Schizophrenia is a common mental disorderReference Saha, Chant, Welham and McGrath1 that accounts for a tremendous healthcare burden,Reference Charlson, Ferrari, Santomauro, Diminic, Stockings and Scott2 and is frequently comorbid with substance use disorders (SUDs).Reference Hunt, Large, Cleary, Lai and Saunders3 Substance use not only increases the risk of developing psychotic symptoms, but also negatively affects the course of illness.Reference Heiberg, Jacobsen, Nesvåg, Bramness, Reichborn-Kjennerud and Næss4,Reference Schmidt, Hesse and Lykke5 Possible consequences of comorbid substance use include worsening of psychotic symptoms, treatment non-adherence, pharmacodynamic interactions with prescribed agents, increased risk of medical comorbidities, increased violence (both as offenders and victims) and suicides.Reference Schmidt, Hesse and Lykke5–Reference Margolese, Malchy, Negrete, Tempier and Gill7 Patients with schizophrenia and comorbid SUD also show increased service utilisation (e.g. emergency room visits, hospital admissions) and premature mortality.Reference Schmidt, Hesse and Lykke5–Reference Margolese, Malchy, Negrete, Tempier and Gill7 Although schizophrenia and SUDs are associated with premature death, their comorbidity shows additive effects on mortality. Generally, patients with co-occurring schizophrenia and SUD are considered relatively challenging to treat because of severe positive symptoms, more frequent relapses and frequently observed medication non-adherence.Reference Large, Mullin, Gupta, Harris and Nielssen8
To our knowledge, the European First Episode Schizophrenia Trial (EUFEST) and Clinical Intervention Trial of Antipsychotics Effectiveness (CATIE) are the largest clinical trials that have investigated SUD subgroup differences in treatment response for patients with schizophrenia. In EUFEST, SUDs were not associated with outcome,Reference Wobrock, Falkai, Schneider-Axmann, Hasan, Galderisi and Daivdson9 whereas in CATIE, patients with moderate substance use showed relatively poor response to antipsychotics.Reference Kerfoot, Rosenheck, Petrakis, Swartz, Keefe and McEvoy10 Similarly inconsistent results have been found in several small-scale studies, including small randomised clinical trials, case reports and small observational studies.Reference Buckley, Thompson, Way and Meltzer11–Reference Zimmet, Strous, Burgess, Kohnstamm and Green14 Although researchers and clinicians have suggested that atypical antipsychotics and, in some cases, clozapine should be preferred for patients with comorbid schizophrenia and SUD,Reference Krause, Huhn, Schneider-Thoma, Bighelli, Gutsmiedl and Leucht15 to date there is little evidence to inform prescribing guidelines.Reference Ziedonis, Smelson, Rosenthal, Batki, Green and Henry16,Reference Clerici, de Bartolomeis, de Filippis, Ducci, Maremmani and Martinotti17 In a recent systematic review, authors cautioned against the use of conventional long-acting injectables (LAIs) in this patient group because of the risk of increased drug craving, although the authors note that evidence on the use of LAIs in this patient group is still very scarce.Reference Zhornitsky and Stip18 Since then, at least one study with a follow-up of 1 year has investigated the effectiveness of atypical LAIs in this patient group and found favourable effects on quality of life, general functioning (as evaluated with Clinical Global Impression rating scales) and substance craving.Reference Cuomo, Kotzalidis, de Persis, Piacentino, Perrini and Amici19 Patients with schizophrenia and comorbid SUD have also been shown to have more extrapyramidal symptoms than patients without an SUD,Reference Potvin, Pampoulova, Mancini-Marië, Lipp, Bouchard and Stip20 which would then favour clozapine treatment, as clozapine may decrease the risk and severity of extrapyramidal symptoms. Finally, a recent meta-analysis noted important limitations to the current evidence for the use of antipsychotics in schizophrenia and comorbid SUD, highlighting the need for large-scale, good-quality studies into this topic.Reference Krause, Huhn, Schneider-Thoma, Bighelli, Gutsmiedl and Leucht15
In summary, research on the effectiveness of pharmacotherapies for schizophrenia and comorbid SUD is very sparse, and more importantly, non-existent on the prevention of the development of SUDs in patients with schizophrenia. Therefore, we studied which antipsychotics are associated with the lowest risk of the initial onset of SUD in schizophrenia, and the most effective treatments for preventing relapses in schizophrenia and comorbid SUD. To that end, we collected cohorts totalling more than 45 000 patients with schizophrenia, from two independent national registries.
