistent with a neurodevelopmental model of schizophrenia involving localised periventricular deficiency of critical neuronal pathways. We suggest that maldevelopment of such pathways may be evidenced initially by delayed achievement of developmental milestones in association with ventricular enlargement and later by the emergence of psychotic symptoms.
Specificity of findings
Developmental delay was not correlated exclusively with either third or lateral ventricular volume. Both lateral and third ventricular volumes showed a significant association with the developmental measure. The absence of a specific association between third ventricle volume and a measure of developmental delay may be interpreted in a number of ways. First, contrary to previous reports (Reference Bornstein, Schwartzkopf and OlsonBornstein et al, 1992), third ventricle enlargement may not be a more specific finding in schizophrenia relative to lateral ventricle enlargement. Such a finding in previous studies may have been confounded by inconsistent measurement of ventricular volumes or atypical patient samples. Jones et al (Reference Jones, Harvey and Lewis1994b ) have suggested that third ventricle enlargement may be more specific to affective psychosis than schizophrenia or schizoaffective psychosis. Second, specific associations may exist between third and lateral ventricle volumes and disparate aetiological factors in schizophrenia, but these processes may not have an impact on the developmental characteristics measured in this study. Third, our study may have lacked sufficient power to detect a true difference in the relationships between third and lateral periventricular pathology and developmental aspects of the early prepsychotic phase of the illness. Our findings are consistent with a common periventricular mechanism affecting both third and lateral ventricles and associated with developmental disturbance in childhood. Abnormality in periventricular structures early in childhood may result in both ventricular enlargement and disturbed development, perhaps owing to aberrant development of proximate neural pathways. This discussion focuses on the significance of third ventricle abnormality.
Behaviour and cognition
Previous computed tomography (CT) studies have shown contradictory findings in relation to third ventricle changes and are of uncertain clinical relevance. However, in this study we show preliminary evidence that among patients with first-episode psychosis, enlargement of ventricles detected in adulthood is related to developmental delay in childhood. This is consistent with earlier work showing that behavioural abnormalities in childhood, analogous to negative schizophrenic symptomatology, are associated with third ventricle widening in people who have a high genetic risk of the illness (Reference Dykes, Mednick and MachonDykes et al, 1992). Third ventricle enlargement has been shown to be correlated significantly with poor neuropsychological performance (especially on tests of frontal lobe function, attention and concentration) and may be found in a more severely ill subgroup of patients (Reference Bornstein, Schwartzkopf and OlsonBornstein et al, 1992).
Genetics
The possible aetiological significance of third ventricle abnormality is evident in the results of family studies. Sharma et al (Reference Sharma, Deboulay and Lewis1997) have shown significantly increased third ventricle volume in obligate carriers among families multiply affected with schizophrenia. Keshavan et al (Reference Keshavan, Montrose and Pierri1997) demonstrated that first-degree relatives of patients with schizophrenia have enlarged third ventricles. More recent work has shown enlargement of the third ventricle to be correlated with epithalamus calcifications and cortical atrophy, suggestive of a lesion of the third periventricular region (Reference Caputo, Ghiringhelli and DieciCaputo et al, 1998). The origin of this finding is suggested by the association of third ventricular pathology with the absence of the adhesio interthalamica in first-episode patients, indicative of early developmental disturbance (Reference Snyder, Bogerts and WuSnyder et al, 1998).
Neurodevelopmental disorder
An association between ventricular enlargement and developmental delay in childhood is consistent with a neurodevelopmental disorder and in keeping with other lines of evidence pointing to disturbance of normal brain maturation in schizophrenia. Such evidence includes the first presentation of illness typically occurring in adolescence or early adulthood and the presence of structural and functional abnormalities close to or prior to the onset of illness. Similarly, premorbid intellectual deficits (Reference Jones, Rogers and MurrayJones et al, 1994a ) point to early developmental disturbance, as do minor physical anomalies (Reference Lane, Kinsella and MurphyLane et al, 1997) and markers of aberrant prenatal growth (Reference Davis and BrachaDavis & Bracha, 1996). The finding of more ‘normal’ third and lateral ventricle volumes, relative to controls, in those patients without a history of developmental delay is consistent with a model of schizophrenia that includes neurodevelopmental and non-developmental sub-types (Reference Murray, O'Callaghan and CastleMurray et al, 1992). The most plausible interpretation of our findings is that abnormality of periventricular brain structure and associated childhood developmental disturbance are both attributable to altered neurological development much earlier in life. Paus et al (Reference Paus, Zijdenbos and Worsley1999) reported evidence for protracted structural maturation of fibre pathways supporting motor and speech functions during childhood and adolescence. The proposed model hypothesises that insult to neurodevelopment results in a lesion to periventricular brain structure and malfunction of cognitive and neurological function presenting as developmental delay in childhood.
