Cognitive–behavioural therapy (CBT)Reference Castell, Kazantzis and Moss-Morris1–Reference Wiborg, van Bussel, van Dijk, Bleijenberg and Knoop4 has been shown to significantly reduce fatigue severity and functional impairment in patients with chronic fatigue syndrome (CFS). However, face-to-face CBT is an intensive treatmentReference Castell, Kazantzis and Moss-Morris1 and treatment capacity is limited. Internet-based CBT (iCBT) might help to lower the threshold for seeking help and reduce the burden of the intervention for patients. iCBT makes treatment easier to access for more patientsReference Hedman, Ljotsson and Lindefors5 and might promote self-efficacy. However, iCBT for CFS has only been tested in adolescents,Reference Nijhof, Bleijenberg, Uiterwaal, Kimpen and van de Putte6 where it was found to be effective. But, as adolescents are more inclined to use e-health,Reference Andreassen, Bujnowska-Fedak, Chronaki, Dumitru, Pudule and Santana7 these outcomes cannot automatically be translated to adults. Guided variants of internet treatment are more effective than unguided variants,Reference Spek, Cuijpers, Nyklicek, Riper, Keyzer and Pop8 but require therapist time to deliver them. Most guided variants require patients and therapists to respond at pre-set intervals determined by a protocol. We tested the efficacy of iCBT with this type of guidance in a protocol-driven feedback condition. We also tested the efficacy of iCBT in which guidance was only given when patients asked for it, i.e. a feedback-on-demand condition. Both interventions were compared with a waiting-list condition and to each other. We also considered the therapist time needed to deliver the intervention for both forms of iCBT.Reference Janse, Worm-Smeitink, Bussel-Lagarde, Bleijenberg, Nikolaus and Knoop9
Method
Trial design
The efficacy of iCBT was determined in a three-arm parallel randomised controlled trial (RCT), using randomisation with two versions of iCBT and a waiting list (1:1:1) comparing baseline outcomes to those obtained in a second assessment (T 1) 6 months post-randomisation. Six months was the regular waiting time before patients could start with routine clinical treatment. The study has been described in a protocol paperReference Janse, Worm-Smeitink, Bussel-Lagarde, Bleijenberg, Nikolaus and Knoop9 and registered with the Netherlands Trial Register (NTR4013).
Participants
A total of 240 patients participated in the study. They were consecutively referredReference Janse, Worm-Smeitink, Bussel-Lagarde, Bleijenberg, Nikolaus and Knoop9 to the Expert Center for Chronic Fatigue, a tertiary treatment facility for chronic fatigue at a university hospital. Before referral, consultants at the out-patient clinic of the department of Internal Medicine assessed their medical status to decide whether they had been sufficiently examined to rule out a medical explanation for their fatigue. If their medical evaluation was deemed insufficient, patients were seen again for anamnesis, full physical examination, case-history evaluation and laboratory tests following the national CFS guidelines,10 which are in accordance with the Centers for Disease Control and Prevention (CDC) guidelines.Reference Fukuda, Straus, Hickie, Sharpe, Dobbins and Komaroff11, Reference Reeves, Lloyd, Vernon, Klimas, Jason and Bleijenberg12 If patients met the CDC criteria for CFS, they were referred to our centre. Psychiatric comorbidity that could explain the fatigue was ruled out using the Mini International Neuropsychiatric Interview.Reference Lecrubier, Sheehan, Weiller, Amorim, Bonora and Sheehan13
After referral, all eligible patients were informed and invited to participate in the trial during the standard clinical assessment. Patients were eligible if they met the following inclusion criteria: (a) aged 18 years or older; (b) being severely fatigued as indicated by a score of 35 or higher on the fatigue subscale of the Checklist Individual Strength (CIS);Reference Vercoulen, Swanink, Fennis, Galama, van der Meer and Bleijenberg14, Reference Dittner, Wessely and Brown15 (c) being severely disabled, operationalised as a score of 700 or higher on the Sickness Impact Profile 8 (SIP8);Reference Bergner, Bobbitt, Carter and Gilson16 (d) able to speak, read, and write Dutch; (e) able to use a computer and having access to the internet. Exclusion criteria were: (a) being involved in legal procedures concerning disability benefit claims; (b) participating in other CFS research. All patients were asked to refrain from seeking treatment for their fatigue elsewhere for the duration of the study.