Method
Study cohorts
We had access to two nationwide cohorts of persons with schizophrenia from Finnish and Swedish national registries, and refer to them as the Finnish and the Swedish cohorts throughout this paper. The Finnish cohort included all persons treated for schizophrenia (ICD-10 codes F20 and F2521 and ICD-8 and -9 code 295) in in-patient care during 1972–2014 in Finland, who entered the cohort at age <46 years. The cohort was identified from the Hospital Discharge Register (HDR) maintained by the National Institute of Health and Welfare. Data were collected from the HDR (all hospital care periods with diagnoses, 1972–2017), Prescription Register (reimbursed prescription drug purchases, 1995–2017) and Causes of Death Register from Statistics Finland (1972–2017). The follow-up period started on 1 January 1996 for persons diagnosed before that, and at the first discharge from in-patient care for persons diagnosed during 1996–2014. The follow-up period ended at death or 31 December 2017, whichever occurred first. This Finnish cohort included 30 860 persons with schizophrenia.
The Swedish cohort included all persons with schizophrenia diagnoses (ICD-10 codes F20 and F25) and registered schizophrenia treatment contact between 1 July 2006 until 31 December 2013 in Sweden, who entered the cohort at age <46 years. Schizophrenia diagnoses were derived from the National Patient Register (maintained by the National Board of Health and Welfare, in-patient and specialised out-patient care) and the MiDAS Register (disability pensions and sickness absence, maintained by the Swedish Social Insurance Agency). Data were collected from the National Patient Register (all hospital care periods and specialised out-patient visits with diagnoses, July 2005 to December 2016), the Prescribed Drug Register (maintained by the National Board of Health and Welfare, prescription drug purchases July 2005 to December 2016), the Causes of Death Register (maintained by the National Board of Health and Welfare, causes of death 2006–2016) and the LISA register (maintained by Statistics Sweden, demographic characteristics). The follow-up period started on 1 July 2006 for persons diagnosed before that, and at the first recorded diagnoses for persons diagnosed during July 2006 to December 2013. The follow-up period ended at death or 31 December 2016, whichever occurred first. The Swedish cohort included 14 616 persons. The cohort and methods have been described previously.Reference Tiihonen, Mittendorfer-Rutz, Majak, Mehtälä, Hoti and Jedenius22
The main differences between the cohorts are the shorter follow-up time for the Swedish cohort (11 v. 22 years, i.e. years 2006–2016 for the Swedish cohort and years 1996–2017 for the Finnish cohort) and the Finnish cohort only including individuals with a history of hospital admission owing to schizophrenia (the Swedish cohort also included patients identified using diagnoses obtained from disability pensions, sickness absences and specialised out-patient care contacts). The age limit for inclusion was set at <46 years to reduce both risk of survival bias and the number of iatrogenic SUDs arising from benzodiazepine and opioid prescriptions (e.g. for patients undergoing major surgery or starting to suffer from geriatric sleep disorders).
The Regional Ethics Board of Stockholm approved this research project (decision number 2007/762–31). Permissions were also granted by pertinent institutional authorities at the Finnish National Institute for Health and Welfare (permission number THL/847/5.05.00/2015), the Social Insurance Institution of Finland (permission number 65/522/2015) and Statistics Finland (permission number TK53-1042-15). The study was registry-based and no contact was made with the participants of the study; therefore, according to legislation in both countries and as per our previous publications, obtaining informed consent from participants was not required.
Substance misuse
SUDs were defined as ICD-10 diagnoses F10–F19 excluding F17 (nicotine abuse), utilising both in-patient and specialised out-patient care registers. F17 (nicotine abuse) was excluded because it is very severely underreported in the registries, and including it would have led to serious skewing of the data. SUD was defined categorically: a person was considered as not having an SUD until the first recorded diagnoses, and having an SUD after the first recording. Some persons already had an SUD at cohort entry, whereas some persons were transferred from a ‘non-SUD’ to SUD group during follow-up. In the Finnish cohort, the non-SUD group included 22 750 persons and the SUD group included 8642 persons (2853 new onsets of an SUD) during follow-up. In the Swedish cohort, there were 10 102 persons in the non-SUD group and 4836 persons in the SUD group (1043 new onsets of SUD) during follow-up. The group compositions are shown in Supplementary Figure 1 available at https://doi.org/10.1192/bjp.2022.117.
