With an annual number of around four million users, cocaine is currently the second most frequently used illicit drug in Europe. 1 Given its addictive potential Reference Nutt, King, Saulsbury and Blakemore2,Reference Wagner and Anthony3 and negative health consequences, Reference Nutt, King, Saulsbury and Blakemore2,Reference Degenhardt and Hall4 the use of cocaine is regarded as a major public health issue. Reference Nutt, King, Saulsbury and Blakemore2,Reference Degenhardt and Hall4 For more than two decades, research has tried to examine the long-term impact of cocaine by focusing on dependent cocaine users. Evidence has accumulated that addictive cocaine use leads to neuroadaptive changes and dopaminergic alterations, mainly exerted in the frontostriatal network. Reference Beveridge, Gill, Hanlon and Porrino5-Reference Bolla, Ernst, Kiehl, Mouratidis, Eldreth and Contoreggi9 Imaging studies in chronic cocaine users have repeatedly reported reductions in grey matter density in the dorsolateral prefrontal cortex, the anterior cingulate cortex and the orbitofrontal cortex, Reference Ersche, Barnes, Jones, Morein-Zamir, Robbins and Bullmore10-Reference Sim, Lyoo, Streeter, Covell, Sarid-Segal and Ciraulo14 areas critically involved in several cognitive functions. Reference Cabeza and Nyberg15 Accordingly, cognitive deficits in chronic cocaine users have been linked to structural and functional alterations primarily of the prefrontal cortex. Reference Beveridge, Gill, Hanlon and Porrino5,Reference Garavan and Hester6,Reference Bolla, Ernst, Kiehl, Mouratidis, Eldreth and Contoreggi9,Reference Goldstein, Leskovjan, Hoff, Hitzemann, Bashan and Khalsa16 The recent literature is characterised by a consensus that cocaine dependence is associated with significant neuropsychological impairment, although the aetiology and the severity of these impairments are a matter of ongoing debate. Reference Beveridge, Gill, Hanlon and Porrino5,Reference Goldstein, Leskovjan, Hoff, Hitzemann, Bashan and Khalsa16-Reference Woicik, Moeller, Alia-Klein, Maloney, Lukasik and Yeliosof18 Existing studies with dependent users indicate persisting cognitive impairments including deficits predominantly in the domains of attention, working and declarative memory, and, less consistently, in the ill-defined concept of executive functions. Reference Beveridge, Gill, Hanlon and Porrino5,Reference Ersche, Barnes, Jones, Morein-Zamir, Robbins and Bullmore10,Reference Goldstein, Leskovjan, Hoff, Hitzemann, Bashan and Khalsa16-Reference Pace-Schott, Morgan, Malison, Hart, Edgar and Walker21 However, given that these previous studies differed in their inclusion and exclusion criteria regarding comorbid psychiatric diseases, polytoxic drug-use history, abstinence time and verification of self-reported drug intake, the specific impact of chronic cocaine use on cognitive processes has been difficult to determine.
Whereas most of these studies focused on the chronic misuse of cocaine, relatively little is known about the substantial number of recreational but non-dependent cocaine users (recreational users). 1 Moreover, in comparison with studies with dependent cocaine users, the investigation of recreational users has several advantages, as they (a) are not (or not yet) addicted, (b) are less burdened by psychiatric comorbidities, Reference Smith, Thirthalli, Abdallah, Murray and Cottler22 (c) usually unmedicated with psychotropic drugs, and (d) mostly display less polytoxic drug use. It is only recently that research has started to systematically investigate the possible cognitive effects of recreational cocaine use. Reference Colzato, van den Wildenberg and Hommel23 Preliminary data from small samples of recreational users indicate that small and infrequent doses of cocaine affect different cognitive components such as attention, memory or components of executive functions. Reference Colzato, van den Wildenberg and Hommel23-Reference Soar, Mason, Potton and Dawkins29 However, these studies lacked a unique definition of recreational cocaine use (recreational cocaine use was either defined by cocaine use below a certain level or by not matching dependency criteria according to DSM-IV 30 ), mostly relied on simple self-reported drug use without objective verification, or tested only very small and predominantly male samples with mainly polytoxic drug use patterns. As a consequence, after more than two decades of research and despite concern about public health effects, there is still no clarification on the relationship between the extent of cocaine use and the characteristics of cognitive impairments.
To date, analyses of regular cocaine users categorised by groups of differing consumption patterns have been lacking. Our aim, therefore, was to investigate a large sample of recreational users, dependent users and matched stimulant-naive healthy controls with a comprehensive neuropsychological test battery to examine whether cognitive performance is impaired in relatively pure recreational and dependent cocaine users. Any differences in cognitive performance would have implications notably with regard to risk markers, prevention and treatment implications. Reference Beveridge, Gill, Hanlon and Porrino5,Reference Lucantonio, Stalnaker, Shaham, Niv and Schoenbaum12,Reference Cunha, Nicastri, Gomes, Moino and Peluso20 We expect to find considerable cognitive deficits in dependent users and similar, but less pronounced, cognitive impairments in recreational users, as we recently reported deficits in early information processing and blue-yellow colour vision in recreational users suggesting alterations of catecholamine neurotransmission. Reference Hulka, Wagner, Preller, Jenni and Quednow31,Reference Preller, Ingold, Hulka, Vonmoos, Jenni and Baumgartner32 Although psychiatric comorbidities such as attention-deficit hyperactivity disorder (ADHD) and depression are frequently found in dependent cocaine users, Reference Perez de Los Cobos, Sinol, Puerta, Cantillano, Lopez Zurita and Trujols33,Reference Swendsen and Merikangas34 their impact has scarcely been investigated so far. Thus, we conducted a comprehensive psychiatric diagnostic interview and additionally assessed symptoms of ADHD and depression with self-report questionnaires. Finally, by performing urine and hair toxicology analyses, we were uniquely able to objectively characterise not only recent drug use but also drug use over the past 6 months.
Method
Participants
A total of 68 recreational cocaine users, 30 dependent users and 68 cocaine-naive controls were included in the study (recruitment and selection details in online data supplement Method DS1). The three groups did not differ significantly in age, gender, smoking habits and verbal IQ. Exclusion criteria for all participants were acute or previous neurological disorders or head injury, any clinically significant medical diseases and use of prescription drugs affecting the central nervous system. Additional exclusion criteria for the control group were all acute or previous Axis I DSM-IV psychiatric disorders 30 including ADHD and any form of addiction, except nicotine, or regular illegal drug use (lifetime use >15 occasions) with the exception of occasional cannabis use. Specific exclusion criteria for the cocaine user groups were use of opioids, a polytoxic drug use pattern according to DSM-IV and acute or previous Axis I DSM-IV adult psychiatric disorders with the exception of cocaine, cannabis and alcohol misuse, history of affective disorders (acute major depression was excluded) or ADHD. None of the cocaine users was help-seeking in our department. Inclusion criteria for the two user groups were cocaine as the primary drug, cocaine use of >0.5 g per month, and an abstinence duration of <6 months. Cocaine dependence was diagnosed according to the DSM-IV criteria, 30 with only dependent cocaine users fulfilling these criteria. Participants were asked to abstain from illegal substances for at least 72 h and not to consume alcohol for 24 h before the testing session. Adherence with these instructions was controlled by urine and 6-month hair toxicologies (online Method DS2). The study was approved by the Cantonal Ethics Committee of Zurich. All participants provided written informed consent and were compensated for their participation.
