Around a third of physical symptoms presented in medical care settings are medically unexplained. Reference Kroenke and Mangelsdorff1,Reference Jackson and Passamonti2 Cases that present in this way are heterogeneous and may be associated with depression, anxiety or somatoform disorders, Reference Jackson and Passamonti2–Reference Toft, Fink, Oernboel, Christensen, Frostholm and Olesen5 but in some there is neither physical nor mental disorder. Reference Jackson and Passamonti2,Reference Wessely6 Therefore, existing psychiatric classifications are unsatisfactory, and hinder understanding and management of this complex problem. Reference Mayou7,Reference Kroenke, Spitzer, Williams, Linzer, Hahn, deGruy and Brody8 Many studies of medically unexplained symptoms have used selective entry criteria, resulting in samples with high rates of mental disorders, high service use or symptom syndromes. Reference Smith, Gardiner, Lyles, Sirbu, Dwamena, Hodges, Collins, Lein, Given, Given and Goddeeris9–Reference Creed and Barsky12 Hence, the term ‘medically unexplained symptoms’ is used in this study to capture the presentation of patients in a clinical setting, Reference Sumathipala13–Reference Sumathipala15 with no specific emphasis on a diagnostic category. There is evidence for the effectiveness of interventions for this demanding group using antidepressant medication and cognitive–behavioural therapy (CBT), Reference O'Malley, Jackson, Santoro, Tomkins, Balden and Kroenke16–Reference Sumathipala18 but few intervention studies have been conducted in primary care. Reference Sumathipala18
Medically unexplained symptoms have a similar prevalence and consequences across widely different cultural settings. Reference Gureje, Simon, Ustun and Goldberg19 In Sri Lanka, patients with such symptoms were compared with other primary care attenders, Reference Sumathipala13 using the 30-item General Health Questionaire (GHQ–30). Reference Goldberg and Blackwell20 The prevalence ratio was 2.38 (95% CI 1.78–3.18) and the mean duration of illness was 39 months. Symptoms were not relieved in 72% of these patients in spite of 17 visits to different categories of doctor of their choice per year, compared with 4 visits per year in the control group.
A pilot randomised controlled trial preceding this study to test the effectiveness of an intervention based on CBT principles is the only published example of such an evaluation from a low- to middle-income country. Reference Sumathipala, Hewege, Hanwella and Mann14 The results of the pilot study indicated that brief CBT carried out by a psychiatrist in a primary care setting was efficacious compared with treatment as usual in reducing symptoms (difference in symptom count=2.3, 95% CI 0.85–3.7, P=0.001), psychological morbidity (GHQ score difference=4.1, 95% CI 0.5–7.6, P=0.04) and consultation frequency (difference=4.8, 95% CI 1.3–8, F=9.1, P=0.004). However, Sri Lanka has only 1.3 psychiatrists per million people. 21 The larger trial described here tests the same CBT intervention in more pragmatic circumstances, delivered by primary care physicians. The pilot trial, with treatment as usual as its control condition, was open to the criticism that the treatment effect might have been linked to non-specific elements, rather than being a specific effect of CBT. Therefore, we replaced treatment as usual by structured care, offering sessions with similar duration, frequency and attention given by doctors similar to those providing CBT. We tested the hypothesis that for patients with medically unexplained symptoms attending a general out-patient clinic, would be more efficacious than structured care.
Method
Study design
The study was a randomised controlled trial, with individuals randomised to CBT or structured care. The primary outcome was psychological morbidity, measured by the GHQ–30. Secondary outcomes were the number of symptoms reported by the participants, the score on the Bradford Somatic Inventory (BSI), Reference Mumford, Bavington, Bhatnagar, Hussain, Mirza and Naraghi22 and the number of patient-initiated visits to healthcare providers of their choice. The study received ethical clearance from the ethics committee at the Institute of Psychiatry and approval from the board of management of Sri Jayewardenepura Hospital in Sri Lanka.
Setting and participants
The trial was conducted in a general out-patient clinic at Sri Jayewardenepura General Hospital, Colombo, where patients initiate their own visits without prior appointments. The clinic is a primary care facility with eight doctors, and patients use it as the first point of contact for healthcare. Consecutive attenders were screened to identify those meeting the inclusion criteria for the trial.
Inclusion and exclusion criteria
Patients aged 16–65 years who had had five or more medically unexplained symptoms for a period of at least 6 months were eligible for inclusion. Symptoms of shorter duration are particularly likely to resolve spontaneously, therefore such patients were excluded. Reference Craig, Boardman, Mills, Daly-Jones and Drake23 Medically unexplained symptoms were defined on the basis of at least one of the following:
-
(a) incompatibility of the clinical presentation with a known physical illness
-
(b) absence of relevant positive physical signs
-
(c) laboratory investigations not supporting a diagnosis of a physical illness.
Symptoms (e.g. pain) experienced at different anatomical sites were counted as separate symptoms, as were different symptoms at the same anatomical site. Those with dementia, psychosis or alcohol dependence were excluded from the trial, as were those currently receiving treatment for a psychiatric disorder.
Recruitment procedures
Recruitment took place among consecutive out-patient department attenders. The eight primary care physicians were instructed verbally as well as by a printed A4 sheet on how to recognise medically unexplained symptoms, and identified patients with repeated consultations for such symptoms. These patients were referred to the trial coordinator (A.S.) and the trial physician (S.S.), who made independent assessments to establish eligibility, each administering two open-ended questions (‘What are your symptoms?’ ‘Are there any other symptoms/problems?’) to elicit the number of symptoms and the number of visits over the previous 6 months. Reference Sumathipala, Hewege, Hanwella and Mann14 A comprehensive physical examination was carried out by S.S., who also reviewed previous laboratory investigation results. Patients with overt disease were excluded. If both A.S. and S.S. agreed that the patient was eligible to be recruited, non-clinical research assistants obtained informed consent. Patients who refused or who did not fulfil the inclusion criteria were referred back to the primary care doctor.