Exposure
Antipsychotics were defined as Anatomical Therapeutic Chemical classification codes N05A (lithium N05AN01 excluded), and further categorised into oral versus LAIs according to their drug formulation (oral antipsychotics referred to in the text if not explicitly stated as ‘LAI’). Polytherapy refers to the concomitant use of two or more antipsychotics. Antipsychotics were categorised as aripiprazole, clozapine, olanzapine, quetiapine, risperidone, other oral, any LAI or antipsychotic polytherapy. Drug purchases recorded in the register data were modelled into drug use periods with the PRE2DUP method.Reference Tanskanen, Taipale, Koponen, Tolppanen, Hartikainen and Ahonen23 The method estimates drug use on a day-by-day basis and is based on the calculation of sliding averages of the daily dose in defined daily dosages according to individual drug use patterns, and it takes time periods of hospital care into account (when drugs are provided by the caring unit and not recorded in the registers). All exposures were defined time dependently, i.e. they are updated every time anything changes. Time-dependent or time-varying exposure means that changes in medication use versus non-use and changes in the medication regimen were followed up and updated in the models. For each time interval, medication treatments were categorised as currently ongoing or not.
Outcomes
Outcomes were onset of SUD (diagnosed either in in-patient or specialised out-patient care settings) for patients with schizophrenia but no history of SUD (ICD-10 codes F10–F19, excluding F17); and psychiatric hospital admission (ICD-10 codes F20–F29) and SUD-related hospital admission (ICD-10 codes F10–F19, excluding F17) for patients with schizophrenia and comorbid SUD. Hospital admissions were used as a proxy for relapse.
Overview of statistical analyses
All statistical analyses were conducted independently in the Finnish and Swedish cohorts, using SAS for Windows version 9.4. Further details on the analysis models are given and pictured in the Supplementary Methods (Supplementary Figs 1 and 2). Results are presented as adjusted hazard ratios (aHRs) with 95% confidence intervals. The P-values for the analyses were corrected for multiple comparisons on a per-graph basis, using the Benjamini–Hochberg false discovery rate correction with a 0.05 threshold for statistical significance. Correlations between the countries for outcome measures for the different antipsychotic exposures used were calculated with Pearson's correlation (statistical significance threshold: P < 0.05; R Statistical Software version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/).
Statistical analyses to compute risk of initial development of an SUD in patients with schizophrenia (between-individuals model)
Traditional multivariate-adjusted Cox regression models were used for analyses of the outcome development of initial SUD. This means that individuals undergoing certain exposures (receiving clozapine) were compared with individuals not undergoing this exposure (not receiving clozapine). This analysis may be affected by bias arising from permanent or long-term individual characteristics if the exposure groups differ with regards to these characteristics (e.g. if patients prescribed clozapine are more often male than those who are not prescribed clozapine, this may cause gender-based bias). To reduce risk of such bias, the analyses were therefore adjusted for gender, age at cohort entry, number of previous hospital admissions owing to psychosis, time since first schizophrenia diagnosis, continuously updated variables for current versus no use of medications (lipid-modifying agents, opioid analgesics, non-opioid analgesics, anticholinergic anti-Parkinson drugs, prior use of LAI) and continuously updated variables for the following diagnoses: cardiovascular disease, diabetes, asthma/chronic obstructive pulmonary disease, previous cancer or previous suicide attempt.
Statistical analyses to compute risk of hospital admission in patients with schizophrenia and comorbid SUD (within-individual model)
Among persons with schizophrenia and comorbid SUD, the outcomes psychiatric hospital admission and SUD-related hospital admission were analysed as recurrent events (i.e. events that may happen multiple times for the same person) and analyses were conducted with within-individual design, using stratified Cox regression.Reference Tiihonen, Mittendorfer-Rutz, Majak, Mehtälä, Hoti and Jedenius22 This means that individuals are compared against themselves when undergoing different exposures, and can contribute to data sets of different exposures if they switched their medication regimens during follow-up (e.g. using oral olanzapine for the first 10 months, then 5 months using no medication, then 3 months using clozapine followed by 2 years of polytherapy with clozapine and aripiprazole). The risks derived from these comparisons within individuals are then pooled for each exposure. Since an individual is compared against themselves, this analysis method eliminates bias from permanent or long-term characteristics. However, only persons having an outcome event can contribute to within-individual analyses, which leads to a lower number of individuals in the analyses than in between-individual comparisons. The analyses were adjusted for time-varying covariates, which were sequential order of treatments use of antidepressants, use of benzodiazepines or Z-drugs, use of mood stabilisers and lithium, and time since cohort entry. For analyses on psychiatric hospital admission, the most common (five or ten, depending on analysis) specific antipsychotics were included, in addition to polytherapy, and the rest were grouped as ‘other (first/second generation) orals’. Sensitivity analyses were conducted and are outlined in more detail in the Supplementary Methods.
