Introduction
Quinoa (Chenopodium quinoa Willd.) is a crop that was domesticated in South America 1000 years ago (Repo-Carrasco et al., Reference Repo-Carrasco, Espinoza and Jacobsen2003; Costa Tártara et al., Reference Costa Tártara, Manifesto, Bramardi and Bertero2012). This species is classified into ecotypes (including the altiplano, sea-level and valley types) based on their place of origin (Galwey, Reference Galwey1989; Wilson, Reference Wilson1990; Fuentes et al., Reference Fuentes, Martinez, Hinrichsen, Jellen and Maughan2009). The sea-level type quinoa originated in Southern Chile, and the valley type quinoa originated in the high altitude terrains (2000–3000 m above sea level) of Bolivia (Risi and Galwey, Reference Risi and Galwey1984, Reference Risi and Galwey1989). Several studies have reported differences in the effect of day length on seed formation and seed growth among ecotypes after flowering (Risi and Galwey, Reference Risi and Galwey1991; Bertero et al., Reference Bertero, King and Hall1999; Ujiie et al., Reference Ujiie, Sasagawa, Yamashita, Isobe and Ishii2007; Cheristiansen et al., Reference Cheristiansen, Jacobsen and Jørgensen2010; Hirich et al., Reference Hirich, Choukr-Allah and Jacobsen2014).
Several studies have reported that day length after flowering may affect seed-set, embryo development and seed development in numerous annual crops (Thomas and Raper, Reference Thomas and Raper1976; Cure et al., Reference Cure, Patterson, Raper and Jackson1982; Summerfield et al., Reference Summerfield, Roberts, Ellis and Lawn1991; Linnemann, Reference Linnemann1993; Lobell et al., Reference Lobell, Fambrini, Baraldi, Lercari and Pugliesi2000; Lagercrantz, Reference Lagercrantz2009). For example, in soybean the seed dry weight and seed number increased under a 15 h day length regime (Cure et al., Reference Cure, Patterson, Raper and Jackson1982). The critical photoperiod requirement for seed-filling in the early-maturing cultivars was reported to be longer than that in the late-maturing cultivars (Thomas and Raper, Reference Thomas and Raper1976). The seed-set percentage in sunflowers grown under a 24 h day length regime was very low compared with that of plants grown under a 16 h day length period (Lobell et al., Reference Lobell, Fambrini, Baraldi, Lercari and Pugliesi2000). In bambara groundnut, embryo growth ceased when plants were grown under an 11.5 h day length regime but was allowed to continue when plants were grown under a 14.0 h day length regime after flowering (Linnemann, Reference Linnemann1993).
The seed developmental stages in quinoa are as follows: (1) pollen germination and pollen tube elongation from stigma to ovule; (2) fertilization and embryo formation; and (3) embryo and seed growth. In quinoa, seed growth of sea-level type quinoa was not suppressed under either an 11 or 14 h day length regime after flowering. In contrast, seed growth of the valley type quinoa was suppressed under a 14 h day length regime, but not under an 11 h day length regime after flowering (Ujiie et al., Reference Ujiie, Sasagawa, Yamashita, Isobe and Ishii2007; Isobe et al., Reference Isobe, Sugiyama, Okuda, Murase, Harada, Miyamoto, Koide, Higo and Torigoe2016). However, the reason behind inhibition of seed development under long days after flowering in the valley type quinoa remains unclear. Because the nutritional characteristics of quinoa seeds were reassessed, an increase in the amount of quinoa consumption is foreseen (Armeja et al., Reference Armeja, Tanwar and Chauhan2015). There is good potential for increased production of quinoa in the world. At present, quinoa is not cultivated in Japan. However, we believe that the cultivation of quinoa in Japan may become more important in the future. The objective of this study was to evaluate the effects of day length after flowering on pollen tube elongation, embryo formation and seed growth in two quinoa varieties.