Procedure
The present data were collected as part of the longitudinal Zurich Cocaine Cognition Study (ZuCo2St). Reference Preller, Ingold, Hulka, Vonmoos, Jenni and Baumgartner32 Trained psychologists conducted a Structured Clinical Interview (SCID-I) Reference Wittchen, Wunderlich, Gruschwitz and Zaudig35 according to DSM-IV procedures. Drug use was assessed by means of a structured and standardised Interview for Psychotropic Drug Consumption. Reference Quednow, Kuhn, Hoenig, Maier and Wagner36 For the estimation of verbal intellectual performance, the Mehrfachwahl-Wortschatz-Intelligenztest (MWT-B) was applied. Reference Lehrl37 The brief version of the Cocaine Craving Questionnaire (CCQ) was used to capture current cocaine craving. Reference Sussner, Smelson, Rodrigues, Kline, Losonczy and Ziedonis38 Smoking habits were assessed by the Fagerström Test of Nicotine Dependence. Reference Heatherton, Kozlowski, Frecker and Fagerstrom39 The Beck Depression Inventory (BDI) Reference Beck, Ward, Mendelson, Mock and Erbaugh40 measured the current severity of depression and the ADHD Self-Rating scale (ADHD-SR) Reference Rosler, Retz, Retz-Junginger, Thome, Supprian and Nissen41 focused on the diagnosis of ADHD in adulthood according to DSM-IV criteria. Subsequently, participants underwent a comprehensive neuropsychological test battery as described below. Participants were allowed to take a break at any time and smoking was permitted during the breaks.
Neuropsychological assessment
The test battery comprises four tests of the Cambridge Neuropsychological Test Automated Battery (CANTAB): Reference Strauss, Sherman and Spreen42 rapid visual processing (RVP), spatial working memory (SWM), intra/extra-dimensional set shifting (IED), paired associates learning (PAL); a German version of the Rey Auditory Verbal Learning Test (RAVLT); Reference Helmstaedter, Lendt and Lux43 and the Letter Number Sequencing Task (LNST). Reference Wechsler44 With regard to data reduction and specific analyses, 15 predefined main cognitive test parameters were z-transformed on the basis of means and standard deviations of the control group. These parameters were reduced to the four cognitive domains commonly used in cocaine research: Reference Goldstein, Leskovjan, Hoff, Hitzemann, Bashan and Khalsa16-Reference Woicik, Moeller, Alia-Klein, Maloney, Lukasik and Yeliosof18,Reference Pace-Schott, Morgan, Malison, Hart, Edgar and Walker21 attention, working memory, declarative memory and executive function according to theoretical a priori considerations (a detailed description can be found in online Method DS3). Furthermore, these four z-scored domains were equally integrated into a global cognitive index (GCI).
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics 19.0 for Windows. Frequency data were analysed by means of Pearson's chi-squared test and quantitative data by analyses of variance (ANOVA). Based on significant main effects, Sidak post hoc comparisons were performed. To control for demographic inequalities, the variables age and verbal IQ were introduced as covariates in analyses of covariance (ANCOVA) with linear group contrasts. Correlation analyses (Pearson's product-moment) to relate drug-use parameters to cognitive performance were conducted across a combined user group. Cumulated cocaine use and weekly use in grams were ln-transformed for statistical analyses because of the highly skewed distribution and the resulting deviation from the normal distribution (Shapiro-Wilk W<0.001). The effect of depression, ADHD, cocaine craving, recent cocaine use (positive urine test) and age at onset on cognitive performance was examined by correlation analyses and ANCOVA subgroup comparisons additionally corrected for severity of cocaine use. The effect of craving status was investigated because previous studies reported that craving for food and nicotine has an impact on cognitive functioning. Reference Kemps and Tiggemann45,Reference Sayette, Schooler and Reichle46 Multiple logistic regressions were used to estimate odds ratios associated with the use of cocaine and cognitive performance. The odds ratios were left unadjusted because the values decisive for the group assignment were already adjusted for age and verbal IQ.
Results
Demographic characteristics and drug use
The groups did not differ regarding age, gender distribution, smoking status and verbal IQ but dependent users had fewer years of education than the controls and recreational users (Table 1). As expected, dependent users displayed higher BDI and ADHD-SR sum scores than controls and recreational users, whereas recreational users showed higher scores than controls.
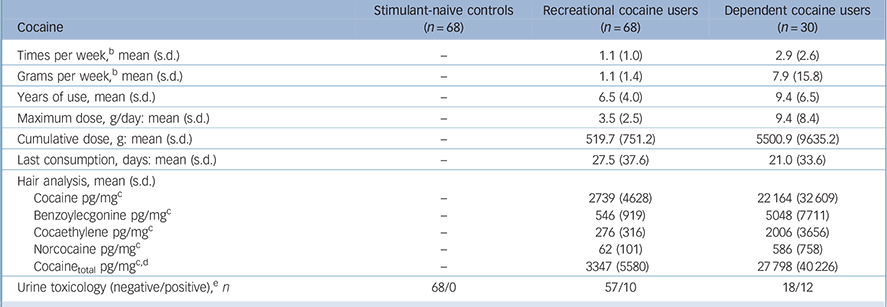
Dependent cocaine users had more than eightfold higher concentrations of cocaine and metabolites compared with recreational users in their hair samples (Table 2). Nonetheless, recreational users were regular users with a mean weekly consumption of about 1 g of cocaine but without fulfilling the DSM-IV criteria for dependence (41 recreational users met the criteria for cocaine abuse). The main route of administration was intranasal; only three dependent users were primarily inhaling the drug (2 free-base, 1 coca paste). In the urine samples, 10 recreational users and 12 dependent users tested positive for cocaine. However, we decided not to exclude them but to investigate the acute and post-acute effects of the drug. Online Table DS1, a more detailed version of Table 2 that also includes details of the patterns and amount of other drug and alcohol use by the three groups, shows that the hair samples revealed a clear domination of cocaine compared with other illegal drug use.
Table 1 Demographic dataFootnote a
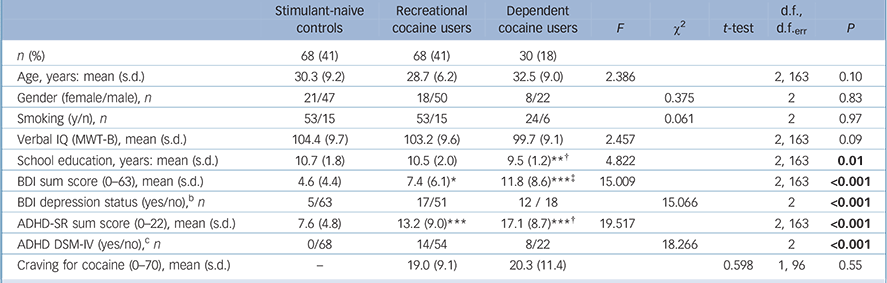
| Stimulant-naive controls |
Recreational cocaine users |
Dependent cocaine users |
F | χ2 | t-test | d.f., d.f.err |
P | |
|---|---|---|---|---|---|---|---|---|
| n (%) | 68 (41) | 68 (41) | 30 (18) | |||||
| Age, years: mean (s.d.) | 30.3 (9.2) | 28.7 (6.2) | 32.5 (9.0) | 2.386 | 2, 163 | 0.10 | ||
| Gender (female/male), n | 21/47 | 18/50 | 8/22 | 0.375 | 2 | 0.83 | ||
| Smoking (y/n), n | 53/15 | 53/15 | 24/6 | 0.061 | 2 | 0.97 | ||
| Verbal IQ (MWT-B), mean (s.d.) | 104.4 (9.7) | 103.2 (9.6) | 99.7 (9.1) | 2.457 | 2, 163 | 0.09 | ||
| School education, years: mean (s.d.) | 10.7 (1.8) | 10.5 (2.0) | 9.5 (1.2)**† | 4.822 | 2, 163 | 0.01 | ||
| BDI sum score (0-63), mean (s.d.) | 4.6 (4.4) | 7.4 (6.1)* | 11.8 (8.6)***‡ | 15.009 | 2, 163 | <0.001 | ||
| BDI depression status (yes/no),Footnote b n | 5/63 | 17/51 | 12 / 18 | 15.066 | 2 | <0.001 | ||
| ADHD-SR sum score (0-22), mean (s.d.) | 7.6 (4.8) | 13.2 (9.0)*** | 17.1 (8.7)***† | 19.517 | 2, 163 | <0.001 | ||
| ADHD DSM-IV (yes/no),Footnote c n | 0/68 | 14/54 | 8/22 | 18.266 | 2 | <0.001 | ||
| Craving for cocaine (0-70), mean (s.d.) | - | 19.0 (9.1) | 20.3 (11.4) | 0.598 | 1, 96 | 0.55 |
MWT-B, Mehrfachwahl-Wortschatz-Intelligenztest; BDI, Beck Depression Inventory; ADHD, attention-deficit hyperactivity disorder; ADHD-SR, ADHD Self-Rating scale.