Interventions, treatment adherence and treatment integrity
The two iCBT conditions tested in this trial are based on a face-to-face CBT for CFS protocol.Reference Knoop and Bleijenberg17 The cognitive–behavioural model of CFS assumes that fatigue-related behaviours and beliefs perpetuate fatigue and impairment (for further details of the treatment see supplementary Data 1, available at http://dx.doi.org/10.1192/bjp.2017.22). The treatment is tailored to a patient's current activity pattern as assessed with actigraphy. Patients received a private username and password, ensuring private communication with their therapist. iCBT consisted of seven modules aimed at change of fatigue-related behaviours and beliefs.Reference Janse, Worm-Smeitink, Bussel-Lagarde, Bleijenberg, Nikolaus and Knoop9 The iCBT modules were opened when patients had read general treatment information and had set their personal goals. The times at which their therapist would contact them were given on the first page of the portal. The final treatment module that covered relapse prevention became accessible after patients had completed the digital evaluation assignment.
Therapist guidance was manipulated in that in the protocol-driven feedback condition, patients were asked by their therapist to report on their progress by email according to a prescribed schedule of at least fortnightly. The therapist provided feedback and sent reminders if the schedule was not adhered to. Therapist adherence to the feedback schedule was monitored (at least fortnightly for 6 months with a minimum of 12 messages).
In the second treatment arm, the feedback-on-demand condition (referred to as support on demand in the protocol paper), support was tailored to the individual needs of the patient in that feedback was only provided when the patient indicated a need for advice. Patients did not receive any reminders.
Treatment-adherence criteria for the patients allocated to the protocol-driven condition were strict. It was verified whether all treatment modules had been accessed and whether email contact was made at least fortnightly. For the patients following the feedback-on-demand condition monitoring was restricted to checking whether each module had been opened. An integrity check was performed and for this the content of 5% of all emails the therapists had sent were evaluated. Two authors (A.J. and H.K.) coded treatment delivery dichotomously, discerning interventions delivered according to protocol and interventions not delivered according to protocol. In cases of disagreement, the item was discussed until consensus was reached. Finally, how the web-portal was used was assessed by recording the number of times patients logged in, mean duration of sessions, number of opened treatment modules and number of emails sent.
Therapists
All 12 therapists delivering the interventions were experienced clinical psychologists trained in treating patients with CBT for CFS.Reference Janse, Worm-Smeitink, Bussel-Lagarde, Bleijenberg, Nikolaus and Knoop9
Outcome measures
Primary outcome measure
Fatigue was assessed with the fatigue severity subscale of the CIS, which consists of eight items scored from 1 to 7, with subscale scores varying between 8 and 56. A score higher than 35 indicates severe fatigue. The CIS has proven to be a reliable and valid instrument in patients with CFS.Reference Vercoulen, Swanink, Fennis, Galama, van der Meer and Bleijenberg14, Reference Dittner, Wessely and Brown15
Secondary outcome measures
Level of functional impairment was assessed with the total score of the SIP8. The SIP8 gauges overall functional impairment in the following eight domains: ambulation, home management, mobility, alertness behaviour, sleep and rest, work, social interactions and recreation and pastimes. A weighted total score was computed from the scores on the eight subscales, (range 0–5799). Higher scores are indicative of more severe overall impairment. This widely used measure has good reliabilityReference Bergner, Bobbitt, Carter and Gilson16 and validity.Reference Knoop, Bleijenberg, Gielissen, van der Meer and White18
Physical functioning was assessed with the physical functioning subscale of the Medical Outcomes Survey Short Form-36 (SF-36)Reference Stewart, Hays and Ware19 where scores also range from 0 (maximum limitations) to 100 (no limitations). The SF-36 is a reliable and valid instrument to assess self-reported health status in patients with CFS.Reference Stewart, Hays and Ware19
Psychological distress was assessed with the Symptom Checklist 90 (SCL-90),Reference Derogatis20 whose 90 items are answered on a five-point Likert scale. Total scores range from 90 to 450, with higher scores being indicative of more psychological distress. The SCL-90 has good reliability and discriminating validity.Reference Arrindell and Ettema21
Fatigue scores in the normal range were defined as a score of <35 on the CIS fatigue severity subscale at second assessment, together with a reliable change index (RCI) >1.96.