Materials and methods
Cultivation conditions and plots
The quinoa varieties tested in this study were Amarilla de Marangani (valley type) and NL-6 (sea-level type). Ten seeds were sown in 1/5000a pots containing 3 kg field soil (Andosol), and 4.57 g compound fertilizer (N-P2O5-K2O = 14-14-14). The seedlings were thinned to two plants per pot at the expansion stage (plants showing two or three leaves). After sowing, the quinoa plants were placed in growth cabinets (Koito Kogyo; FR-535A-S2). From sowing to maturity, the day temperature was set to 25°C, and the night temperature to 22°C. From sowing to flowering, the plants were exposed to a 15 h day length regime. After flowering, the plants were grown under either a 15 h (15 h plot) or an 11 h day (11 h plot) length regime. At flowering, sepals on each flower were marked with different colours to determine the flowering date.
Assessment of pollen tube elongation in the style
Two days after flowering, 150 flowers (50 flowers × 3 plants) of each plot were collected. After collection, the flowers were fixed in Carnoy's solution (ethanol:chloroform:glacial acetic acid = 6:3:1). Subsequently, the flower samples were soaked in 1N-KOH at 60°C for 10 min, and stained in a 1% aniline blue solution for 24 h. The elongation of pollen tubes in the style was observed under a fluorescence microscope (Leica DM-2500). The ratio of elongated pollen tubes to the number of styles (the number of total elongated pollen tubes the number of observed styles) was calculated for each plot and variety of quinoa.
Assessment of embryo growth
Flowers from each plot were collected at 2, 4, 6, 8 and 14 days after flowering. After collection, the flowers were fixed in FAA solution (50% ethanol:acetic acid:formalin = 18:1:1), and then dehydrated by immersion in an alcohol series ranging from 40% n-butanol, 25% ethanol in water to absolute n-butanol. The sections were then embedded in paraffin. Serial transversal sections (10–20 µm thick) were cut and stained using Mayer's Hematoxylin solution for 20 min. The sections were observed under a light microscope (Olympus BX50-F4).
Seed diameter
At 8 and 14 days after flowering, 30 flowers (50 flowers × 3 plants) from each plot were collected, and seed diameter was measured using a stereoscopic microscope (Olympus SZX12).
Flower number and seed number at maturity
At maturity, 10 plants of each plot were harvested and the number of flowers, and the seeds per plant were enumerated. The seed-set ratio (the number of seeds divided by the number of flowers) was calculated. In this experiment, seeds with a diameter >1.0 mm were considered viable.
Statistical analysis
All values are expressed as means. Significant differences between the 11 h and the 15 h day length plots for each variety were determined by t-test. The data pertaining to pollen tube elongation ratio and seed per flower ratio were statistically analysed after angular transformation. Statistical analysis was performed using Kaleida Graph version 4.1 software; P < 0.05 was considered significant.
Results
Assessment of pollen tube elongation in the style
When a pollen tube was elongated from stigma to the ovule, a fluorescent line was observed in the style (Fig. 1). In NL-6, the ratio of elongated pollen tubes per style was significantly higher under the 11 h than under the 15 h day length (Fig. 2). In Amarilla de Marangani, the ratio of elongated pollen tubes per style was significantly higher under the 11 h compared with 15 h day length (100 and 75%, respectively) regime (Fig. 2).