a. Significant P-values are shown in bold. Statistical tests: ANOVA (all groups), χ2 test (all groups) for frequency data or independent t-test (cocaine users only).
b. Cut-off ⩾11.
c. Cut-off DSM-IV criteria.
Significant Sidak post hoc test v. control group: * P<0.05; ** P<0.01; *** P<0.001.
Significant Sidak post hoc test v. recreational users group: † P<0.05; ‡ P<0.01.
Neurocognitive measures
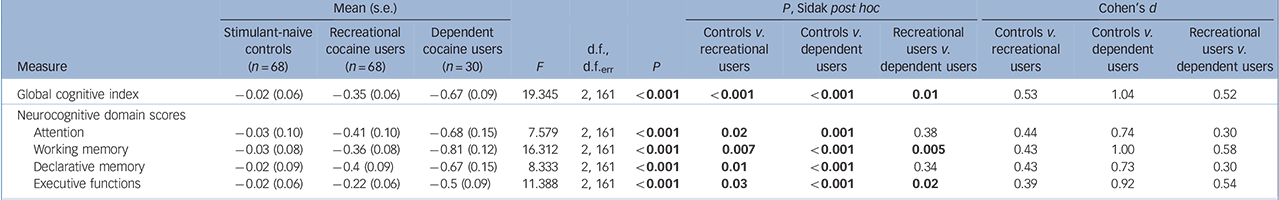
The ANCOVA for the GCI showed a significant group effect including a clear linear trend (P<0.001), and significant pair-wise comparisons between all three groups (Fig. 1, Table 3 and online Fig. DS1), indicating global cognitive impairment in both cocaine user groups. Likewise, all four domains (P<0.001) and 12 of 15 test parameters (P<0.05-0.00001, except the two IED parameters and the SWM strategy score) displayed significant linear trends, suggesting robust dose-response relationships (Table 3 and online Table DS2 for a more detailed analysis including all parameters). In all domains, recreational and dependent users differed significantly from controls. Additionally, the domains working memory and executive functions showed significant group differences between recreational and dependent users. The single test parameters within the attention, working memory and declarative memory domains (detailed RAVLT analysis, online Fig. DS2) showed similar results. However, the effect in the executive function domain was mainly driven by a strong effect regarding RAVLT recall consistency and, to a lesser degree, by the SWM strategy score, whereas the two IED parameters did not show any substantial group differences (detailed IED analysis, online Fig. DS3).
Table 2 Pattern and amount of drug useFootnote a
| Cocaine | Stimulant-naive controls (n = 68) |
Recreational cocaine users (n = 68) |
Dependent cocaine users (n = 30) |
|---|---|---|---|
| Times per week,Footnote b mean (s.d.) | - | 1.1 (1.0) | 2.9 (2.6) |
| Grams per week,Footnote b mean (s.d.) | - | 1.1 (1.4) | 7.9 (15.8) |
| Years of use, mean (s.d.) | - | 6.5 (4.0) | 9.4 (6.5) |
| Maximum dose, g/day: mean (s.d.) | - | 3.5 (2.5) | 9.4 (8.4) |
| Cumulative dose, g: mean (s.d.) | - | 519.7 (751.2) | 5500.9 (9635.2) |
| Last consumption, days: mean (s.d.) | - | 27.5 (37.6) | 21.0 (33.6) |
| Hair analysis, mean (s.d.) | |||
| Cocaine pg/mgFootnote c | - | 2739 (4628) | 22 164 (32 609) |
| Benzoylecgonine pg/mgFootnote c | - | 546 (919) | 5048 (7711) |
| Cocaethylene pg/mgFootnote c | - | 276 (316) | 2006 (3656) |
| Norcocaine pg/mgFootnote c | - | 62 (101) | 586 (758) |
| Cocainetotal pg/mgFootnote c , Footnote d | - | 3347 (5580) | 27 798 (40 226) |
| Urine toxicology (negative/positive),Footnote e n | 68/0 | 57/10 | 18/12 |
a. See online Table DS1 for a more detailed version of this table that includes details for other drug and alcohol use. Use frequency, duration of use and cumulative doses are averaged within the total group.
b. Average use during the past 6 months.
c. Cut-off value for cocaine: 500 pg/mg.47 Hair samples were voluntary and data are missing for three controls and one recreational user.
d. Cocainetotal (= cocaine + benzoylecgonine + norcocaine) is a more robust procedure for discrimination between incorporation and contamination of hairs. Reference Hoelzle, Scheufler, Uhl, Sachs and Thieme48
e. Cut-off value for cocaine: 150 ng/ml. 49 Urine toxicology test data are missing for one recreational user.
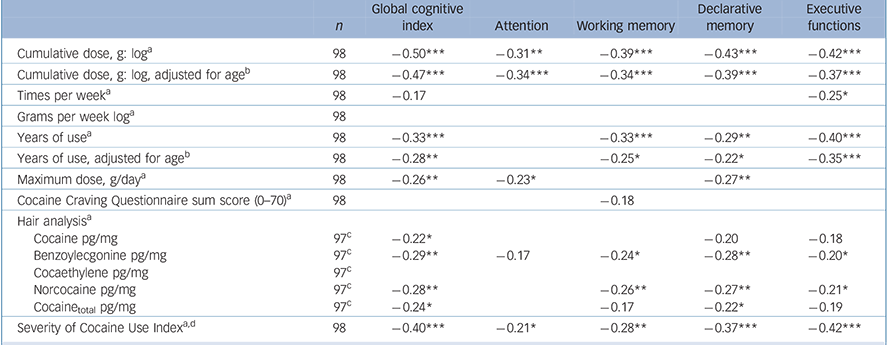
Correlation analyses within the total group of cocaine users (n = 98) revealed that the GCI and the domains working memory, declarative memory and executive functions were all inversely associated with cumulative cocaine dose, duration of cocaine use, cocaine metabolites benzoylecgonine and norcocaine in the hair, and a composite index reflecting the severity of cocaine use (Table 4; intercorrelation of cocaine use parameters, online Table DS3). Interestingly, the domain attention was only strongly correlated with the cumulative cocaine dose. The relatively high correlations in the domain executive functions were again driven by both the RAVLT and SWM parameter, whereas no associations were found for the two IED measures (single test correlation analysis, online Table DS4).