Results
Descriptive statistics
In the Finnish cohort, a total of 30 860 persons with schizophrenia were included, of whom 8110 (26%) had a diagnosis of SUD (SUD group: mean age 32.9, s.d. 7.8 years, 71.9% men; non-SUD group: mean age 33.8, s.d. 7.8 years, 52.5% men). The Swedish cohort (n = 14 616) followed a similar pattern, although the prevalence of SUD was slightly higher (31%, n = 4514) and they were slightly older (SUD group: mean age 34.3, s.d. 7.6, 70.4% men; non-SUD group: mean age 35.2, s.d. 7.4, 58.2% men) than in the Finnish cohort. A flowchart of the study cohorts and groups is shown in Supplementary Figure 1. Persons without an SUD had a slightly higher proportion of their out-patient time spent on antipsychotics (80.3% in the Finnish cohort, 78.7% in the Swedish cohort) than persons with an SUD (75.7% in the Finnish cohort, 73.3% in the Swedish cohort).
Risk of developing an initial SUD
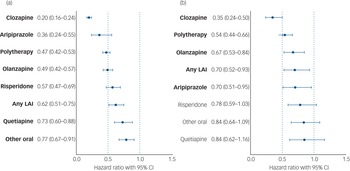
During follow-up (Finnish cohort: median 18.2 years, interquartile range (IQR) 10.0–22.0; Swedish cohort: 9.9 years, IQR 6.7–10.5), 2853 out of 25 603 (11.1%) patients in the Finnish non-SUD cohort and 1043 out of 11 145 (9.4%) patients in the Swedish non-SUD cohort were diagnosed with their first SUD (Table 1), and time to developing an SUD is described in Supplementary Figure 3. Persons who developed an SUD were more likely to be men, younger and have previous suicide attempts than those who did not develop an SUD (Table 1). In the Swedish cohort (where information was available), the proportion on disability pension was similar (57.1% SUD, 58.2% non-SUD). Among persons without SUD, use of clozapine was associated with the lowest risk of developing an initial SUD in both countries (Finland: aHR 0.20, 95% CI 0.16–0.24; Sweden: aHR 0.35, 95% CI 0.24–0.50). In both countries, use of aripiprazole (Finland: aHR 0.36, 95% CI 0.24–0.55; Sweden: aHR 0.70, 95% CI 0.51–0.95), antipsychotic polytherapy (Finland: aHR 0.47, 95% CI 0.42–0.53; Sweden: aHR 0.54, 95% CI 0.44–0.66), olanzapine (Finland: aHR 0.49, 95% CI 0.42–0.57; Sweden: aHR 0.67 (0.53–0.84) and any LAI (Finland: aHR 0.62, 95% CI 0.51–0.75; Sweden: aHR 0.70, 95% CI 0.52–0.93) were also associated with lower risk of developing an initial SUD, whereas risperidone, quetiapine and other oral antipsychotics showed associations with reduced risk in the Finnish, but not the Swedish cohort (Fig. 1). Of all of the specific monotherapies, use of quetiapine was associated with the highest SUD risk. The results were highly consistent between the two countries (Pearson's r = 0.87, P = 0.005; Fig. 2(a)). Clozapine use was also associated with the lowest risk of development of an initial SUD when compared head-to-head with the most common antipsychotic, olanzapine, in the Finnish cohort (aHR 0.30, 95% CI 0.20–0.44) and the Swedish cohort (aHR 0.34, 95% CI 0.12–0.92; Supplementary Fig. 4). In this comparison analysis, clozapine and polytherapy were the only treatments consistently outperforming olanzapine across both countries (Supplementary Fig. 4).