Sample size calculation
The sample size calculation was based on the assumption that only a relatively large effect size associated with the intervention was likely to influence policy. The priorities for hard-pressed primary care services in Sri Lanka remain infectious disease, heart disease, hypertension and diabetes. Although repeated attendance of patients with multiple symptoms is a well-recognised problem, a psychological intervention would have to be highly efficacious to stand a realistic chance of being adopted. Therefore the sample size was set at 55 in each group to confer 80% power at 5% significance of detecting a true effect size of 0.5 (generally designated as a moderate effect) for detectable differences in mean scores on the primary outcome measure (the GHQ score) between the two groups. Allowing for 30% attrition, 72 patients were needed in each group, rounded to 75 in each arm. In the pilot trial a large effect size was observed when the psychiatrist provided CBT. We assumed such a larger effect size was unrealistic when primary care doctors provided the therapy.
Randomisation
The six doctors comprised four who were entirely based in the out-patient department and two who were employed in the hospital but also worked as general practitioners in the community. The doctors were allocated at random to deliver CBT or structured care, in such a way that three doctors were allocated to each intervention, with two hospital-based physicians and one general practitioner in each group.
Trial participants were first randomised to the two intervention groups using a random permuted block design, with a block size of four. Next, participants were randomly allocated to one of the three doctors selected to deliver the intervention to which they had been allocated. Randomisation codes were generated by a statistician in the UK and passed on to the independent epidemiologist (M.R.N.A.) in Sri Lanka, who executed the random allocation of treatment condition.
Throughout the trial both the physician (S.S.) and the research assistants for the project remained masked to the group status of the patients. Details of allocation of all patients were concealed from them until the end of the trial. The research assistants did not know which primary care doctors provided which treatment. Neither the primary care doctors who delivered the interventions nor the patients who received them could be masked to their allocation because of the nature of the interventions. Similarly, the trial coordinator (A.S.) was not masked to the group status. However, he was not involved in registration, randomisation, treatment allocation, data collection or main outcome analysis.
Trial procedures
The primary care physician was responsible for arranging the subsequent treatment sessions. An administrator facilitated the appointments and follow-up assessments. The full baseline assessment was repeated 3 months, 6 months and 12 months post-baseline. A part assessment was done at 9 months to maintain continuity. Patients who were not present for re-assessments were sent reminders by post or were contacted over the telephone. If they were unable to attend, assessments were carried out at the person's home.
Assessments and instruments
The trial physician (S.S.) and the trial coordinator (A.S.) ascertained the number of medically unexplained symptoms using the procedure described above. Participants also completed the following clinical assessments.
General Health Questionnaire
The GHQ–30 is a scalable measure of psychological morbidity, and was used as a continuous variable because it is useful for comparisons across groups. Reference Odell, Surtees, Wainwright, Commander and Sashidharan24 This questionnaire has been translated into Sinhala, validated, Reference Jackson and Passamonti2,Reference De Silva and Samarasingha25,Reference Samarasingha26 and used successfully in previous studies in primary care. Reference Sumathipala13,Reference Sumathipala, Hewege, Hanwella and Mann14
Bradford Somatic Inventory
The BSI is a structured assessment of the presence and the severity of 21 commonly occurring somatic symptoms. Reference Mumford, Bavington, Bhatnagar, Hussain, Mirza and Naraghi22 The symptoms were derived from psychiatric case-notes of British patients of indigenous and Pakistani origin, with clinical diagnoses of anxiety, depression, hypochondriasis and somatoform disorders. It has been validated in Britain and Pakistan, and used widely in the detection of psychiatric disorders among Asian patients presenting with somatic symptoms. Symptoms are coded as absent (0), or present on less than 15 days (1) or more than 15 days (2) in the past month. Possible scores range from 0 to 42. The BSI was also adapted and validated for Sri Lanka by A.S. Reference Sumathipala and Murray27
Interventions
Assessment as part of the interventions
The Semi-Structured Explanatory Model Interview (SEMI) developed by Lloyd et al is a framework for eliciting salient information relevant to the management of medically unexplained symptoms. Reference Lloyd, Jacob and Patel28 The SEMI was part of both the CBT and the structured care intervention. Using the SEMI for exploration of the patient's and clinician's explanatory model is valuable in developing culturally appropriate interventions. Reference Bhui and Bhugra29 This instrument uses open-ended questions to elicit patients' explanatory models. It generates data on the respondents' assumptions, beliefs, thoughts about their illness and its causes, and fears about their future. It includes details of healthcare utilisation, and patients' expectations of treatment and satisfaction with their care. Both groups were interviewed at baseline by A.S. using the SEMI, and its case vignettes and information were passed on to all the primary care physicians with the case-notes. In addition, the physicians providing CBT received a summary and a formulation based on SEMI findings prepared by A.S. and were trained to use this information to inform the strategy for their CBT intervention.