ADHD, age at onset, craving, depression and acute drug effects
Our analysis of ADHD and craving subgroups, by further splitting user groups according to predefined criteria Reference Rosler, Retz, Retz-Junginger, Thome, Supprian and Nissen41 (yes/no fulfilling DSM-IV criteria on ADHD-SR) or median split (low/high, CCQ ⩽16) suggested an impact of these variables on cognitive performance (Fig. 2). The ANCOVAs showed significant group effects for ADHD (F(4,158) = 9.56, P<0.001) and craving subgroups (F(4,158) = 9.35, P<0.001). The presence of craving additionally decreased cognitive performance only in recreational users (d = 0.26) (Fig. 2b), an ADHD diagnosis had a detrimental effect on cognitive functioning in both recreational (d = 0.30) and dependent users (d = 0.33) (Fig. 2a). Notably, recreational and dependent users without ADHD still significantly differed from controls. A combined analysis of ADHD and craving status in an integrated group of cocaine users confirmed this assumption by revealing a significant main effect for group (F(4,158) = 7.66, P<0.001), whereby the controls differed significantly from all the cocaine user groups (Fig. 2c).
Age at onset of cocaine use played a crucial role (F(2,160) = 10.92, P<0.001), as users starting cocaine use before the age 19 years performed significantly worse than users with a later age at onset (d = 0.66). Both users groups differed substantially from the control group (d ⩽18 = 1.10, d >18 = 0.43) (Fig. 3).
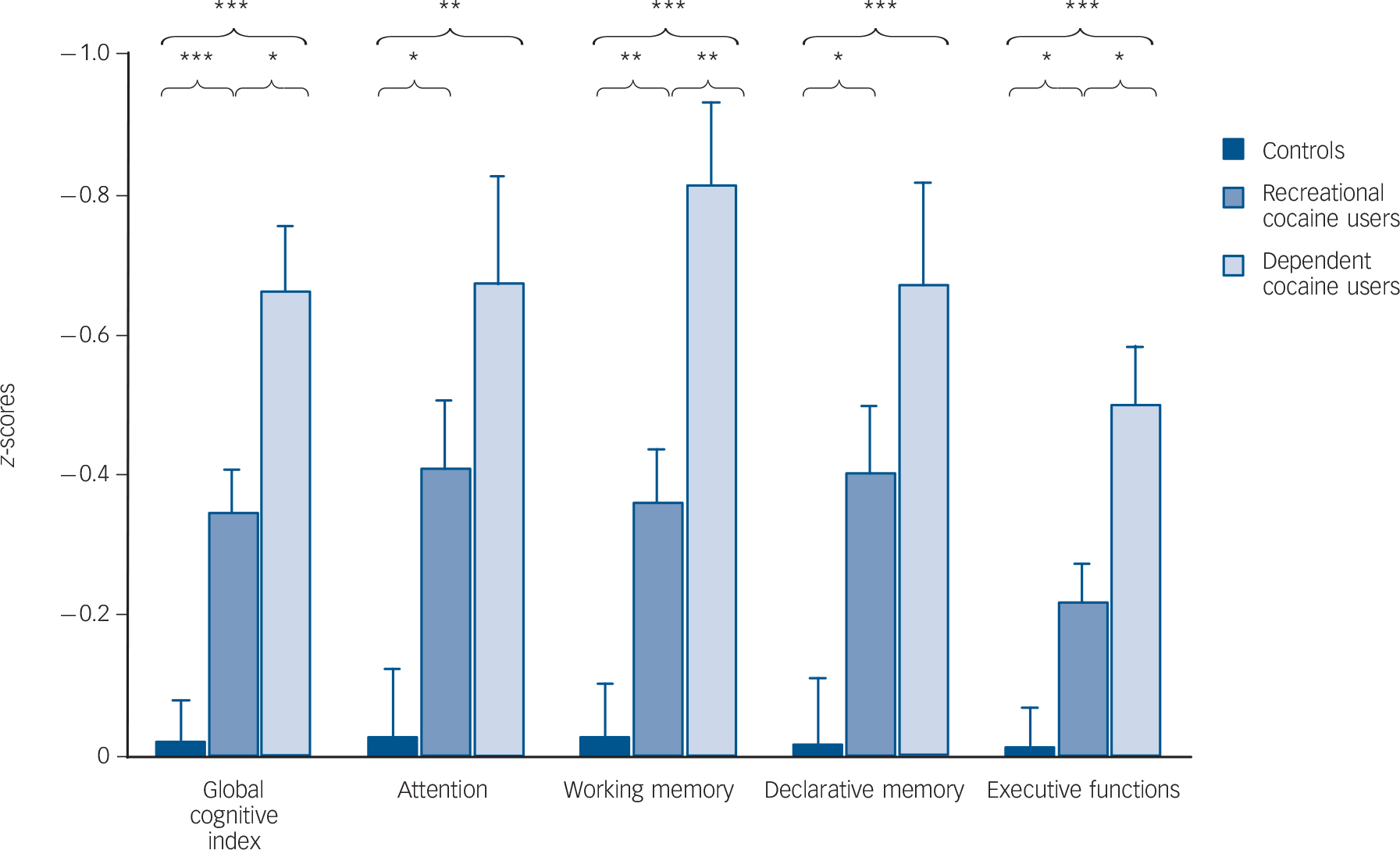
Fig. 1 Mean z-scores and standard errors for the global cognitive index (GCI) and the four cognitive domains (values corrected for age and verbal IQ).
Sidak post hoc tests: * P<0.05, ** P<0.01, *** P<0.001.
Splitting the user groups and controls according to a predefined depression criterion Reference Hautzinger, Bailer, Worall and Keller50 (low/mild-strong, BDI ⩾11) showed a significant group effect (F(5,157) = 7.41, P<0.001) reflecting a weak additive impact of depressive symptoms on cognitive performance only in recreational users (d = 0.28). Also, cocaine users without depression differed significantly from controls without depression (online Fig. DS4).
To test the influence of recent cocaine use, cocaine users were divided into users with positive (n = 22, range 217-24 888 ng/ml, mean 3 873ng/ml, s.d. = 6 461ng/ml) and users with negative urine samples (n = 75) and compared with controls (n = 68). Results revealed significant group effects for the GCI (F(2,160) = 14.76, P<0.001). Pair-wise Sidak comparisons yielded still significant and relatively strong differences between controls and both user groups (dneg = 0.63, dpos = 0.84), and users with positive urine samples showed slightly but non-significantly lower GCI scores than users with negative urine samples (d = 0.22). Similar patterns were found for all four domains (online Fig. DS5).
Multiple regression analyses conducted only in cocaine users confirmed that cumulative dose and duration of cocaine use were the best predictors of cognitive performance in contrast to psychopathological symptoms (online Table DS5).
Risk threshold for cognitive impairments
As the use of cocaine proved to be an important determinant for cognitive performance, odds ratios (ORs) were calculated to assess the risk of impairment when using cocaine. If a progressive clinical criterion of −1 standard deviation was applied to define a cognitive decline, the use of cocaine indicated significant relative risks for deficits in attention (OR = 3.52, 95% CI 1.60-7.72, P<0.01), working memory (OR = 3.08, 95% CI 1.47-6.49, P<0.01), declarative memory (OR = 2.40, 95% CI 1.11-5.19, P<0.05) and executive functions (OR = 3.28, 95% CI 1.53-7.04, P<0.01). In summary, cocaine users were 3.8 times more likely to manifest global cognitive deficits (GCI) than controls (OR = 3.80, 95% CI 1.81-7.97, P<0.001). If a conservative clinical criterion of −2 standard deviations was applied, 1.5% (n = 1) of the controls, 11.8% (n = 8) of the recreational users and 30% (n = 9) of the dependent users revealed strong global cognitive impairment.