Invested therapist time in hours and minutes per patient, was recorded by therapists on an excel sheet for comparison with CBT provided face-to-face or by telephone, for which a mean of 12 h is reported in the literature.Reference Castell, Kazantzis and Moss-Morris1, Reference van Der Schaaf, Schmits, Roerink, Geurts, Toni and Roelofs22 Additionally, 2 h of time was added to the therapist time spent per patient representing the two diagnostic assessment sessions that are part of clinical routine.
Adverse events were assessed 6 months post-randomisation (T 1). All participants were asked if they had experienced new symptoms or an increase of existing symptoms during therapy or the waiting period. Patients who received iCBT were asked if they had experienced negative side-effects of the therapy. The adverse-event assessment was added to the test battery after an update of the internet portal in March 2014. Clinically significant exacerbation was computed for fatigue severity, level of functional impairment, physical functioning and psychological distress. Indicating an RCI >1.96 between two measurements, we set clinically significant exacerbation at a RCI <–1.96Reference Jacobson and Truax23 (for visual illustration see supplementary Fig. 1).
After trial registration but before the start of the study, we added the Chalder Fatigue Questionnaire (CFQ)Reference Chalder, Berelowitz and Pawlikowska24 and Work and Social Adjustment Scale (WSAS)Reference Mundt, Marks and Shear25 to the assessment battery as both instruments are often used in CFS intervention studies. Adding them aids comparison of treatment effects between studies (see also Worm-Smeitink et al)Reference Worm-Smeitink, Nikolaus, Goldsmith, Wiborg, Ali and Knoop26 Another deviation of the original study protocol was the decision not to determine quality-adjusted life-years; because of limited resources we were unable to perform a cost-effectiveness study. The quality of life questionnaire (the EQ-6D)Reference Hoeymans, van Lindert and Westert27 was, however, still part of the assessment battery.
Sample size
We expected a mean difference on the CIS fatigue severity score between each web-based CBT format and the waiting-list condition of 6.7 points with a standard deviation of 12.1 for the iCBT conditions and 8.7 for the waiting-list condition.Reference Knoop, van der Meer and Bleijenberg28 Assuming a power of 0.95 and a two-sided alpha of 0.025, 76 patients were needed in each study condition when a t-test was used. This sample size could be multiplied with (1 – 0.3422) for using ANCOVA.Reference Borm, Fransen and Lemmens29 This meant that 68 patients with complete data were needed per condition. Assuming a drop-out rate of 15%, a sample size of 80 patients was needed per study condition. In an exploratory analysis, we determined whether there was a difference in efficacy between both iCBT conditions. Based on 68 patients with complete data per condition, a two-sided P-value of 0.05 and a power of 0.80, a difference between both iCBT conditions could be detected, corresponding to a moderate effect size (Cohen's d of 0.48).
Randomisation
All patients attended two therapist-conducted intake sessions and completed a baseline assessment (T 0) as part of the clinical routine in our treatment centre. If eligible and willing to participate at the second session, patients were asked to give their written consent to the therapist who performed the intake sessions, after which they were randomly allocated (computer-generated; in blocks of 12, which was only known to the researcher and the statistician) to one of the three trial conditions. The randomisation was performed by a study assistant in the presence of the patient and therapist.