Two diaries were issued to every participant for the period of the intervention. The first was for any doctor consulted over the period of the trial to record consultations, symptoms, investigations and treatment. The other diary was for participants to record their own symptoms, associated cognitions and behaviours. For participants in both study groups the diaries afforded a mechanism for expressing distress. Information in the diary was available to the physicians in both study arms. In the CBT intervention the doctors were trained to use the participants' diaries to identify dysfunctional cognitions and to monitor symptoms. The doctors who provided structured care were not given training as to the purpose or potential therapeutic use of the diaries.
Cognitive–behavioural therapy
The intervention strategy was based on the therapy developed and manualised for the previous pilot trial. Reference Sumathipala, Hewege, Hanwella and Mann14,Reference Sumathipala, Siribaddana, Mangava and De Silva30,Reference Patel and Sumathipala31 It aimed to contain the patient's help-seeking behaviour by offering structured regular visits to one health professional, thus reducing unstructured visits to different practitioners who might reinforce dysfunctional cognitions and behaviours through inappropriate advice and investigations. The treatment was based on the principles of CBT and reattribution technique, Reference Salkovoskis, Hawton, Salkovoskis, Kirk and Clark32–Reference Goldberg, Gask and O'Dowd34 modified to suit the local socio-cultural context. Where possible, the support of the spouse or other close relative was elicited to discourage inappropriate discussions with ill-informed relatives and friends, who could reinforce the patient's preoccupation with fears of serious illness. Reference Sharpe, Peveler and Mayou33 A treatment manual was used to standardise the intervention. Reference Sumathipala, Siribaddana, Mangava and De Silva30 In the pilot study we offered six therapy sessions; however, 90% of the participants who attended three or more sessions stayed in the study, improved and also were available for outcome assessment. Hence, in this study, CBT was offered in three half-hourly structured sessions over the 3 weeks following the baseline assessment; these sessions were mandatory and those who did not complete them were considered non-adherent. A further three optional fortnightly follow-up sessions were offered.
The CBT training was a short course consisting of five sessions covering the basis of medically unexplained symptoms; the relevance of the explanatory model, elicited by the SEMI, to the CBT model of such symptoms; and the CBT treatment approach. Training was accomplished through lectures by P.d.S. and A.S., supplemented by case vignettes and role-play of therapeutic sessions by simulated patients based on case scenarios from the pilot trial, all with reference to the intervention manual. To ensure that CBT was delivered appropriately, the three doctors in the intervention arm received regular supervision from A.S.
Structured care
The components of the treatment packages and follow-up assessments received by the two groups differed in only one respect: participants in the structured care group did not receive CBT components detailed in the manual. Reference Sumathipala, Siribaddana, Mangava and De Silva30 The structured care also consisted of six half-hour appointments with one primary care physician. As in the CBT intervention, the first three weekly sessions were mandatory and the next three fortnightly sessions were optional. Another similarity was the use of diaries, which provided a mechanism for expressing distress. The three physicians were free to manage the patients as they wished within the sessions. No training or supervision was provided for these doctors, and the intervention was not manualised.
Follow-up
At the end of the intervention, participants in both groups received a written summary of their history and the intervention and were asked to produce this if they consulted any other doctor within the next 12 months. No further appointment for CBT or structured care was booked, but participants had the option of visiting the doctor who offered the intervention or to visit any other doctor of their choice. This is the usual practice in Sri Lanka, as a formal general practice system does not exist. However, an administrator facilitated the appointments for follow-up outcome assessments.
Statistical analysis
An interim analysis was not done. M.D., who was masked to randomised group allocation, analysed the scores from the four fixed time points (3 months, 6 months, 9 months and 12 months after randomisation) using a mixed effects model. We included as fixed effects group allocation, baseline score, time, and the interaction of group and time. Time was coded as months after the 3-month time point, giving values of 0, 3, 6 and 9, so that in the presence of the interaction the effect of group represents the difference at 3 months. We included the patient as a random effect. We also fitted models with a random effect of time, with various covariance patterns, with treating doctor as an effect, and pattern mixture models to allow for the different drop-out patterns. We report here the simpler models as they fit as well as any of the more complex ones. We also examined model residuals. A mixed effects model was used as this enables effective use of all the information even from participants who had some missing scores. We used r for the analysis, Reference Ihaka and Gentleman35 with the nlme package for fitting the mixed effects models. Reference Pinheiro and Bates36
Results
A total of 150 participants were recruited, 75 each randomly allocated into the CBT and the structured care groups (Fig. 1). The baseline characteristics of participants in the two groups are given in Table 1. A large majority were women. Ages ranged from 16 years to 58 years with a mean of 35 years. Most participants were well educated, with 61% completing GCE Ordinary Level examination (11 years of education) and another 27% Advanced Level (13 years of education). As expected, trial recruits were chronically ill and high users of healthcare services. The mean duration of symptoms was 42 months (95% CI 35.5–48.3). Only 40% of participants were employed, and 95% reported requiring one or more assistants for help in their day-to-day activities. The SEMI findings revealed that 95% had considerable illness worries; 37% believed their symptoms indicated moderately serious illness and 58% thought they indicated very serious illness. More specifically, 33% harboured fear of death, 20% fear of paralysis, 13% fear of having cancer and the rest had fearful concerns about unspecified incurable illness. There was no substantial difference in baseline characteristics between the groups allocated to CBT and structured care.

Fig 1 Flow of participants through the trial.