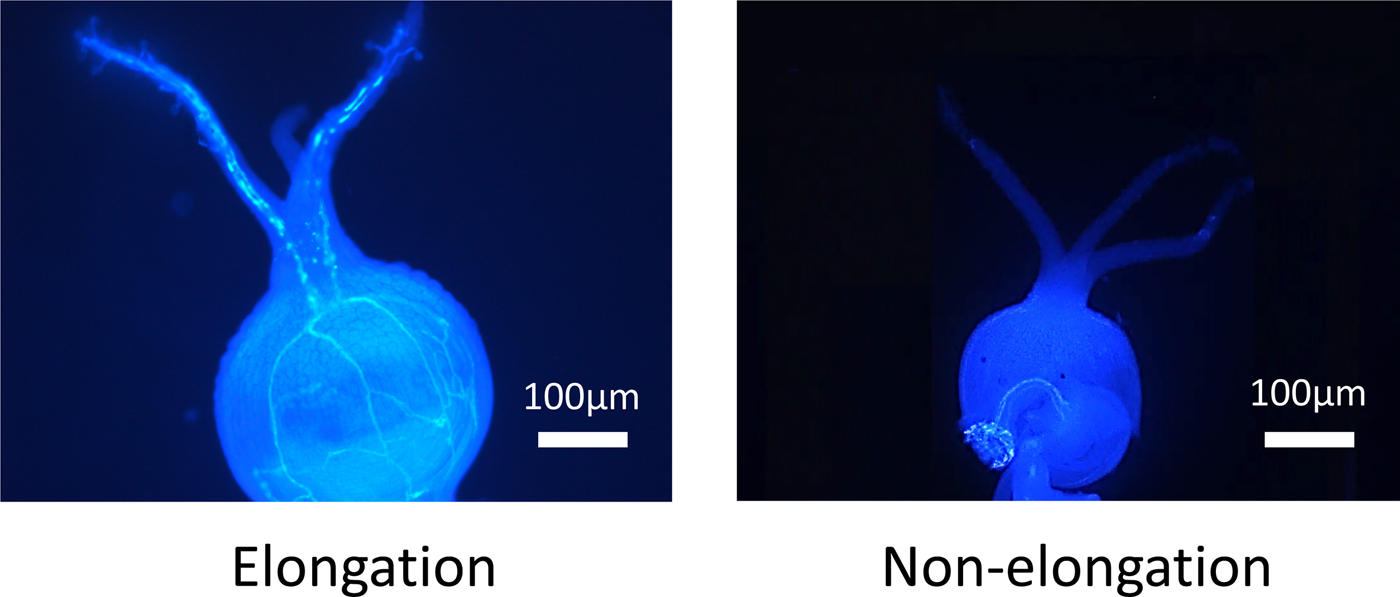
Figure 1. The elongation or non-elongation of pollen tube from stigma to ovule.
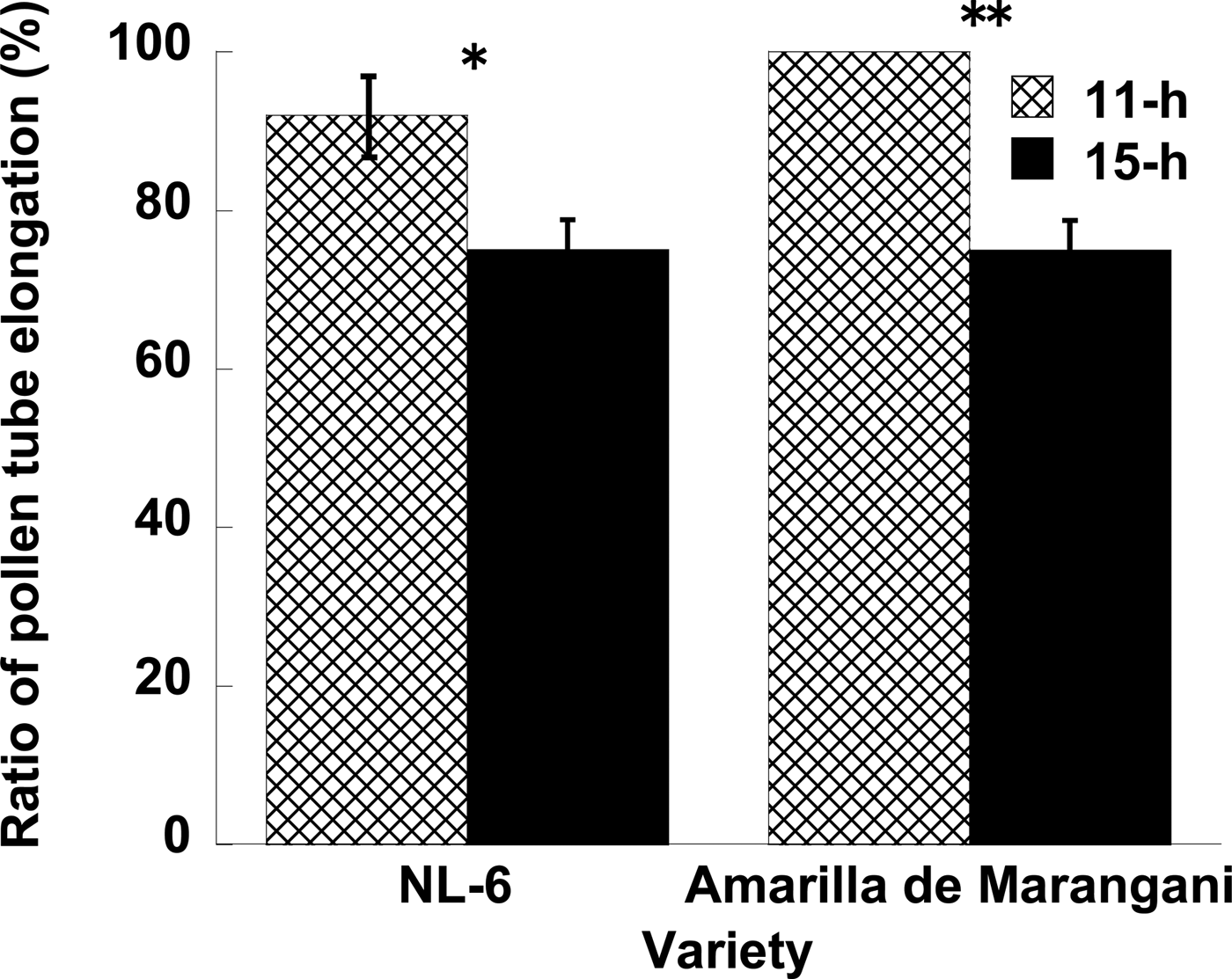
Figure 2. Effects of day length after flowering on the pollen tube elongation. **1% and *5% level of significance between 11 and 15 h by t-test, respectively. Replications = 3.
Embryo growth
NL-6 and Amarilla de Marangani under an 11 h day length regime
At 2 days after flowering, the early embryo was observed in the ovules of both