Patients were not informed about the existence of the two iCBT treatments to avoid contamination between the two treatment arms. Further contact between the study assistant and the patients was restricted to one standardised email containing the link to the T 1 assessment. Patients allocated to the waiting list started face-to-face CBT after T 1. Statistical analysis was performed on locked data files masking the researchers for allocation to condition. The analysis was done by an independent researcher. Post-analysis, the data manager unmasked the data to enable the authors to interpret results.
Statistical methods
It was hypothesised that both iCBT conditions would lead to improvements in fatigue and secondary outcomes compared with a waiting-list control. ANCOVA was performed with the CIS fatigue scores at T 1 as the dependent variable, its T 0 score as covariate, and condition as fixed factor. Differences in the proportion of patients with fatigue scores in the normal range were assessed using chi-squared tests. We also used ANCOVA for our secondary outcome measures and for comparisons between the iCBT conditions. The latter analysis was exploratory as was already mentioned in the protocol paperReference Janse, Worm-Smeitink, Bussel-Lagarde, Bleijenberg, Nikolaus and Knoop9 because there were no previous findings to determine the power needed to test our hypothesis. However, we expected more improvement in primary and secondary outcomes following iCBT with protocol-driven feedback than following iCBT with feedback on demand. We also expected that more therapist time would be needed to deliver iCBT with protocol-driven feedback.
Difference in therapist time between both iCBT conditions was analysed with an independent t-test and additional bootstrap procedure if the therapist times were too skewed. We conducted post hoc ANCOVAs for the CFQ, the WSAS and the assessment of physical activity using actigraphy to determine the effects of the intervention. Furthermore, we used ANCOVA with T 0 assessment and gender as covariate and CIS fatigue at T 1 as dependent in testing the potential correlation to outcome. The number of patients per study arm reporting adverse events and/or clinically significant symptom exacerbation was compared using chi-squared tests.
Our outcome analyses were based on ITT with multiple imputation (20 imputed data-sets) for missing observations in primary and secondary outcomes, assuming that data were missing at random. Separate from the imputation, we performed two stand-alone sensitivity analyses for missing data on our primary outcome: (a) we hypothesised the mean change in the control group for missing data in that group and hypothesised no change for missing data in the iCBT conditions; (b) we hypothesised an improvement for the control group and deterioration for the iCBT conditions. Specifically, the progression of the iCBT conditions was used for missing data in the waiting-list condition and the maximum score on the CIS fatigue subscale was used for missing iCBT data.
A per-protocol analysis was performed for fatigue severity including completers only, i.e. patients who had started treatment and had complete data without receiving treatment elsewhere. There was no data-monitoring board, but data entry was checked by an independent data manager who was also responsible for data encryption and storage. IBM SPSS statistics (version 22) were used for all statistical analyses.
Results
We assessed 398 patients between 24 April 2013 and 24 June 2015 who were consecutively referred to our clinic because of severe fatigue and impairment and met CDC criteria for CFS. Of these 398, 240 eligible patients remained and were randomly allocated to the three study conditions (Fig. 1).

Fig. 1 Flow of patients through the study.
The primary outcome measure was completed by 234 patients (97.5%). See Table 1 for the baseline patient characteristics. With independent t-tests, chi-squared tests or Mann–Whitney U-tests we tested potential baseline differences between all study conditions. Patients on the waiting list had a significantly higher education level (P = 0.0022) than patients from the feedback-on-demand iCBT group. Significantly more patients reported unrefreshing sleep in the protocol-driven iCBT than in the feedback-on-demand group (P = 0.0125).
Table 1 Baseline patient characteristics per study conditiona

iCBT, internet-based cognitive–behavioural therapy; IQR, interquartile range.
a. Test statistics were: the independent t-test where variables are mean (s.d.); chi-squared for n (%); and the Mann–Whitney U-test for median (IQR). Percentages were rounded to whole numbers.
b. Checklist Individual Strength.
c. Sickness Impact Profile 8.
d. Medical Outcomes Survey Short Form–36.
e. Symptom Checklist-90.
f. Beck Depression Inventory for Primary Care, total score ≥4.Reference Beck, Guth, Steer and Ball30
g. Body dysmorphic disorder (mild), bulimia nervosa, obsessive–compulsive disorder, somatisation disorder, conversion disorder.