Table 1 Comparison of baseline characteristics between the two study groups
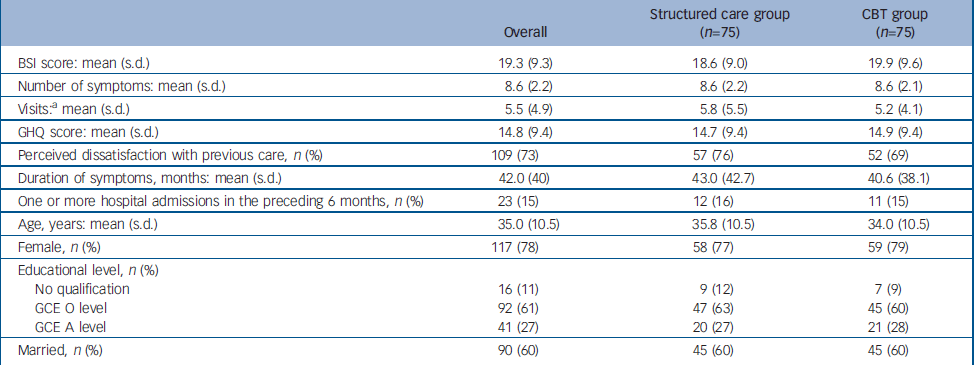
| Overall | Structured care group (n=75) | CBT group (n=75) | |
|---|---|---|---|
| BSI score: mean (s.d.) | 19.3 (9.3) | 18.6 (9.0) | 19.9 (9.6) |
| Number of symptoms: mean (s.d.) | 8.6 (2.2) | 8.6 (2.2) | 8.6 (2.1) |
| Visits:a mean (s.d.) | 5.5 (4.9) | 5.8 (5.5) | 5.2 (4.1) |
| GHQ score: mean (s.d.) | 14.8 (9.4) | 14.7 (9.4) | 14.9 (9.4) |
| Perceived dissatisfaction with previous care, n (%) | 109 (73) | 57 (76) | 52 (69) |
| Duration of symptoms, months: mean (s.d.) | 42.0 (40) | 43.0 (42.7) | 40.6 (38.1) |
| One or more hospital admissions in the preceding 6 months, n (%) | 23 (15) | 12 (16) | 11 (15) |
| Age, years: mean (s.d.) | 35.0 (10.5) | 35.8 (10.5) | 34.0 (10.5) |
| Female, n (%) | 117 (78) | 58 (77) | 59 (79) |
| Educational level, n (%) | |||
| No qualification | 16 (11) | 9 (12) | 7 (9) |
| GCE O level | 92 (61) | 47 (63) | 45 (60) |
| GCE A level | 41 (27) | 20 (27) | 21 (28) |
| Married, n (%) | 90 (60) | 45 (60) | 45 (60) |
Uptake of the interventions
In each arm, 64 participants (85%) completed the three mandatory sessions. Uptake of optional sessions was low; four sessions out of 20 (27%) in the CBT group compared with 14 (19%) in the structured care group, and five sessions out of 15 (20%) in the CBT group compared with 13 (17%) in the structured care group. Significantly, uptake of all six sessions was higher for the latter group (37%, n=28) than for the CBT group (20%, n=15; χ2=4.69, P=0.03). In contrast, a higher percentage of those allocated to structured care did not attend any of the sessions, mandatory or optional (9% v. 3%; χ2=1.89, P=0.17).
Availability for follow-up assessment
Every attempt was made to follow-up all 150 participants regardless of whether they completed the treatment. Availability of participants at each of the follow-up assessments is presented in Fig. 1 and in Table 2. The proportion attending all four follow-up assessments was higher among those allocated to CBT, but this was not statistically significant. The 24 participants (16%) who missed all four follow-up assessments could not be traced to the original addresses, directly refused, did not engage any further or had gone abroad. There were no reported deaths.
Table 2 Comparison of patterns of attendance for follow-up assessment between the two study groups
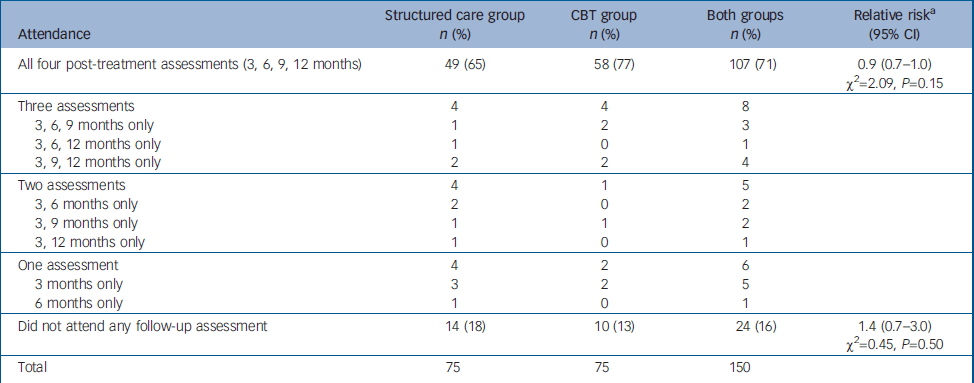
| Attendance | Structured care group n (%) | CBT group n (%) | Both groups n (%) | Relative riska (95% CI) |
|---|---|---|---|---|
| All four post-treatment assessments (3, 6, 9, 12 months) | 49 (65) | 58 (77) | 107 (71) | 0.9 (0.7-1.0) χ2=2.09, P=0.15 |
| Three assessments | 4 | 4 | 8 | |
| 3, 6, 9 months only | 1 | 2 | 3 | |
| 3, 6, 12 months only | 1 | 0 | 1 | |
| 3, 9, 12 months only | 2 | 2 | 4 | |
| Two assessments | 4 | 1 | 5 | |
| 3, 6 months only | 2 | 0 | 2 | |
| 3, 9 months only | 1 | 1 | 2 | |
| 3, 12 months only | 1 | 0 | 1 | |
| One assessment | 4 | 2 | 6 | |
| 3 months only | 3 | 2 | 5 | |
| 6 months only | 1 | 0 | 1 | |
| Did not attend any follow-up assessment | 14 (18) | 10 (13) | 24 (16) | 1.4 (0.7-3.0) χ2=0.45, P=0.50 |
| Total | 75 | 75 | 150 |
Relationship between treatment completion and availability at follow-up
The majority (n=13) of the 22 patients who did not complete the three mandatory sessions (protocol violators) were also lost to follow-up and did not attend any of the four follow-up assessments. However, 7 of the remaining 9 protocol violators were available for all four follow-up assessments. Of those who completed the three mandatory sessions (n=64 in each arm), 53 (83%) in the CBT group and 47 (73%) in the structured care group were available for all four follow-up assessments (RR=1.1, 95% CI 0.9–1.4; χ2=1.1, P=0.29). Those who did not receive a sufficient dose of treatment (three mandatory sessions) were more likely to be lost to follow-up.