Table 3 Neurocognitive global and domain z-scoresFootnote a
| Mean (s.e.) | P, Sidak post hoc | Cohen's d | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Stimulant-naive controls (n = 68) |
Recreational cocaine users (n = 68) |
Dependent cocaine users (n = 30) |
F | d.f., d.f.err |
P | Controls v. recreational users |
Controls v. dependent users |
Recreational users v. dependent users |
Controls v. recreational users |
Controls v. dependent users |
Recreational users v. dependent users |
| Global cognitive index | –0.02 (0.06) | –0.35 (0.06) | –0.67 (0.09) | 19.345 | 2, 161 | <0.001 | <0.001 | <0.001 | 0.01 | 0.53 | 1.04 | 0.52 |
| Neurocognitive domain scores | ||||||||||||
| Attention | –0.03 (0.10) | –0.41 (0.10) | –0.68 (0.15) | 7.579 | 2, 161 | <0.001 | 0.02 | 0.001 | 0.38 | 0.44 | 0.74 | 0.30 |
| Working memory | –0.03 (0.08) | –0.36 (0.08) | –0.81 (0.12) | 16.312 | 2, 161 | <0.001 | 0.007 | <0.001 | 0.005 | 0.43 | 1.00 | 0.58 |
| Declarative memory | –0.02 (0.09) | –0.4 (0.09) | –0.67 (0.15) | 8.333 | 2, 161 | <0.001 | 0.01 | <0.001 | 0.34 | 0.43 | 0.73 | 0.30 |
| Executive functions | –0.02 (0.06) | –0.22 (0.06) | –0.5 (0.09) | 11.388 | 2, 161 | <0.001 | 0.03 | <0.001 | 0.02 | 0.39 | 0.92 | 0.54 |
a. Please see online Table DS2 for a more detailed version of this table that includes neuropsychological test scores. The ANCOVAs for all groups were corrected for age and verbal IQ. Significant P-values are shown in bold. Global cognitive index and cognitive domain scores are z-transformed values. The robustness of these parametric tests was confirmed using bootstrap simulations with 1000 replications.
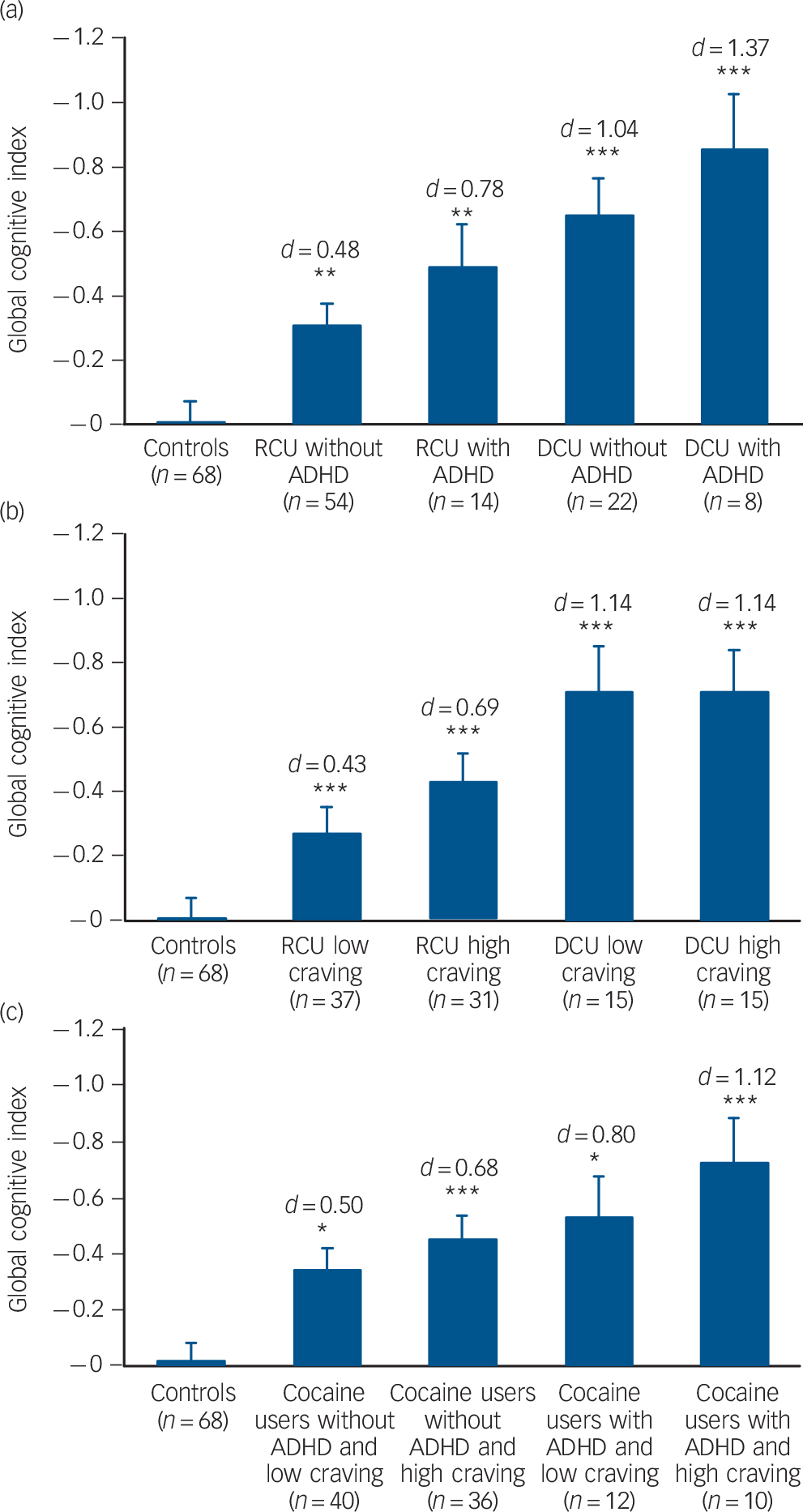
Fig. 2 Mean global cognitive index (GCI) scores and standard errors in groups stratified for cocaine use and confounding variables (values corrected for age, verbal IQ and cocaine g/week).
Significant Sidak post hoc test v. control group: * P<0.05, ** P<0.01, *** P<0.001. Cohen's d v. control group. (a) Attention-deficit hyperactivity disorder (ADHD), DSM-IV criteria based on ADHD-Self Report (ADHD-SR). (b) Cocaine Craving Questionnaire (CCQ), craving for cocaine status based on median split ⩽16. (c) Combined user group (n = 98) stratified for ADHD DSM-IV criteria based on ADHD-SR and CCQ, craving for cocaine status based on median split ⩽16. RCU, recreational cocaine users; DCU, dependent cocaine users.
Figure 4 illustrates a clearly increasing risk of cognitive impairment with increasing cumulative doses of cocaine. Although this analysis emphasised the long-term impact of cocaine use on all four cognitive domains, declarative memory is the latest, whereas working memory is generally the earliest and most affected domain. Interestingly, a lifetime consumption of more than 1 kg cocaine seemed to strongly enhance the risk for cognitive impairment (Fig. 4a), whereas a lifetime consumption of more than 100 g was associated with an approximately 50% risk for mild cognitive impairment (Fig. 4b).