Six patients were included with less than four CDC symptoms (one being randomised to the waiting list and five to the on-demand treatment). Twenty-five patients started another treatment for CFS during the study (n = 8/8/9; equally spread over conditions with medical, psychological and alternative treatments). Median treatment length was 27 weeks for both iCBT conditions and mean waiting time was 26 weeks for the waiting list.
At T 1, patients who followed protocol-driven iCBT were significantly less fatigued than those awaiting treatment (CIS fatigue; Table 2 and Fig. 2). The two sensitivity analyses and per-protocol analysis confirmed this finding (supplementary Table 1). Compared with waiting-list controls, patients reported less overall functional impairment (SIP8), less psychological distress (SCL-90) but no significant improvement on physical functioning (SF-36) following protocol-driven iCBT. Significantly more patients had fatigue scores in the normal range following protocol-driven iCBT than controls (protocol-driven 29/80 (36%) v. waiting list 12/80 (15%): χ2(1,n = 160) = 9.5, P = 0.0021; number needed to treat (NNT) = 4.7) (supplementary Fig. 1).
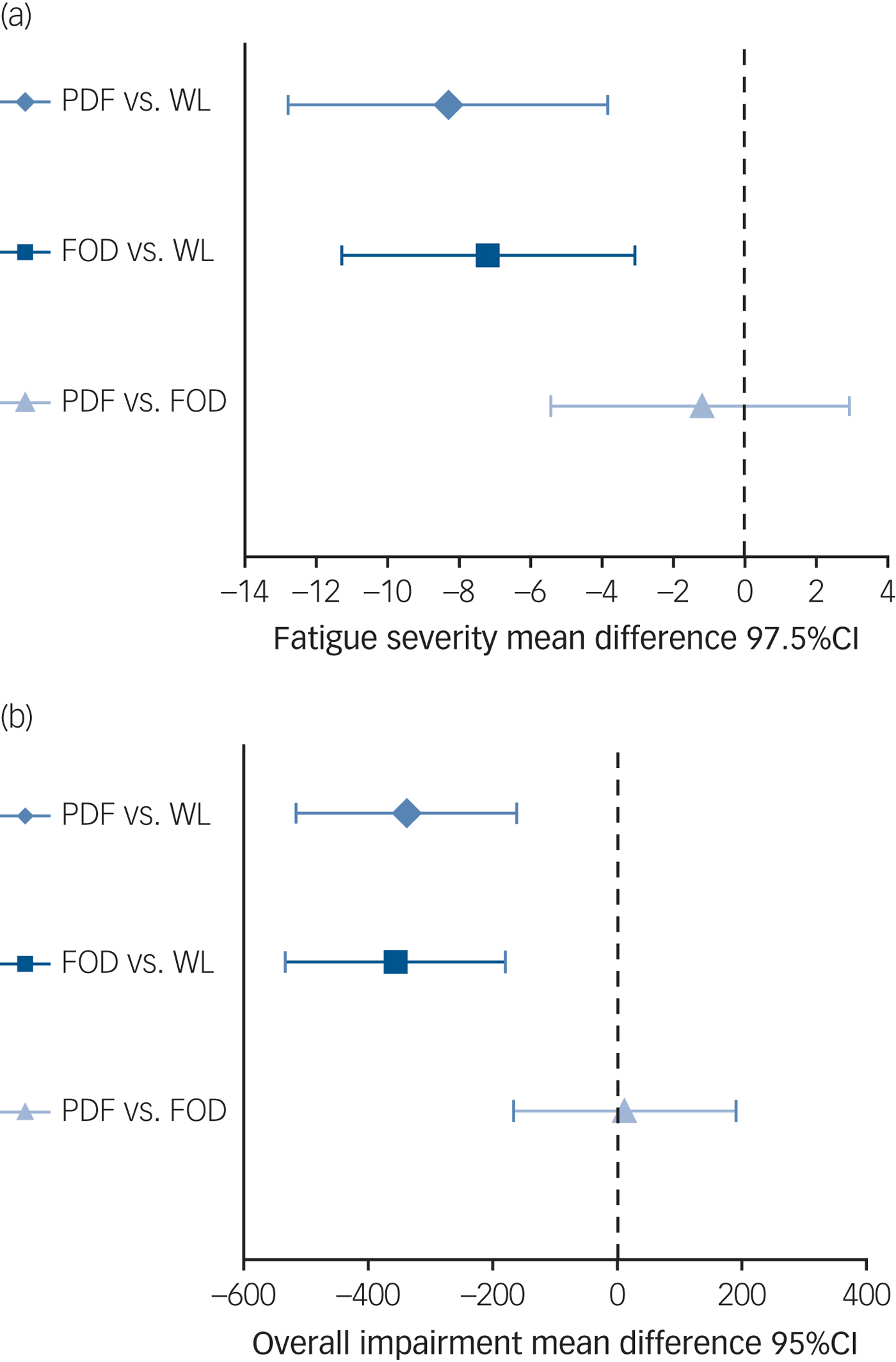
Fig. 2 Treatment differences for (a) fatigue and (b) overall impairment at 6 months.
Table 2 Effects for the patients receiving internet-based cognitive–behavioural therapy (iCBT) and the controls based on intention-to-treat analyses

a. Checklist Individual Strength
b. Sickness Impact Profile 8.
c. Medical Outcomes Survey Short Form–36.
d. Symptom Checklist-90.