Outcomes
Table 3 provides the coefficients and 95% confidence intervals for the mixed effects models outcome scores at 3 months, 6 months, 9 months and 12 months after baseline. Coefficients were estimated for the fixed effects of group allocation, baseline score, time and the interaction of group and time. In the presence of an interaction the group coefficient represents the difference at 3 months. For both groups, mean scores for all outcomes declined sharply from baseline to the first 3-month outcome assessment, and then remained essentially constant over time thereafter (Fig. 2). As can be seen from the coefficients, none of the group differences at 3 months was statistically significant, nor was there any difference in the effect of time (after the 3-month outcome) between groups (i.e. none of the interactions between time and group was statistically significant). Given the observed changes in outcome over time, we calculated, post hoc, the effect sizes for the change scores between baseline and 3 months for each outcome, with each randomised allocation. These indicated substantial and statistically significant reductions from baseline (Table 4).
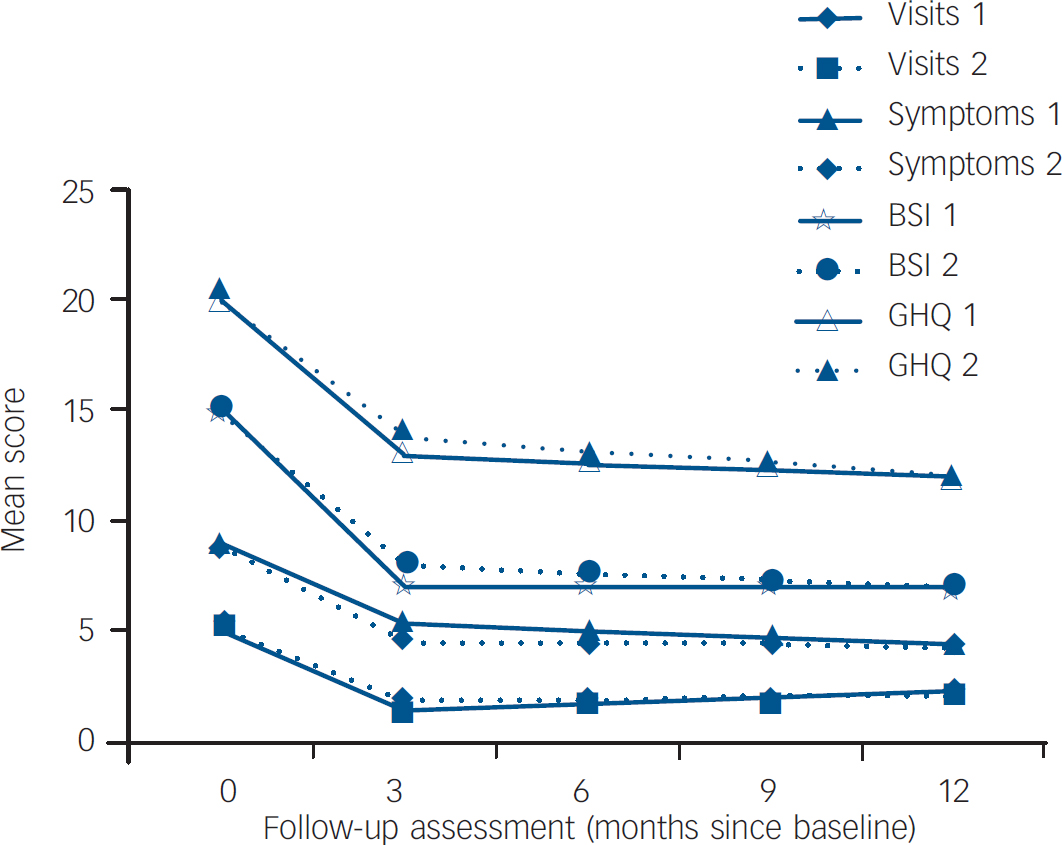
Fig. 2 Study outcomes for the cognitive–behavioural group (group 1) and the structured care group (group 2). BSI, Bradford Somatic Inventory; GHQ, General Health Questionnaire.