varieties. The globular embryo was observed at 4 days after flowering. At 8 days, the cotyledon and the radicle were observed in the ovules of both varieties. Between 8 and 14 days after flowering, the cotyledon and the radicle elongated, and the endosperm widened in the seeds of both varieties (Fig. 3).
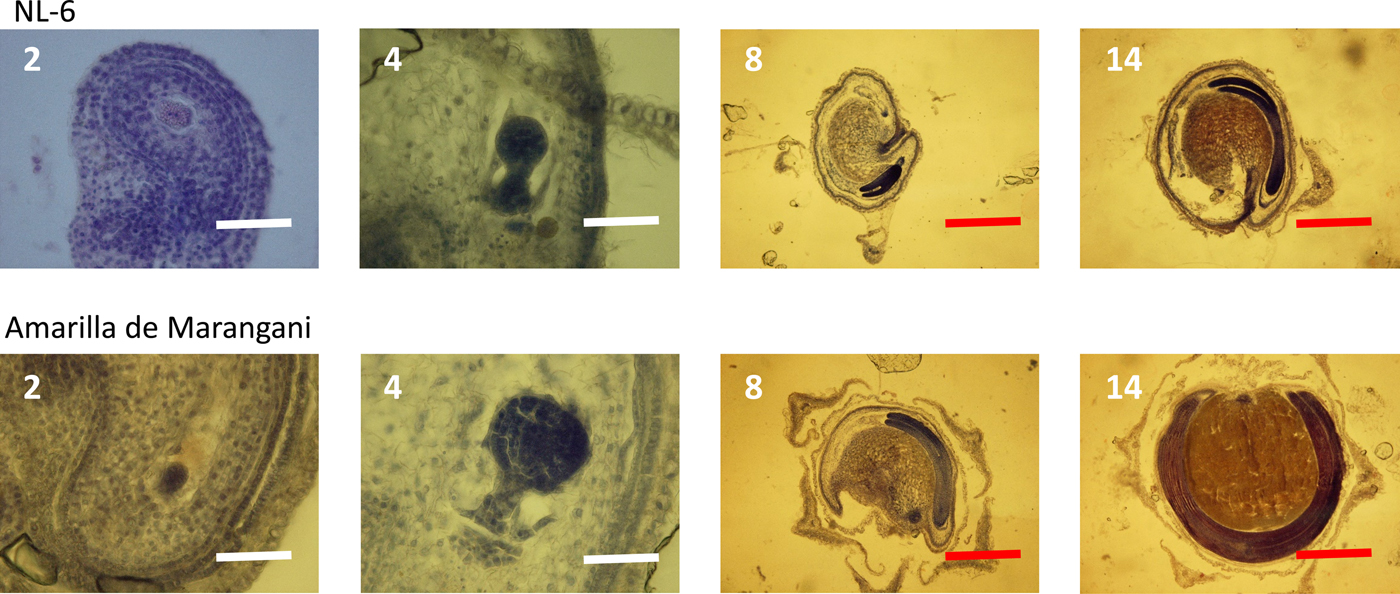
Figure 3. Embryo development under 11 h day length regime after flowering. 2, 4, 8, 14: days after flowering. White scale bar, 100 µm; red scale bar, 1000 µm.