At T 1, patients that followed on-demand iCBT were significantly less fatigued than those awaiting treatment (Table 2 and Fig. 2). The two sensitivity analyses and per-protocol analysis confirmed this result (supplementary Table 1). Compared with waiting-list controls, patients reported less overall functional impairment, significant improvement in physical functioning and less psychological distress following feedback-on-demand iCBT. The covariate gender was added to the model and all aforementioned analyses were repeated. This did not change the pattern of results, the covariate gender did not reach significance. Significantly more patients had fatigue scores in the normal range following treatment than the controls (on demand 34/80 (43%) v. waiting list 12/80 (15%): χ 2(1,n = 160) = 14.8, P = 0.0001; NNT = 3.6) (supplementary Fig. 1).
We found no significant differences on all outcome measures between both iCBT formats (fatigue: B = −1.2; t(157) = −0.6, P = 0.5589; overall functional impairment B = 11.2; t(157) = 0.1, P = 0.9027 (Fig. 2); physical functioning B = −3.4; t(157) = −1·1, P = 0.2628; psychological distress B = −1.6; t(157) = −0.3, P = 0.7466 and with fatigue scores in the normal range χ 2(1,n = 160) = 0.6, P = 0.4392). With this small treatment outcome difference between both formats on fatigue severity (Cohen's d = 0.04, 95% CI −0.03 to 0.11), a large number of patients (n > 10 000) would have needed to participate in order to reach significance.
The independent t-test indicated a significant difference in therapist time between both iCBT conditions (t = −4.13, P < 0.0001). The bootstrap procedure confirmed this result, i.e. protocol-driven iCBT required significantly more time to deliver than feedback-on-demand iCBT (mean 6 h 9 min, s.e. = 2 h 17 min and mean 4 h 37 min, s.e. = 2 h 23 min, respectively; mean difference: −92; bias, −0.45, bias-corrected and accelerated (Bca) 95% CI = −132 to −51, P = 0.001).