Table 3 Coefficient estimates from the mixed models

| GHQ Estimate (95% CI) | BSI Estimate (95% CI) | Symptoms Estimate (95% CI) | Visits Estimate (95% CI) | |
|---|---|---|---|---|
| Intercept | 0.64 (-2.03 to 3.31) | 0.89 (-2.0 to 3.79) | 3.16 (1.24 to 5.07) | 0.79 (0.16 to 1.41) |
| Base | 0.34 (0.21 to 0.46) | 0.57 (0.46 to 0.69) | 0.19 (-0.01 to 0.39) | 0.05 (-0.01 to 0.11) |
| Time | -0.02 (-0.21 to 0.17) | -0.08 (-0.26 to 0.09) | -0.10 (-0.20 to 0.00) | 0.11 (0.03 to 0.19) |
| Randomised allocation (structured care v. CBT) | 1.18 (-1.44 to 3.8) | 0.89 (-1.59 to 3.37) | -0.83 (-1.91 to 0.25) | 0.53 (-0.24 to 1.29) |
| Interaction (time × randomised allocation) | -0.08 (-0.36 to 0.19) | -0.05 (-0.31 to 0.20) | 0.07 (-0.08 to 0.22) | -0.09 (-0.20 to 0.03) |
Table 4 Comparison of outcome clinical measures between the two study groups over all time periods

| Structured care group | CBT group | |||||
|---|---|---|---|---|---|---|
| n | Mean (s.d.) | n | Mean (s.d.) | Difference (95% CI) | P | |
| GHQ score | ||||||
| Baseline | 75 | 14.7 (9.4) | 75 | 14.9 (9.4) | 0.2 (-2.8 to 3.2) | 0.9 |
| 3 months | 60 | 6.1 (8.3) | 65 | 5.5 (7.7) | -0.6 (-3.4 to 2.2) | 0.6 |
| 6 months | 54 | 7.2 (9.7) | 60 | 6.2 (8.3) | -1.0 (-4.3 to 2.2) | 0.5 |
| 9 months | 53 | 6.3 (8.2) | 63 | 5.6 (7.9) | -0.7 (-3.6 to 2.3) | 0.6 |
| 12 months | 53 | 5.7 (9.5) | 60 | 5.6 (8.0) | -0.1 (-3.3 to 3.1) | 0.9 |
| Complaints | ||||||
| Baseline | 75 | 8.6 (2.2) | 75 | 8.6 (2.2) | 0.0 (-0.72 to 0.7) | 0.9 |
| 3 months | 59 | 3.9 (2.3) | 63 | 4.8 (3.9) | 0.8 (-0.3 to 1.9) | 0.2 |
| 12 months | 49 | 3.8 (2.7) | 57 | 3.9 (2.8) | 0.8 (-0.94 to 1.13) | 0.8 |
| BSI score | ||||||
| Baseline | 75 | 18.6 (9.0) | 75 | 19.9 (9.6) | 1.3 (-1.7 to 4.3) | 0.4 |
| 3 months | 60 | 12.4 (8.9) | 65 | 12.4 (9.6) | 0.0 (-3.3 to 3.3) | 0.1 |
| 6 months | 54 | 11.8 (8.9) | 60 | 11.5 (9.0) | -0.3 (-3.7 to 3.0) | 0.8 |
| 9 months | 53 | 12.0 (9.0) | 63 | 11.7 (9.8) | -0.2 (-3.7 to 3.0) | 0.8 |
| 12 months | 53 | 11.0 (9.1) | 60 | 11.1 (8.7) | 0.2 (-3.1 to 3.5) | 0.9 |
| Visits | ||||||
| -6 months to baseline | 75 | 10.2 (8.0) | 75 | 8.6 (5.7) | -1.5 (-3.7 to 0.7) | 0.18 |
| -3 months to baseline | 75 | 5.8 (5.5) | 75 | 5.2 (4.1) | 0.6 (-2.1 to 0.96) | 0.4 |
| 0-3 months | 60 | 1.6 (3.0) | 63 | 1.1 (1.7) | -0.5 (-1.4 to 0.3) | 0.2 |
| 3-6 months | 52 | 1.6 (2.4) | 57 | 1.2 (1.5) | -0.4 (-1.2 to 0.3) | 0.2 |
| 9-12 months | 51 | 2.0 (2.5) | 57 | 1.8 (2.6) | 0.2 (-0.8 to 1.1) | 0.7 |
Discussion
This study suggests that structured care offered by primary care physicians is neither more nor less efficacious than CBT provided by primary care physicians after a short course of training, having controlled for duration and frequency of treatment sessions. There was no substantial difference between the two groups at 3-month, 6-month, 9-month and 12-month follow-up for the primary clinical outcome measure (GHQ score) or for the secondary outcome measures (BSI score, numbers of symptoms and visits). However, for both groups all outcome measures showed substantial and statistically significant reductions after 3 months compared with baseline, which were maintained for up to 12 months.
Potential explanations of these findings are natural remission of symptoms in both groups and higher baseline scores regressing to the mean. However, in a recent cohort study of patients presenting with physical symptoms to primary care, those with medically unexplained symptoms were unlikely to improve at 5 years if they initially had poor functioning, longer duration of symptoms and illness worries. Reference Jackson and Passamonti2 Similarly, in a 10-year follow-up study of patients with chest pain who had negative coronary angiography, 75% remained symptomatic and disabled. Reference Potts and Bass37 Natural remission or higher baseline scores regressing to the mean are therefore unlikely to be the most plausible explanations, because patients in this trial had a mean duration of symptoms of 42 months, poor functioning (95% requiring one or more assistants for help in their day-to-day activities) and considerable illness worries (harboured by 95%).