NL-6 and Amarilla de Marangani under a 15 h day length regime
At 2 days after flowering, the early embryo was observed in the ovules of both varieties. At 6 days after flowering, the globular embryo was observed in the ovules of NL-6 but not in the ovules of Amarilla de Marangani. At 8 days after flowering, the heart-shaped embryo was observed in the ovule of NL-6 but not in the ovules of Amarilla de Marangani. At 14 days after flowering, the cotyledon and the radicle were observed and the endosperm widened in the seeds of NL-6 (Fig. 4).
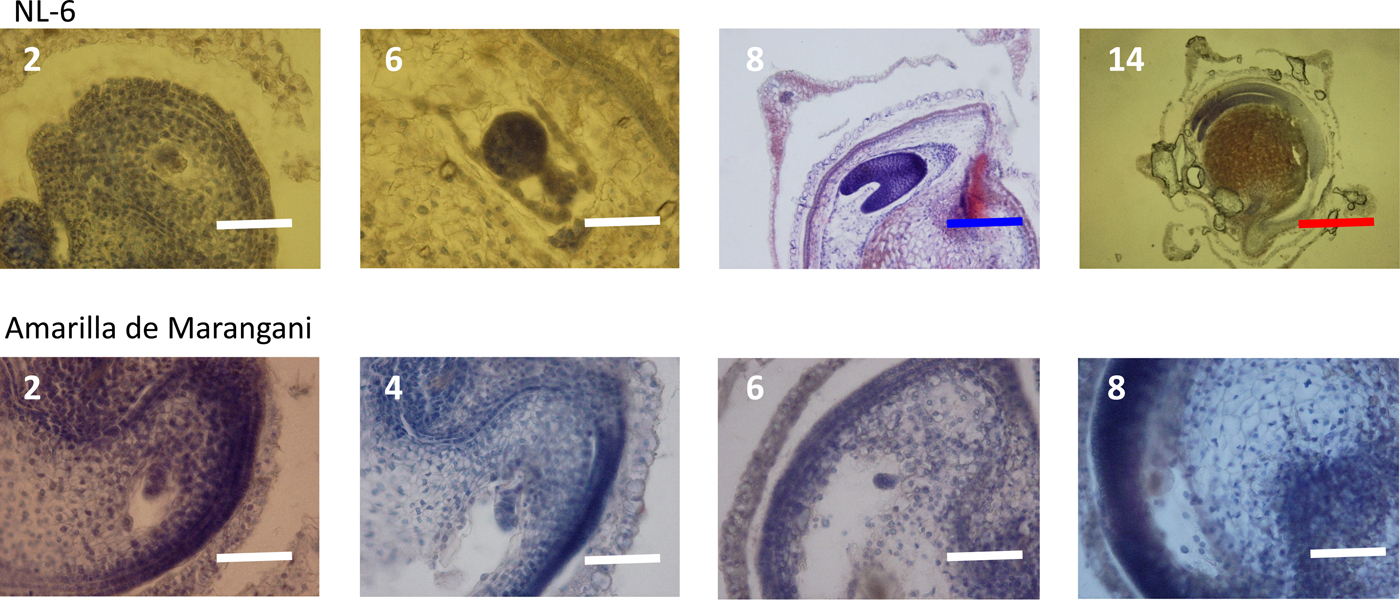
Figure 4. Embryo development under 15 h day length regime after flowering. 2, 4, 6, 8, 14: days after flowering. White scale bar, 100 µm; blue scale bar, 400 µm; red scale bar, 1000 µm.