Fig. 1 Risk of first substance use disorder (among those without substance use disorder) associated with use of specific antipsychotics, in a between-individuals model. (a) Finnish cohort. (b) Swedish cohort. Exposures significant after Benjamini–Hochberg false discovery rate correction for multiple comparisons with a 0.05 threshold are bolded. Hazard ratio are adjusted for covariates. LAI, long-acting injectable.

Fig. 2 (a) Correlation of effectiveness of antipsychotic treatments (i.e. of adjusted hazard ratios) in preventing development of an initial substance use disorder in patients with schizophrenia between the Finnish and Swedish cohorts (Pearson's r = 0.87, P = 0.005, N = 8 antipsychotics). The linear regression curve is depicted with a red line and the 95% confidence interval area for the curve is shown in dark grey. x-axis shows adjusted hazard ratios in Finland and y-axis shows adjusted hazard ratios in Sweden. (b) Correlation of effectiveness of antipsychotic treatments (i.e. of adjusted hazard ratios) in reducing risk for psychiatric hospital admission in patients with schizophrenia and comorbid substance use disorder between the Finnish and Swedish cohorts (Pearson's r = 0.84, P = 0.009, N = 8 antipsychotics). The linear regression curve is depicted with a red line and the 95% confidence interval area for the curve is shown in dark grey. x-axis shows adjusted hazard ratios in Finland and y-axis shows adjusted hazard ratios in Sweden.
Table 1 Baseline characteristics of persons without comorbid substance use disorder at the start of follow-up who did or did not develop substance use disorder during follow-up, for both cohorts of patients with schizophrenia

SUD, substance use disorder.
All sensitivity analyses confirmed the main results (Supplementary Figs 5–7). The numbers of events and person-years in each treatment category are shown in Supplementary Table 1.
Risk of psychiatric hospital admission
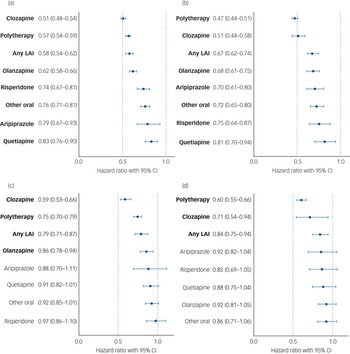
Median follow-up time for hospital admissions in persons with an SUD was 11.6 years (IQR 6.1–17.7) in the Finnish cohort and 8.6 years (IQR 5.5–10.5) in the Swedish cohort; 5948 persons (73.3%) in the Finnish cohort and 2991 persons (66.3%) in the Swedish cohort experienced psychiatric hospital admission at least once. Compared with non-use of antipsychotics (the within-individual model), use of any antipsychotic was associated with a 40% reduction in risk of psychiatric hospital admission in patients with schizophrenia and comorbid SUD (Finland: aHR 0.61, 95% CI 0.58–0.64; Sweden: aHR 0.60, 95% CI 0.56–0.64). For patients with schizophrenia and comorbid SUD, risk of psychiatric hospital admission was lowest during use of clozapine (Finland: aHR 0.51, 95% CI 0.48–0.54; Sweden: aHR 0.51, 95% CI 0.44–0.58), antipsychotic polytherapy (Finland: aHR 0.57, 95% CI 0.54–0.59; Sweden: aHR 0.47, 95% CI 0.44–0.51), any LAI (Finland: aHR 0.58, 95% CI 0.54–062; Sweden: aHR 0.67, 95% CI 0.62–0.74) and olanzapine (Finland: aHR 0.62, 95% CI 0.58–0.66; Sweden: aHR 0.68, 95% CI 0.61–0.75), compared with non-use (Fig. 3(a), 3(b), Supplementary Table 1). Use of any specific antipsychotic treatment was associated with reduced risk of psychiatric hospital admission in both countries, but use of quetiapine was associated with the least reduction in risk (Finland: aHR 0.83, 95% CI 0.76–0.90; Sweden: aHR 0.81, 95% CI 0.70–094). These beneficial associations of reduced risk for psychiatric hospital admission observed for specific antipsychotic treatments in patients with SUD were similar between the two countries (r = 0.84, P = 0.009; Fig. 2(b)).