With the benefit of hindsight, the lack of a trial arm allocated to treatment as usual is an important disadvantage, as the significant change scores for both groups cannot be directly compared with currently available treatment in Sri Lanka. Such treatment is usually symptomatic, with no structured care, so that these patients make around seven visits to 4–10 different categories of doctors of their choice over 6 months. Reference Sumathipala, Hewege, Hanwella and Mann14
Assuming both interventions to be equally efficacious, lack of a difference between the groups at follow-up should not be interpreted exclusively as equal effect of both treatments, because it might be due to a type II error, resulting from inadequate power to detect small differences. Hence, our findings need to be interpreted cautiously. The earlier pilot trial, conducted in a similar setting on a smaller sample with similar characteristics, Reference Sumathipala, Hewege, Hanwella and Mann14 indicated a substantial and statistically significant treatment benefit associated with CBT delivered by a psychiatrist, when compared with treatment as usual (no structured care), using for the most part the same outcomes studied in this trial. The characteristics of the pilot trial and the present trial are presented in Table 5, because the setting, recruitment, inclusion criteria, assessment instruments (including the use of SEMI) and the outcome measures were the same. Although a direct comparison cannot be made between the two trials, the effect sizes associated with CBT on primary and secondary outcomes are similar in both, despite the CBT intervention being administered by primary care physicians in one study and by an experienced psychiatrist in the other. Indeed, the effect on GHQ–30 and BSI scores was larger for the physician-administered CBT. However, the effect sizes associated with structured care given by primary care physicians are similar to those achieved by the CBT intervention in both the pilot trial and the present trial, and are much superior to treatment as usual in the pilot trial. The differences between the findings of the pilot trial and the present trial are therefore more parsimoniously explained by the relative effectiveness of structured care than by an ineffectiveness of CBT when administered by primary care doctors following minimal training. Alternatively, the failure of CBT to show a clear superiority could be due to insufficient treatment intensity or duration (i.e. dosage) or an inadequacy of competency (i.e. duration of training or background knowledge). Also, there was no assessment of CBT fidelity to protocol. The short training provided for doctors might have resulted in a technique-based competency with little understanding of cognitive and behavioural sciences. This might have resulted in a lack of flexibility in treatment to produce the maximum treatment effect. Alternatively, primary care physicians might not be good cognitive–behavioural therapists. In a randomised controlled trial on chronic fatigue, CBT given by general practitioners did not have any effect compared with the control group (who did not have CBT). Reference Huibers, Beurskens, Van Schayck, Bazelmans, Metsemakers, Knottnerus and Bleijenberg38 In a systematic review there was no strong evidence for the effectiveness of psychosocial interventions by general practitioners. Reference Huibers, Beurskens, Bleijenberg and van Schayck39 Another possibility is that CBT is not indicated. Unexplained symptoms may be puzzling and distressing, and the patient might simply need the doctor to be honest about uncertainty and provide simple reassurance, without attempting to change cognitions through CBT.
Table 5 Comparison between the pilot trial and the current trial
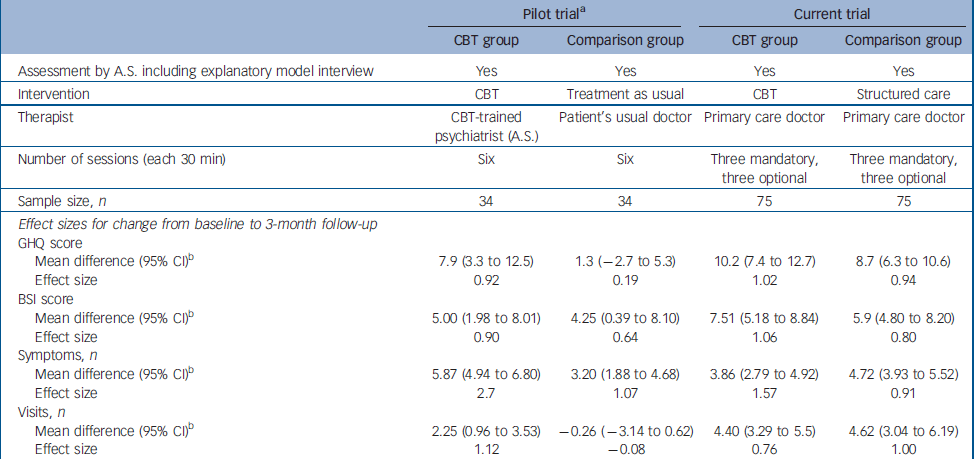
| Pilot triala | Current trial | |||
|---|---|---|---|---|
| CBT group | Comparison group | CBT group | Comparison group | |
| Assessment by A.S. including explanatory model interview | Yes | Yes | Yes | Yes |
| Intervention | CBT | Treatment as usual | CBT | Structured care |
| Therapist | CBT-trained psychiatrist (A.S.) | Patient's usual doctor | Primary care doctor | Primary care doctor |
| Number of sessions (each 30 min) | Six | Six | Three mandatory, three optional | Three mandatory, three optional |
| Sample size, n | 34 | 34 | 75 | 75 |
| Effect sizes for change from baseline to 3-month follow-up | ||||
| GHQ score | ||||
| Mean difference (95% CI)b | 7.9 (3.3 to 12.5) | 1.3 (-2.7 to 5.3) | 10.2 (7.4 to 12.7) | 8.7 (6.3 to 10.6) |
| Effect size | 0.92 | 0.19 | 1.02 | 0.94 |
| BSI score | ||||
| Mean difference (95% CI)b | 5.00 (1.98 to 8.01) | 4.25 (0.39 to 8.10) | 7.51 (5.18 to 8.84) | 5.9 (4.80 to 8.20) |
| Effect size | 0.90 | 0.64 | 1.06 | 0.80 |
| Symptoms, n | ||||
| Mean difference (95% CI)b | 5.87 (4.94 to 6.80) | 3.20 (1.88 to 4.68) | 3.86 (2.79 to 4.92) | 4.72 (3.93 to 5.52) |
| Effect size | 2.7 | 1.07 | 1.57 | 0.91 |
| Visits, n | ||||
| Mean difference (95% CI)b | 2.25 (0.96 to 3.53) | -0.26 (-3.14 to 0.62) | 4.40 (3.29 to 5.5) | 4.62 (3.04 to 6.19) |
| Effect size | 1.12 | -0.08 | 0.76 | 1.00 |
Hence, the lack of statistically significant difference between the two arms of our study should not undermine the clinical importance of its findings, in particular the potentially positive impact of structured care. We cannot directly establish that such care was more efficacious than treatment as usual. However, the comparison of effect sizes between the pilot trial and this study suggests that this is a possibility. Further development of structured care, as a less onerous and cheaper but possibly equally effective alternative to CBT, Reference Sharpe and Carson40 requires some consideration of the elements that were common to both the interventions provided in this trial. These were as follows.