Seed diameter
NL-6
The seed diameter under the 11 h and the 15 h day length regimes was increased from flowering to 14 days after flowering. Therefore, there were no significant differences in the seed diameter at flowering, and at 8 and 14 days after flowering between the 11 h and the 15 h day length plot (Figs 5 and 6).
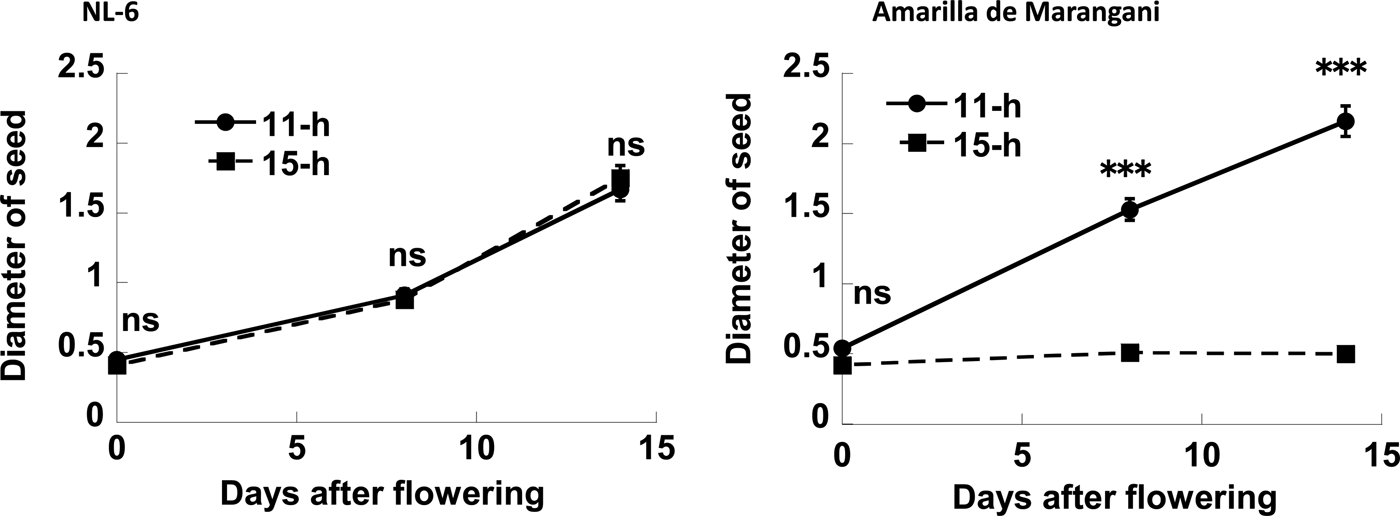
Figure 5. Effects of day length after flowering on the diameter of seed. ns, no significant difference; ***0.1% level of significant difference between 11 and 15 h by t-test, respectively. Replications = 3.
Figure 6. Seed growth at 14 days after flowering. Scale bar, 1 mm.
Amarilla de Marangani
The seed diameter under the 11 h day length regimes was increased from flowering to 14 days after flowering. The seed diameter under the 15 h day length regimes was not increased from flowering to 14 days after flowering. There were no significant differences in seed diameter at flowering between the 11 h and the 15 h plots. However, there were significant differences in seed diameter at 8 and 14 days after flowering between the 11 h and 15 h day length plots (Figs 5 and 6).
Number of flowers and seeds at maturity
NL-6
There were no significant differences in the numbers of flowers and seeds between the 11 h and the 15 h day length regimes. However, the ratio of seeds to flowers was 81.3 and 55.4% under the 11 h and the 15 h day length regimes, respectively, and thus significantly higher under the 11 h day length regime (Table 1).
Table 1. Effects of day length after flowering on flower and seed number
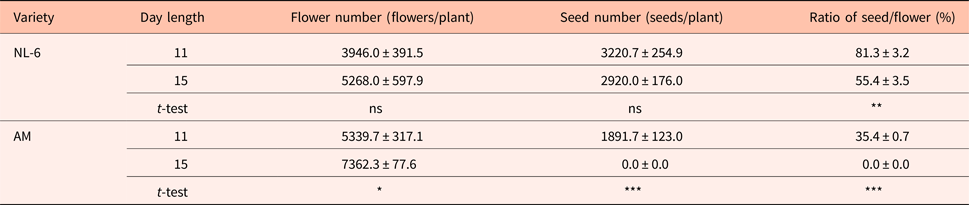
AM, Amarilla de Marangani; ns, no significant difference; ***0.1%, **1% and *5% level of significant difference between 11 and 15 h by t-test, respectively; values are means ± standard error; replications = 10.