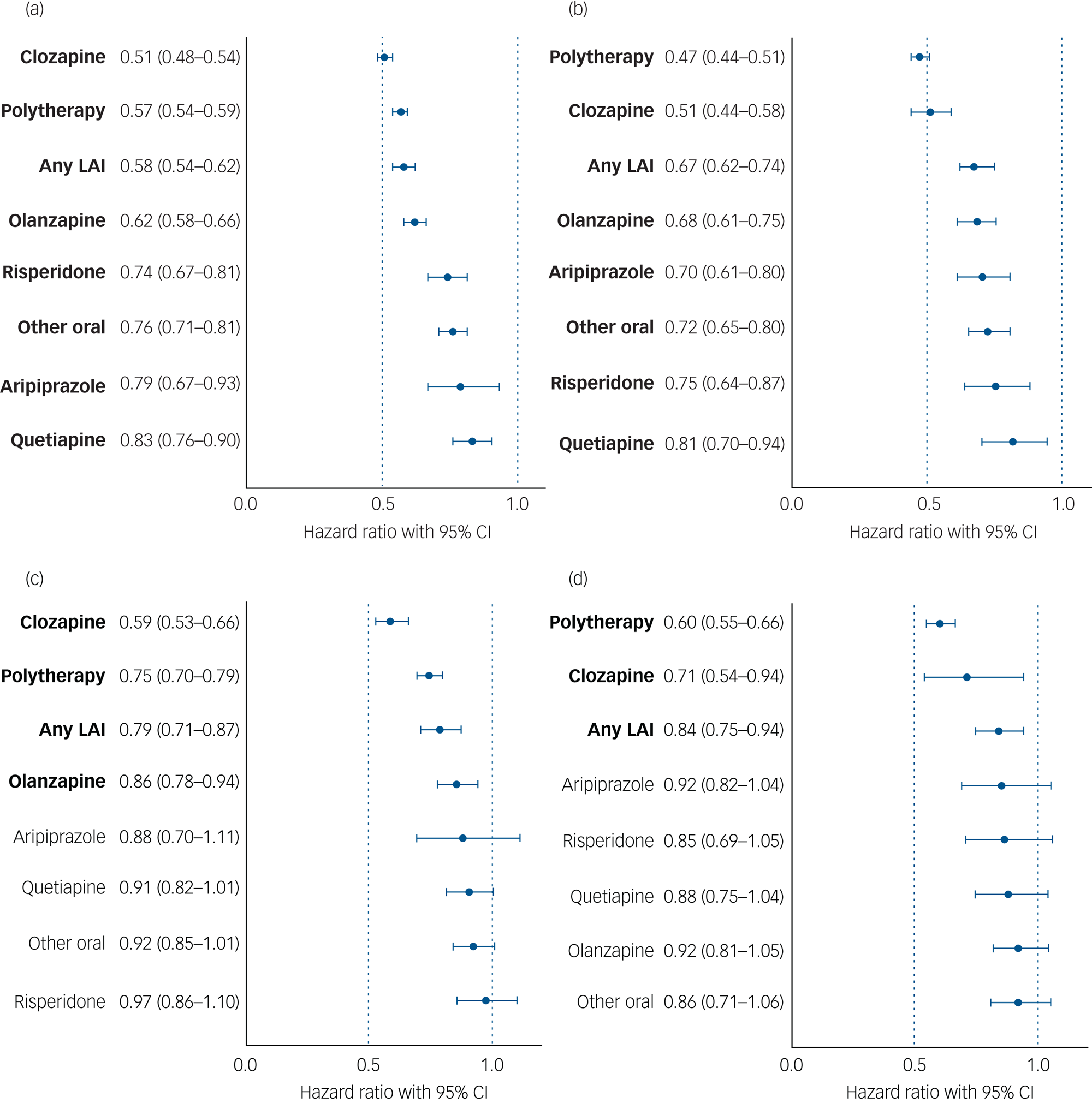
Fig. 3 (a) and (b) Risk of psychiatric hospital admission associated with use of specific antipsychotics among those with comorbid substance use disorder, in a within-individual model. (a) Finnish cohort. (b) Swedish cohort. Exposures significant after Benjamini–Hochberg false discovery rate correction for multiple comparisons with a 0.05 threshold are bolded. LAI, long-acting injectable. (c) and (d) Risk of substance use disorder-related hospital admission associated with antipsychotic use in those with comorbid substance use disorder, in a within-individual model. (c) Finnish cohort. (d) Swedish cohort. Exposures significant after Benjamini–Hochberg false discovery rate correction for multiple comparisons with a 0.05 threshold are bolded. Hazard ratio are adjusted for covariates. LAI, long-acting injectable.