Periventricular lesion?
Magnetic resonance imaging findings have not been generalised to all patients with schizophrenia and structural abnormalities may arise through different mechanisms. With regard to ventricular enlargement, no consistent correlation has been demonstrated between the degree of enlargement and loss of contiguous brain volume. This may be explained by the limitations of current methods of measurement or by the involvement of independent pathological processes in the disease. It may also be due to disproportionate volume loss in localised, periventricular structures (Reference HarrisonHarrison, 1999). Disturbance of the autonomic nervous system (ANS) has been shown to be associated with negative symptom schizophrenia (Reference Frith, Stevens and JohnstoneFrith et al, 1979) and with widening of the third ventricle (Reference Cannon, Fuhrmann and MednickCannon et al, 1988). Widening of the third ventricle has been shown to be associated with negative symptoms (Reference Andreasen, Olsen and DennertAndreasen et al, 1982). It has been suggested, therefore, that damage to the third periventricular area (which accommodates ANS centres) is involved in the neurobiology of schizophrenia (Reference Dykes, Mednick and MachonDykes et al, 1992). Third ventricle enlargement may be indicative of disruption of cortico-striato-thalamo-cortical circuitry critical to limbic functioning (Reference Bornstein, Schwartzkopf and OlsonBornstein et al, 1992) or cortico-subcortical feedback circuits (Reference Caputo, Ghiringhelli and DieciCaputo et al, 1998). Such a model of the disorder is consistent with the view that vulnerability to and expression of schizophrenia are dependent on the disturbance of a number of interconnected brain systems (Reference Bullmore, Frangou and MurrayBullmore et al, 1997).
The nature of the putative insult to periventricular areas is subject to debate. The critical process may relate to axon diameter or myelination or to excessive neuronal pruning. Defects in limbic circuits may account for aberrant functioning, evident as symptoms of schizophrenia such as disruption of higher cognitive processing (attention and memory as well as changes in drive, motility and affect). Diencephalic structures close to or bordering on the third ventricle are prominent relays in the limbic pathways. Third ventricle enlargement may index abnormalities of these surrounding structures. Abnormality of cortico-striato-thalamic circuits caused by loss of cortical efferents or caudate volume might account for lateral ventricular enlargement because frontal and temporal horns are adjacent to limbic circuits. Sensorimotor pathways may be more specifically affected by pathology surrounding the lateral ventricles. Abnormal development of such pathways provides a possible explanation for the findings of our study.
Our data support a model of increased ventricular volume secondary to reduced periventricular matter caused by a lesion of cortico-subcortical circuitry. The specific cause of these deficits is unclear but is evidenced in the cognitive and psychotic features of the disease. A relationship between both lateral and third ventricle volumes and developmental delay may reflect abnormal function in those periventricular pathways serving higher cognitive processes required for normal neurological and psychosocial functioning. Pathways involving midline structures may be especially susceptible in schizophrenia, as indicated by previous work and highlighted by the results of the current study.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Investigation of patients early in their first episode of psychosis reveals structural brain abnormalities that are not attributable to the impact of chronic illness or treatment.
-
▪ Greater structural abnormality appears to be associated with more severe developmental disturbance in childhood, prior to the onset of psychosis.
-
▪ A lesion of third periventricular areas close to the limbic system and to cortico-subcortical pathways may be relevant to the neurobiology of the disease.
Identification of those brain structural abnormalities that are specific to the disease will increase understanding of the aetiology of schizophrenia and provide a means for early detection and treatment.
LIMITATIONS
-
▪ The childhood developmental information was retrospective and based on the mother's recollections. It is therefore subject to bias.
-
▪ No developmental information was gathered from control subjects. Therefore, we failed to rule out a relationship between third ventricle volume and developmental delay in normal subjects without a history of psychosis. The number of patients with developmental delay was small and we cannot exclude the possibility that our finding of a positive correlation between third ventricular volume and developmental delay has arisen by chance. However, this is unlikely given the consistent differences found in lateral and third ventricle volumes between controls and patients, which show progressively greater enlargement in those patients with developmental delay (see Table 3).
-
▪ It is not certain that the presence of developmental problems is linked reliably to specific brain maturation periods or to specific neuropsychiatric diagnoses.
ACKNOWLEDGEMENTS
The authors acknowledge the collaboration of Katy Piper, Sophia Rabe-Hesketh, Jessica Sheringham, Steven Williams, Andrew Simmons and the staff of the Institute of Psychiatry & Maudsley Hospital Neuroimaging Research Group, and the support of Grosvenor House Group Estates and Psychmed Ltd.






eLetters
No eLetters have been published for this article.