-
(a) The recruitment process into the trial included the explanation of medically unexplained symptoms provided in the information sheet.
-
(b) The initial physical examination and case review were carried out by the experienced specialist physician (S.S.).
-
(c) The exploration of patient explanatory models, cognitions and behaviours in the SEMI by the psychiatrist (A.S.) was available to the physicians providing structured care, although they were not provided with a summary of the findings or trained to use it. It is possible that participants might have gained insight through the SEMI alone, and might have brought some of this awareness into the subsequent structured care sessions. There is ample evidence that comprehensive clinical assessments alone may have beneficial effects on clinical outcomes; an assessment itself without formal psychotherapy might have therapeutic effects. Reference Price41,Reference Petrie, Moss-Morris and Weinman42
-
(d) The diaries given to participants to record their symptoms, associated cognitions and behaviours might inadvertently have introduced an element of CBT, reducing the distinctiveness of the two interventions. By the same token, these elements may highlight the potential for a feasible and effective intervention based on structured care without the need for the additional complexity and cost implied in manualisation, training and supervision for CBT.
-
(e) Structured appointments were available with a primary care physician, regardless of whether or not this option was taken up.
-
(f) Therapeutic engagement with the primary care doctor took place over three to six half-hour sessions (routine primary care consultations would typically last around 5–10 min in Sri Lanka). Therefore, simple unambiguous reassurance that did not use specific CBT techniques would have had some effect.
-
(g) Consistent care was provided by a single doctor, with the consequent opportunity to avoid contradictory and ambiguous advice from different care providers, which is one of the most important determinants of perpetuation of symptoms.
-
(h) Regular structured assessment procedures (BSI, GHQ, SEMI) were offered during follow-up every 3 months.
The use of placebo medication was a unique component in the structured care intervention.
Limitations
Contamination (or a spillover effect) of the interventions might have occurred given that the doctors administering both worked in the same primary care centre. Doctors providing structured care might have picked up on cognitive–behavioural techniques from the doctors who provided CBT. Most of the doctors who referred patients for the trial also treated them. This might have biased the inclusion in such a way that only highly motivated patients or patients fitting the treatment were recruited. However, this selection bias would not affect the comparison between the two interventions, but could contribute to the high effect sizes of both interventions. Generalisability to routine primary care may be limited by recruitment confined to chronically ill patients with multiple complaints and repeated visits enrolled from a single clinic. Similarly, even if both interventions were equally efficacious, they were relatively demanding (three to six 30 min sessions) and therefore of questionable generalisability.
Study implications
Either CBT or structured care may improve symptoms of patients with chronic medically unexplained symptoms and frequent attendance to many different healthcare providers. These interventions were not studied directly against treatment as usual in this trial, but the observed change was larger than that seen in a previous trial and deserves further study in comparison with usual treatment. Treatment of patients with medically unexplained symptoms is a complex process, consisting of different components, which may act both independently and interdependently. 43 However, the active component may not be easily defined. Therapist and patient characteristics, delivery, frequency and timing of the trial procedures; recruitment into a trial per se, the information leaflet, the consent process, non-specific effects of structured appointments and the regular structured follow-up assessment all may be active ingredients.
Findings of this trial support the importance of evaluating the ‘effectiveness of medical assessments augmented by inclusion of proven cognitive–behavioural elements’. Reference Sharpe and Carson40 Hence, future research should consider enhanced structured care; medical assessment and structured care incorporating simple elements of CBT principles that can be used by doctors without specific training or CBT skills.
The Wellcome Trust (international programme) provided a project grant (056949/Z/99/Z).
Acknowledgements
We dedicate this paper to Padmal De Silva, internationally renowned psychologist and Buddhist scholar, who died in November 2007. This study was carried out in Padmal's native Sri Lanka and will be one of his last publications. Drs Dhamasa Gunewardene, Rozana Cassim, Champa Karunaratne, Prebath Panduwawala, Madura Manawickrama and Predeepa Thilakartane, the six doctors who provided the interventions in the trial, and Shanthi Lianege, Neela De Silva, Gayathri Gnanathilake and Renuka Pathirana, all of whom are medical officers at the out-patients department of the hospital, extended much-needed support. We also thank Manori Wimelesekara and Lakshmi Abegoonewardena, members of the research team, and Dr Yulia Kovas for her help in reviewing the manuscript and suggestions on presentation of statistics.










eLetters
No eLetters have been published for this article.