Risk of SUD-related hospital admission
Among persons with an SUD, 3971 people (49.0%) in the Finnish cohort and 2199 people (48.7%) in the Swedish cohort were admitted to hospital because of an SUD during follow-up. In individuals with schizophrenia and comorbid SUD, use of any antipsychotic was associated with a 19% reduction of risk of SUD-related hospital admission in Finland (aHR 0.81, 95% CI 0.76–0.85) and a 21% reduction in Sweden (aHR 0.79, 95% CI 0.73–0.85), compared with no use of antipsychotics. Of specific treatments, use of clozapine (Finland: aHR 0.59, 95% CI 0.53–0.66; Sweden: aHR 0.71, 95% CI 0.54–0.94), antipsychotic polytherapy (Finland: aHR 0.75, 95% CI 0.70–0.79; Sweden: aHR 0.60, 95% CI 0.55–0.66) or LAIs (Finland: aHR 0.79, 95% CI 0.71–0.87; Sweden: aHR 0.84, 95% CI 0.75–0.94) were associated with reduced risk in both countries, whereas olanzapine was only associated with reduced risk in the Finnish cohort (Finland: aHR 0.86, 95% CI 0.78–0.94; Sweden: aHR 0.92, 95% CI 0.82–1.04) (Fig. 3(c), 3(d), Supplementary Table 1). These beneficial associations of reduced risk of SUD-related hospital admission observed for specific antipsychotic treatments in patients with schizophrenia and comorbid SUD were similar between the two countries (r = 0.71, P = 0.049; Supplementary Fig. 8).
Discussion
This real-world study provides, for the first time, consistent evidence from two independent cohorts that clozapine use is associated with lower risk of developing an SUD in patients with schizophrenia, compared with no use or use of other antipsychotics. We also show that in patients with schizophrenia and comorbid SUD, clozapine, antipsychotic polytherapy and LAIs are consistently associated with the lowest risks of psychiatric hospital admission and SUD-related hospital admission. Thus, the results are positive for the treatment of schizophrenia, as antipsychotic treatments have now been consistently shown to also be effective for those with comorbid SUD.
Our findings are in line with a recent meta-analysis showing superior efficacy of clozapine in schizophrenia and comorbid SUD,Reference Krause, Huhn, Schneider-Thoma, Bighelli, Gutsmiedl and Leucht15 and other studies pointing toward clozapine's superiority over other antipsychotics in the treatment of individuals with schizophrenia and comorbid SUD.Reference Lee, Dickson, Campbell, Oliphant, Gretton and Dalby13 For example, clozapine has been shown to reduce the subjective effects of cocaine, although it may increase serum cocaine levels, indicating it may be useful in the treatment of cocaine addiction.Reference Farren, Hameedi, Rosen, Woods, Jatlow and Kosten24 Others have found that patients with schizophrenia are more likely to remit from alcohol use when using versus not using clozapine, and when using clozapine versus risperidone.Reference Green, Burgess, Dawson, Zimmet and Strous25
One possible mechanism explaining our findings relates to the effect some antipsychotics may exert on craving. Although not extensively studied, evidence suggests that clozapine reduces craving, whereas other antipsychotic agents have less of an effect on craving.Reference Krause, Huhn, Schneider-Thoma, Bighelli, Gutsmiedl and Leucht15 Given evidence that high D2-receptor occupancy increases substance use risk,Reference de Haan, Booij, Lavalaye, van Amelsvoort and Linszen26 clozapine's relatively low D2-receptor affinity may reduce cue reactivity and craving.Reference Machielsen, Veltman, van den Brink and de Haan27 Reduced craving may result in less frequent and lower amounts of substance use in patients with schizophrenia who are prone to developing SUDs and in patients with schizophrenia and comorbid SUD, diminishing their odds of mental and physical symptom worsening and thus of readmission to hospital. Alternatively, our findings could be an indication that the self-medication hypothesis of substance use in schizophrenia holds true, at least to some extent: it is possible that patients on antipsychotics may experience less symptom severity, and so be at lower risk of using drugs to relieve symptoms and thus developing an SUD.