Table 4 Correlations between neurocognitive global and domain z-scores and cocaine use parameters in cocaine users
| n | Global cognitive index |
Attention | Working memory | Declarative memory |
Executive functions |
|
|---|---|---|---|---|---|---|
| Cumulative dose, g: logFootnote a | 98 | –0.50*** | –0.31** | –0.39*** | –0.43*** | –0.42*** |
| Cumulative dose, g: log, adjusted for ageFootnote B | 98 | –0.47*** | –0.34*** | –0.34*** | –0.39*** | –0.37*** |
| Times per weekFootnote a | 98 | –0.17 | –0.25* | |||
| Grams per week logFootnote a | 98 | |||||
| Years of useFootnote a | 98 | –0.33*** | –0.33*** | –0.29** | –0.40*** | |
| Years of use, adjusted for ageFootnote b | 98 | –0.28** | –0.25* | –0.22* | –0.35*** | |
| Maximum dose, g/dayFootnote a | 98 | –0.26** | –0.23* | –0.27** | ||
| Cocaine Craving Questionnaire sum score (0-70)Footnote a | 98 | –0.18 | ||||
| Hair analysisFootnote a | ||||||
| Cocaine pg/mg | 97Footnote c | –0.22* | –0.20 | –0.18 | ||
| Benzoylecgonine pg/mg | 97Footnote c | –0.29** | –0.17 | –0.24* | –0.28** | –0.20* |
| Cocaethylene pg/mg | 97Footnote c | |||||
| Norcocaine pg/mg | 97Footnote c | –0.28** | –0.26** | –0.27** | –0.21* | |
| Cocainetotal pg/mg | 97Footnote c | –0.24* | –0.17 | –0.22* | –0.19 | |
| Severity of Cocaine Use IndexFootnote a , Footnote d | 98 | –0.40*** | –0.21* | –0.28** | –0.37*** | –0.42*** |
a. Pearson's product-moment correlation.
b. Partial correlation corrected for age.
c. Hair samples were voluntary and data are missing for one recreational user.
d. Severity of Cocaine Index use corresponds to the mean of the z-transformed parameters: cumulative dose, grams per week, years of use, maximum dose and hair analysis Cocainetotal.
Correlations with a P-level below 10% are shown, and significant correlations are marked: * P<0.05; ** P<0.01; *** P<0.001.
Discussion
Main findings
The aim of the present study was to examine whether cognitive performance is impaired in non-dependent recreational cocaine users and dependent cocaine users. In contrast to previous studies, hair toxicologies and comprehensive psychiatric diagnostics allowed the investigation of relatively pure cocaine users with little psychiatric comorbidity. Moreover, this is the largest published sample of neuropsychologically examined cocaine users to date (n = 98) and the first study directly comparing the cognitive performance of stimulant-naive controls with both recreational and dependent users. The major finding of the present study is that intensive recreational users showed small but significant cognitive dysfunction, with dysfunction deteriorating further in dependent users. Recreational users displayed the strongest effects in the attention domain, whereas in dependent users working memory was most affected. Correlation and regression analyses revealed negative associations between cognitive performance and cocaine metabolites in the hair, cumulative cocaine dose and duration of cocaine use, suggesting that cognitive impairments might be partially cocaine-induced.
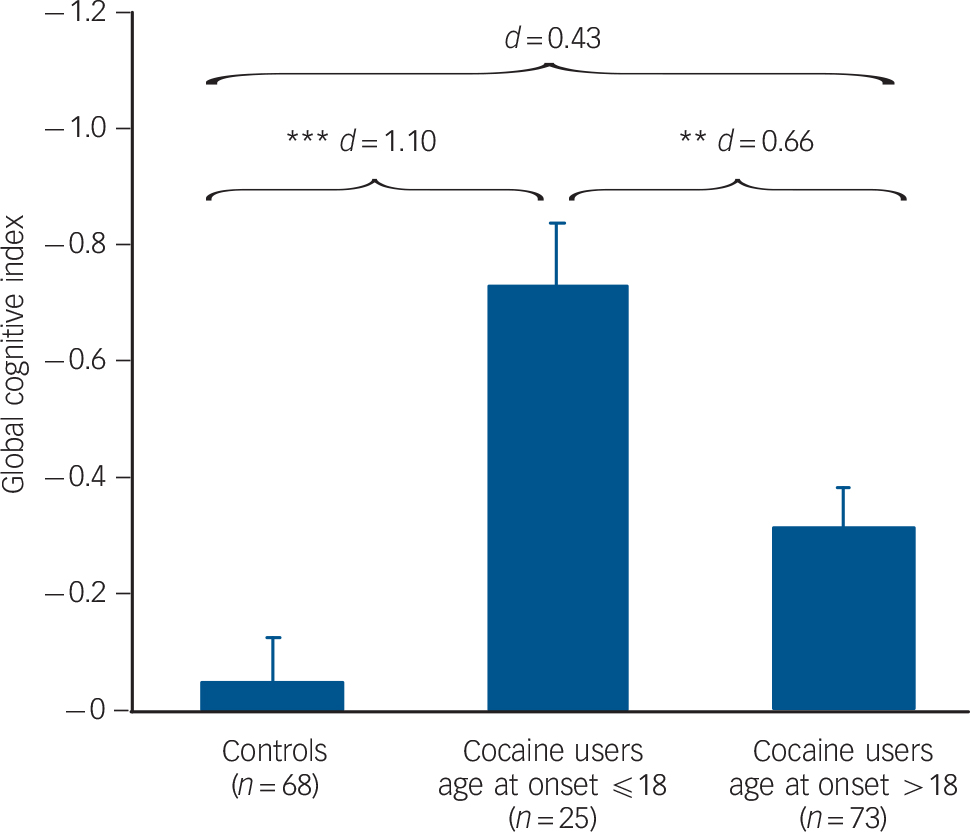
Fig. 3 Mean global cognitive index (GCI) scores and standard errors in groups stratified for age at onset for cocaine use (values are corrected for age, verbal IQ and cocaine use in years).
Group sizes (n) are shown. Significant Sidak post hoc tests are marked: ** P<0.01, *** P<0.001.
The influence of ADHD and cocaine craving on the cognitive functioning of cocaine users had not systematically been investigated before. We found that symptoms of ADHD and depression as well as craving for cocaine are important modulators of cognitive function in cocaine users, whereas recent cocaine use seemed to be less important. However, cognitive dysfunction is still present in cocaine users without the presence of craving, depression or ADHD symptoms. Finally, we demonstrated that the risk for cognitive impairment increases with early age at onset and ascending cumulative cocaine doses, in particular if estimated lifetime doses of 500 g to 1 kg cocaine are exceeded (Fig. 4).