No serious adverse events were reported. Some patients indicated adverse events: 4 of 38 (11%) patients in the protocol-driven condition, 7 of 39 (18%) in the on-demand condition, and 12 of 46 (26%) in the control condition (see supplementary Table 2 for details and the patients’ perception of iCBT side-effects). The chi-squared analysis revealed no significant differences for the three conditions in the proportion of patients reporting an exacerbation of symptoms and/or functional impairments.
Our integrity check showed that 90.3% of the interventions were delivered according to protocol with an interrater reliability (Cohen's Kappa) of 0.96. A total of, 4 of 80 (5%) patients in the protocol-driven condition and 6 of 80 (8%) in the on-demand condition did not start the intervention. Without taking the relapse module into account, 39 (49%) patients in the protocol-driven condition were adherent to following our criteria of emailing fortnightly and having opened all modules. Of the patients in the on-demand condition, 74 (93%) were adherent to following our criterion of having opened all modules. When the relapse module was taken into account, this percentage dropped considerably to 16 and 19%, respectively (see supplementary Table 3). Finally, the post hoc analyses revealed that CFQ scores and social impairment (WSAS) were significantly reduced and objectively assessed activity was significantly increased following the iCBT conditions compared with the waiting list (supplementary Table 1).
Discussion
Main findings
To our knowledge, this study is the first RCT to report on the efficacy of iCBT for adults with CFS. Comparing iCBT outcomes with those of patients allocated to the waiting list, we found a significantly larger reduction of fatigue severity, overall impairment and psychological distress in the treatment groups, with approximately 40% of completers reporting fatigue scores within the normal range. Our results are in line with the findings of studies testing the efficacy of web-based interventions for mental disorders (e.g. depression, post-traumatic stress disorder and anxiety disorders).Reference Andrews, Cuijpers, Craske, McEvoy and Titov31
Comparison of feedback-on-demand with protocol-driven iCBT
Having therapists providing feedback according to a protocol, requiring at least fortnightly patient–therapist email interactions or on demand, resulted in a significant reduction of therapist time compared with the time needed to deliver CBT face-to-face or by telephone (mean therapist time in our study 6 h 9 m and 4 h 37 m, respectively v. 12 h reported in the literature). Furthermore, the therapists needed significantly less time for the on-demand treatment than they did for the protocol-driven treatment without the former treatment being less efficacious. Outcomes for the two iCBT conditions did not significantly differ. Our hypothesis that protocol-driven feedback would be more effective than feedback on demand did not hold. We clearly overrated the influence of set guidance by a therapist over feedback on demand. With the present effect size of Cohen's d = 0.04 between iCBT conditions, the sample size was too small to detect a significant difference between iCBT conditions. However, we think this difference and its confidence interval is of little clinical relevance. One might argue that feedback on demand is superior to the protocol-driven iCBT condition when one takes therapist time spent into account. However, other aspects might be equally or even more important, such as patient satisfaction or being able to plan therapist workload in advance.
Safety of CBT for CFS
Internet makes evidence-based interventions more accessible to more patients, especially those living far from healthcare facilities and those whose mobility is compromised by their condition. There is an ongoing debate in the literature and among patient advocacy groups that challenge the efficacy and safety of CBT for CFS. First, in line with previous studies this study has shown that a subgroup of patients with CFS were able to reduce their fatigue severity to healthy proportions and reduce their overall impairment and improve psychological well-being.Reference Knoop, Bleijenberg, Gielissen, van der Meer and White18, Reference White, Goldsmith, Johnson, Chalder and Sharpe32 Second, this study has shown, in line with previous research, that CBT is a safe intervention.Reference Heins, Knoop, Prins, Stulemeijer, van der Meer and Bleijenberg33, Reference White, Goldsmith, Johnson, Potts, Walwyn and DeCesare34 Unfortunately, only half of the patients in our trial were asked to report on the occurrence of adverse events as this evaluation was not added until a portal update halfway through the study. Still, the available data did not show more patients with adverse events in the iCBT conditions compared with the waiting-list condition and none of the adverse events reported were serious. Furthermore, we found no evidence of a higher prevalence of clinically significant exacerbation in fatigue and other outcomes in the treatment conditions.