Use of clozapine, antipsychotic polytherapy and LAIs were associated with the lowest risks of both SUD-related and psychiatric hospital admissions among persons with schizophrenia and comorbid SUD. The results on polypharmacy are in line with previous results from nationwide cohorts showing a favourable outcome compared with oral monotherapies among persons with schizophrenia in general.Reference Tiihonen, Mittendorfer-Rutz, Majak, Mehtälä, Hoti and Jedenius22,Reference Tiihonen, Taipale, Mehtälä, Vattulainen, Correll and Tanskanen28 Possibly, the additive effects of antipsychotics in those who are prescribed antipsychotic polytherapy increase the beneficial effects of the antipsychotics. Patients with schizophrenia and comorbid SUD have been reported to have lower adherence to antipsychotics than other patients with schizophrenia.Reference Large, Mullin, Gupta, Harris and Nielssen8 Among patients with schizophrenia in general, LAIs have been associated with better adherence and lower risk of hospital admission than their oral counterparts, especially in observational studies.Reference Machielsen, Veltman, van den Brink and de Haan27 However, only a few previous studies examining LAI use among persons with schizophrenia and comorbid SUD exist and this literature is inconsistent (see Supplementary Material).
Strengths and limitations
As an observational study, our results are associations and do not prove causality. However, to corroborate our findings, we used two individually analysed nationwide cohorts from two countries with similar, although not identical, healthcare systems, and stratified our analyses across different calendar time periods. The consistent results across main and sensitivity analyses, as well as across countries and calendar times, lend support to the robustness of our findings and their generalisability to other countries with similar healthcare systems. As the cohorts used were nationwide, the results provided are thus likely to reflect real-world settings. The most significant weaknesses of observational studies are related to confounding by indication. To combat this, the analyses were performed as within-individual analyses (apart from the analyses of developing an SUD, where this was impossible), where an individual is used as their own control, to eliminate bias arising from permanent individual characteristics. Sensitivity analyses were also performed for the between-individual comparisons, and the analyses were corrected for a variety of covariates as well as multiple comparisons. However, the registries used do not contain all of the information used in clinical decision-making, and some residual confounding is bound to remain. For example, we were not able to account for the effects of psychosocial treatment or psychotherapy. Finally, SUDs may sometimes go undiagnosed.
Overall, our study provides consistent evidence across countries that antipsychotic use in patients with schizophrenia is associated with reduced risk of developing an SUD, compared with non-use of antipsychotics. We found that clozapine and antipsychotic polytherapy were most strongly associated with both reduced risk of developing SUDs among patients with schizophrenia and with lower relapse rates in patients with both diagnoses.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1192/bjp.2022.117
Data availability
The data-sets analysed in this study are not publicly available due to participant privacy and security concerns. Researchers can apply for access to these data from the register holders: for Finnish data, the Social Insurance Institution of Finland (Prescription Register), the Finnish National Institute for Health and Welfare (Hospital Discharge Register) and Statistics Finland (Causes of Death Register); for Swedish data, the National Board of Health and Welfare (National Patient Register, Prescribed Drug Register), Statistics Sweden death and sociodemographic data in the LISA Register, and the Swedish Social Insurance Agency (MiDAS Register).
Acknowledgements
We thank Ms Aija Räsänen for secretarial assistance.
Author contributions
All authors conceived the study. H.T. and A.T. performed the statistical analyses. M.L. and J.J.L. wrote the first draft. All authors revised and approved the final draft.
Funding
This study was funded by the Finnish Ministry of Social Affairs and Health through the developmental fund for Niuvanniemi Hospital. H.T. was funded by Academy of Finland (grants 315969 and 320107). M.L. was partly funded by personal grants from the Finnish Medical Foundation and Emil Aaltonen Foundation.
Declaration of interest
J.T., E.M.-R., H.T. and A.T. have participated in research projects funded by grants from Janssen-Cilag and Eli Lilly to their employing institution. H.T. reports personal fees from Janssen-Cilag and Otsuka. J.T. reports personal fees from the Finnish Medicines Agency (Fimea), European Medicines Agency (EMA), Eli Lilly, Janssen-Cilag, Lundbeck and Otsuka; is a member of the advisory board for Lundbeck; and has received grants from the Stanley Foundation and Sigrid Jusélius Foundation. M.L. is a board member of Genomi Solutions and Nursie Health; has received honoraria from Sunovion, Orion Pharma, Otsuka, Janssen-Cilag and Lundbeck; received research funding from The Finnish Medical Foundation and Emil Aaltonen Foundation; and participated in research funded by Janssen-Cilag. A.B. and J.L. declare no competing interests.






eLetters
No eLetters have been published for this article.