Comparison with findings from other studies
The present results indicate impaired attention in both recreational and dependent users, with moderate to strong effect sizes respectively. As attention involves several subprocesses, it should be emphasised that our domain is primarily based on two RVP parameters measuring sustained attention. Our findings replicated previous reports on sustained attention deficits in dependent cocaine users Reference Jovanovski, Erb and Zakzanis17,Reference Pace-Schott, Morgan, Malison, Hart, Edgar and Walker21 but extended the current knowledge regarding relatively pure recreational users, as attentional deficits have previously been indicated only in small samples (n = 13-18) of polytoxic recreational users. Reference Colzato, van den Wildenberg and Hommel23,Reference Colzato and Hommel24,Reference Soar, Mason, Potton and Dawkins29
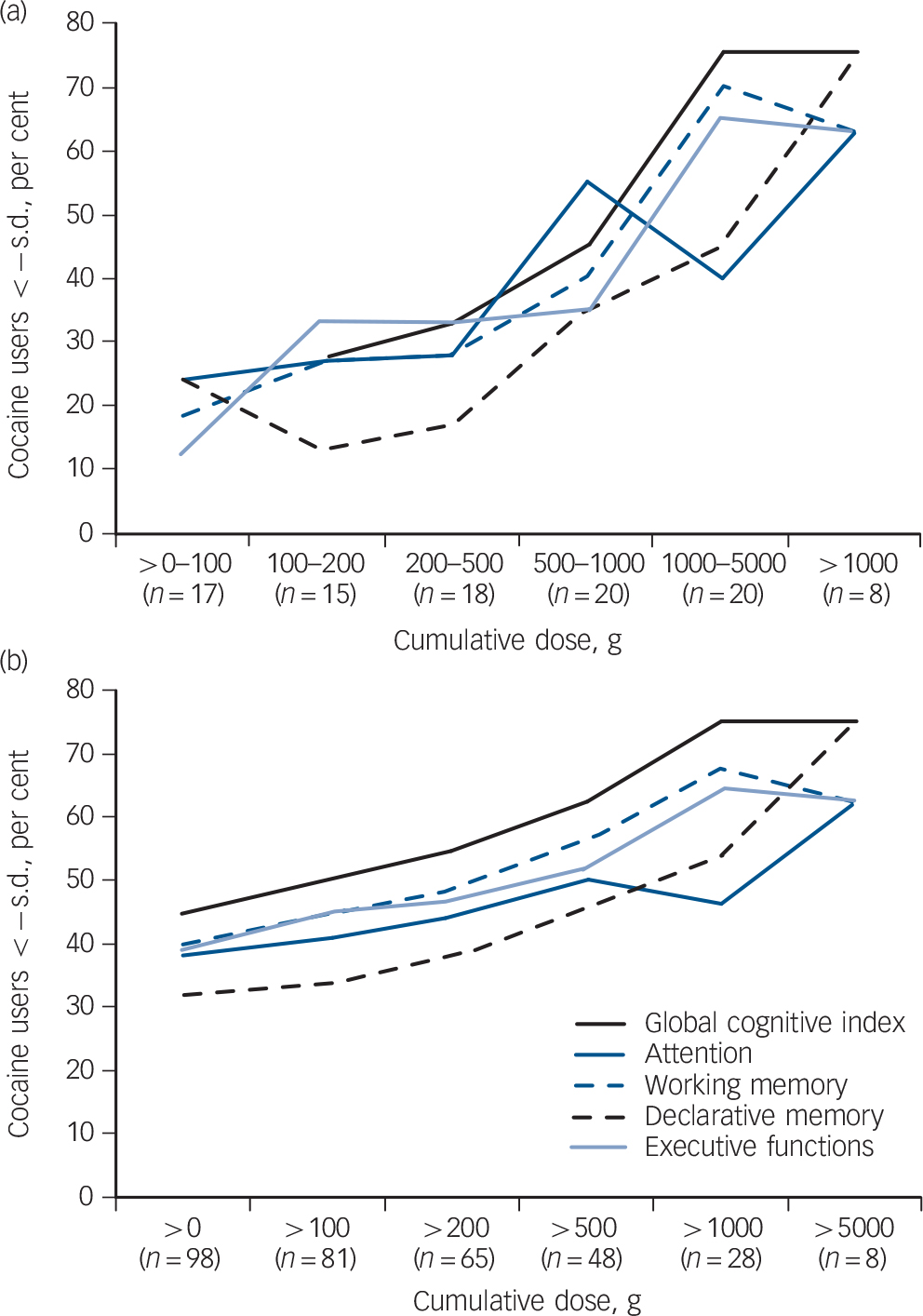
Fig. 4 (a) Percentage of cocaine users fulfilling the clinical cognitive criterion of below −1 standard deviation in the specific cumulative dose group. (b) Percentage of cocaine users fulfilling the clinical cognitive criterion of below −1 standard deviation in groups with (from left to right) ascending cumulative doses.
Domain cut-offs: global cognitive index s.d. = −0.54, attention s.d. = −0.81, working memory s.d. = −0.70, declarative memory s.d. = −0.82, executive functions s.d. = −0.38. Values are corrected for age and verbal IQ.
Regarding working memory, the strong effect sizes found for dependent users confirm previous findings, also mostly drawn from much smaller samples. Reference Jovanovski, Erb and Zakzanis17,Reference Woicik, Moeller, Alia-Klein, Maloney, Lukasik and Yeliosof18 In addition, in accordance with a recent study investigating a small sample of polydrug recreational users (n = 17), we found that recreational cocaine use is associated with subtle visuospatial working memory impairment. Reference Soar, Mason, Potton and Dawkins29 Our results are the first to indicate small to moderate verbal working memory deficits in recreational users.
Furthermore, our study confirmed previous findings of broad deficits in verbal Reference Goldstein, Leskovjan, Hoff, Hitzemann, Bashan and Khalsa16,Reference Cunha, Nicastri, Gomes, Moino and Peluso20,Reference Pace-Schott, Morgan, Malison, Hart, Edgar and Walker21 and visual learning and memory Reference Goldstein, Leskovjan, Hoff, Hitzemann, Bashan and Khalsa16,Reference Jovanovski, Erb and Zakzanis17,Reference Cunha, Nicastri, Gomes, Moino and Peluso20 in dependent users. The only other report that studied recreational users described similar verbal memory deficits for recreational prescription stimulant users with >80% cocaine co-use, but found no significant effects in a small group (n = 13) of recreational users with a low minimal inclusion threshold (use three times in the past 6 months). Reference Reske, Eidt, Delis and Paulus28 Thus, declarative memory dysfunction is associated not only with chronic but also with recreational cocaine use. However, compared with other domains declarative memory seemed to be least affected at cumulative cocaine doses <500 g.
Unlike in the other domains, the single executive function parameters displayed inconsistent results. Both IED parameters indicated no performance deficits in either user groups. On the contrary, the SWM strategy score demonstrated small to moderate, and the RAVLT recall consistency moderate to strong effects in recreational and dependent users. These inconsistencies are typical for the heterogeneous concept of executive functions reflecting varying task requirements and difficulty levels between studies. Reference Jovanovski, Erb and Zakzanis17 Nevertheless, the existing literature reported executive deficits in dependent users on complex but only scarcely on simple tasks. Reference Jovanovski, Erb and Zakzanis17 As 71% of the participants in the user groups achieved the highest IED stage, a ceiling effect can be assumed. Furthermore, we found strong correlations between the executive domain and several cocaine-use parameters confirming similar relationships that were found in earlier studies on dependent Reference Bolla, Rothman and Cadet19 and recreational users. Reference Colzato, van den Wildenberg and Hommel26
Sustained attention and working memory processes are both associated with increased activity in prefrontal, parietal and cingulate brain regions. Reference Cabeza and Nyberg15 Accordingly, the LNST involves the lateral prefrontal cortex, Reference Yochim, Baldo, Nelson and Delis51 SWM performance is associated with the dorsolateral prefrontal cortex and ventrolateral prefrontal cortex, Reference Manes, Sahakian, Clark, Rogers, Antoun and Aitken52,Reference Owen, Evans and Petrides53 and the PAL depends on frontal and medial temporal lobe function. Reference Owen, Sahakian, Semple, Polkey and Robbins54 In-depth analysis of the RAVLT revealed that cocaine users primarily display learning and retrieval deficits, whereas recognition was less affected - a pattern specifically reported for prefrontal cortex lesions. Reference Janowsky, Shimamura, Kritchevsky and Squire55 Likewise, prefrontal cortex lesions have been related to impairments in recall consistency. Reference Alexander, Stuss and Fansabedian56,Reference Benedict, Zivadinov, Carone, Weinstock-Guttman, Gaines and Maggiore57 Finally, glucose metabolism in the dorsolateral prefrontal cortex significantly predicted visual and verbal memory performance in participants who were cocaine addicted and in controls. Reference Goldstein, Leskovjan, Hoff, Hitzemann, Bashan and Khalsa16 Together with previous findings that dependent cocaine users display decreased grey matter volume and glucose metabolism in the orbitofrontal cortex and dorsolateral prefrontal cortex Reference Ersche, Barnes, Jones, Morein-Zamir, Robbins and Bullmore10-Reference Sim, Lyoo, Streeter, Covell, Sarid-Segal and Ciraulo14,Reference Ersche, Jones, Williams, Robbins and Bullmore58-Reference Volkow, Hitzemann, Wang, Fowler, Wolf and Dewey61 the neuropsychological profile therefore suggests that similar but less pronounced alterations in the prefrontal cortex might be present in recreational users.