One could argue that the use of a waiting-list control does not control for non-specific therapy factors and limits the external validity. However, a meta-analysis that studied active placebo conditions for CFS did show low responses,Reference Cho, Hotopf and Wessely35 as was also true for standardised specialist medical care.Reference White, Goldsmith, Johnson, Potts, Walwyn and DeCesare34 If face-to-face CBT was added as a third arm instead of a waiting list, the trial would have shifted toward an effectiveness trial. If iCBT was shown to be less effective than face-to-face therapy we would not have been able to conclude that the more efficient iCBT is an efficacious treatment for a substantial subgroup of patients.
Impact of iCBT on physical functioning
One iCBT condition did not result in a significant increase in physical functioning. This seems remarkable as previous studies did find positive effects of face-to-face CBT on physical functioning (for example White et al).Reference Price, Mitchell, Tidy and Hunot34 Previous studies, however, often used compromised physical functioning as an inclusion criterion, excluding patients who score within the ‘normal range’ on physical functioning. In our study these patients could be included if they reported severe impairments in other domains of functioning, like work or social functioning, as assessed with the SIP8. The fact that our study did not select on the level of physical functioning will make it more difficult to find an effect of iCBT on physical functioning. It would be interesting to directly compare iCBT and face-to-face CBT in a sample of patients with CFS with a compromised physical functioning scoring below a cut-off on the SF-36 to determine if the interventions differ in their effect on physical functioning. A post hoc analysis showed that objectively assessed physical activity significantly increased after iCBT. However, this might be an accidental finding, taking the amount of missing data into account and previous research that did not find an increase in physical activity following CBT.Reference Wiborg, Knoop, Stulemeijer, Prins and Bleijenberg36
Limitations
The effects of iCBT were only assessed 6 months post-randomisation as the medical ethical committee considered it unethical to let patients wait longer than the regular waiting period for start of treatment in usual care. Moreover, step two of our (stepped care) study consisted of face-to-face therapy if patients were still fatigued at the second assessment, therefore ruling out a controlled follow-up. More men participated in this study as compared with other CBT for CFS trials. This can be explained by the inclusion criteria of another study that only included female patients with CFSReference van Der Schaaf, Schmits, Roerink, Geurts, Toni and Roelofs22 out of the same pool of patients. There were no indications that gender was correlated with treatment outcome. As to our strict adherence criteria, a substantial number of patients did not fully adhere to the interventions. Adherence might be improved by sending standardised automatic emails or mobile text reminders.
Face-to-face CBT seems to result in superior fatigue effect sizes (up to d = 1.0)Reference Wiborg, van Bussel, van Dijk, Bleijenberg and Knoop4, Reference Worm-Smeitink, Nikolaus, Goldsmith, Wiborg, Ali and Knoop26 compared with our study results of iCBT with a medium effect size of d = 0.6. This suggests that stepped care may help to optimise treatment effects.Reference Tummers, Knoop and Bleijenberg37 We are currently performing a non-inferiority trial with comparison of a stepped care intervention combining iCBT as a first step and face-to-face CBT as an optional second step (see NTR 4809) with face-to-face CBT only.
Implications
Our current trial was a first attempt to develop and test a web-based CBT for adults with CFS. We think that treatment results can be further optimised, for example by communication via video conferencing and using physical activity apps with affirmative feedback. In the Western world healthcare budgets are overstretched and psychological treatments are increasingly delivered digitally to reduce the costs of intervention. This web-based CBT programme, offers adults with CFS a new and efficacious treatment option.
Supplementary material
Supplementary material is available online at http://dx.doi.org/10.1192/bjp.2017.22.
Acknowledgements
We thank Lianne Vermeeren for her excellent work as independent data manager and all participating therapists for their valuable contributions to this study and Hanneke Meulenbroek (professional translator) for her helpful linguistic suggestions.









eLetters
No eLetters have been published for this article.