We investigated potential cofactors frequently associated with cocaine use or commonly addressed as confounding factors for cognition such as ADHD and depressive symptoms. Reference Perez de Los Cobos, Sinol, Puerta, Cantillano, Lopez Zurita and Trujols33,Reference Gotlib and Joormann62 Moreover, craving for food Reference Kemps and Tiggemann45 and nicotine Reference Sayette, Schooler and Reichle46 has been shown to have an impact on cognitive functioning but the specific impact of cocaine craving has not been investigated to date. Here, high craving and depression scores or an ADHD diagnosis further decreased the cognitive performance within the group of recreational users. Additionally, dependent users with clinically relevant ADHD symptoms displayed stronger cognitive deficits (d = 1.37) than dependent users without ADHD (d = 1.04), whereas neither craving nor depression symptoms had an additional effect in this group. Importantly, cocaine users without clinically relevant ADHD or depression scores and also with low craving scores still displayed significant cognitive deficits, whereas a combination of an ADHD diagnosis and high craving lead to the strongest impairments, similar to our results on early information processing. Reference Preller, Ingold, Hulka, Vonmoos, Jenni and Baumgartner32 Regarding the impact of depression, our findings confirm a previous result reporting no additional effect of dysphoria on cognitive performance in a sample of predominantly dependent cocaine users Reference Woicik, Moeller, Alia-Klein, Maloney, Lukasik and Yeliosof18 but our data additionally indicate a small impact of depression at a recreational level of use.
Attention-deficit hyperactivity disorder is characterised by problems in attentional performance and inhibitory control and patients with ADHD on average perform worse than healthy controls on tests of attention and executive function. Reference Valera, Brown, Seidman, Valera, Brown and Seidman63 Nevertheless, the influence of ADHD symptoms on the cognition of cocaine users, in which ADHD is highly prevalent, had not been investigated previously. The exact pathogenesis underlying ADHD is still unknown, Reference Shen, Liao and Tseng64 but as abnormalities within catecholamine systems and the prefrontal cortex seem to play a major role in ADHD Reference Shen, Liao and Tseng64,Reference Liston, Malter Cohen, Teslovich, Levenson and Casey65 and cocaine use, Reference Volkow, Fowler, Wang, Baler and Telang7,Reference Volkow, Fowler, Wang and Swanson8 it can be assumed that similar pathologies might lead to a mutual aggravation of detrimental effects on cognitive performance.
In contrast to a previous finding, showing that cocaine users with a positive urine toxicology have slightly improved cognitive performances, Reference Woicik, Moeller, Alia-Klein, Maloney, Lukasik and Yeliosof18 users with positive cocaine urine tests displayed slightly worse cognitive scores in the present study. As urine toxicologies were performed by immunoassays, which are only presumptive and potentially biased by external factors, Reference Moeller, Lee and Kissack66 positive urine tests do not necessarily prove a violation of the requested 3-day cocaine retention period.
Study limitations and future research
The study has some limitations. First, cocaine dependency was diagnosed according to DSM-IV criteria. 30 These criteria depend on introspection and self-report but do not consider features such as duration and amount of cumulative doses. Thus, some participants in the recreational users group might be misclassified as non-dependent. Second, although this is one of the first investigations employing hair analysis in a neurocognitive study with cocaine users, we can only rely on self-reports for all illegal drug use prior to 3-6 months (depending on hair length). This is, however, an inevitable constraint of all studies with illegal drug users. Reference Curran67 Third, a cross-sectional design cannot determine whether the cognitive deficits found in the cocaine users were pre-existent traits (vulnerability or resilience), drug-induced consequences or both. Hence, to answer this question we need to await the findings of the second part of the ZuCo2St longitudinal study in 2013. Finally, cocaine users participating voluntary in a study session lasting several hours require a certain level of motivation and cognitive functionality; we assume that the cocaine users in our sample are therefore not the most impaired individuals, and probably even perform relatively well. Thus, the cognitive impairments shown here might partially be underestimates for both recreational and dependent users.
In conclusion, our results confirmed that dependent cocaine use is associated with broad cognitive impairments in the domains attention, working memory, declarative memory and parts of executive functions. In all four domains, recreational users' performance was intermediate between that of controls and dependent users, and they displayed significant deficits, predominantly in the domains attention and working memory. This is in line with our previous work indicating catecholamine dysfunction at a recreational level of use. Reference Hulka, Wagner, Preller, Jenni and Quednow31,Reference Preller, Ingold, Hulka, Vonmoos, Jenni and Baumgartner32 Furthermore, all cognitive domains displayed correlations with the long-term intake parameters duration and amount of cocaine use and specifically early age at onset was linked to considerable cognitive dysfunction. The neuropsychological profile suggests prefrontal cortex dysfunction as the common denominator of these cognitive impairments, which is in line with previous findings showing alterations to the frontostriatal dopamine system in addicted cocaine users. Reference Bolla, Ernst, Kiehl, Mouratidis, Eldreth and Contoreggi9,Reference Ersche, Jones, Williams, Robbins and Bullmore58,Reference Ersche, Jones, Williams, Turton, Robbins and Bullmore59 Additionally, cocaine use and ADHD seem to have mutually aggravating effects on cognitive impairments. Altogether these results indicate gradual impairments in both recreational and dependent cocaine users, and clinically relevant cognitive deficits seem to arise with long-term cocaine use, as best reflected by cumulative cocaine dose.
Funding
The study was supported by grants from the Swiss National Science Foundation (SNSF; grant No. PP00P1-123516/1) and the Olga Mayenfisch Foundation.
Acknowledgements
We are grateful to the following for support with recruitment: Alex Bücheli (Streetwork Zürich), Roland Kowalewski (Research Group Substance Use Disorders, Clinic for General an Social Psychiatry, University Hospital of Psychiatry), Lars Stark and Thilo Beck (ARUD, Zurich), Eric La Serra (Klinik St. Pirminsberg, Psychiatrie-Dienste Süd, Kanton St. Gallen), and Michael Schaub (Research Institute for Public Health and Addiction, Zürich). Moreover, we thank Joëlle Barthassat, Christina Gruber, Kathrin Küpeli, Franziska Minder and Claudia Schulz for excellent support and Dominique Eich-Höchli for critical comments on the manuscript.











eLetters
No eLetters have been published for this article.