Amarilla de Marangani
The number of flowers per plant was significantly higher under the 15 h day length regime (P = 0.05). The number of seeds per plant was 1891.7 and 0.0 under the 11 h and the 15 h day length regimes, respectively, and thus significantly higher under the 11 h day length. The seed to flower ratio was significantly higher under the 11 h day length regime (Table 1).
Discussion
Quinoa (Chenopodium quinoa Willd.) is classified into certain ecotypes (including the altiplano, sea-level and valley types) based on their place of origin (Galwey, Reference Galwey1989; Wilson, Reference Wilson1990; Fuentes et al., Reference Fuentes, Martinez, Hinrichsen, Jellen and Maughan2009). In the valley types, a shorter day length after flowering is required for seed-set and seed growth (Ujiie et al., Reference Ujiie, Sasagawa, Yamashita, Isobe and Ishii2007; Isobe et al., Reference Isobe, Ujiie, Hitomi, Furuya and Ishii2012, Reference Isobe, Sugiyama, Okuda, Murase, Harada, Miyamoto, Koide, Higo and Torigoe2016). This characteristic of the valley type quinoa was one of the most important limitations for the expansion of its cultivated area. In Japan, the longest day length in Tokyo (latitude 35.7°N) in summer, is approximately 14 h 30 min, but in Sapporo (latitude 43.1°N), it is over 15 h. In this study, the elongation of pollen tube and the formation of early embryo in Amarilla de Marangani and NL-6 were not inhibited under the 11 h and the 15 h day length regimes (Figs 2–4). Moreover, embryo growth of NL-6 was not inhibited under the 15 h day length regime, which was not the case for Amarilla de Marangani (Fig. 4). In addition, in Amarilla de Marangani, the seed diameter at 8 and 14 days after flowering under the 11 h day length regime was larger than that of seeds obtained under the 15 h day length regime (Figs 5 and 6). This result is in agreement with previous reports (Ujiie et al., Reference Ujiie, Sasagawa, Yamashita, Isobe and Ishii2007; Isobe et al., Reference Isobe, Ujiie, Hitomi, Furuya and Ishii2012, Reference Isobe, Sugiyama, Okuda, Murase, Harada, Miyamoto, Koide, Higo and Torigoe2016). Thus, the cultivation of Amarilla de Marangani is difficult in northern areas of Japan, for example Sapporo. The decrease in seed number under the 15 h day length regime (Table 1) may be caused by the suspension of embryo growth after fertilization. However, we used only one sea-level type variety (NL-6) and one valley type variety (Amarilla de Marangani) in this experiment. Thus, to find out whether these varieties are representative of sea-level type and valley type quinoa, we should carry out similar experiments with many sea-level and valley type varieties in the future. Moreover, we observed only the elongation of pollen tube and early embryo formation under a 15 h day length regime. Evidently, these results do not guarantee success of fertilization. To prove success of fertilization, the determination of the ploidy of the embryo is necessary in future experiments.
Many studies have reported a change in the concentration of plant hormones or carbohydrates according to day length after the reproductive stage (Dorais et al., Reference Dorais, Yelle and Gosselin1996; Nan et al., Reference Nan, Carman and Salisbury2002; Gibson, Reference Gibson2004). For example, Dorais et al. (Reference Dorais, Yelle and Gosselin1996) studied changes in photosynthetic efficiency and carbon partitioning under extended photoperiods (from 8 to 24 h) in tomato (Lycopersicon esculentum Mill.) and sweet pepper (Capsicum annuum L.). Extending the photoperiod by means of supplemental lighting resulted in an increase in total carbohydrates (CH2O) produced in both species. In tomato, extended photoperiod principally affects shoot development. In contrast, an extended photoperiod did not increase shoot dry weight of sweet pepper plants. Nan et al. (Reference Nan, Carman and Salisbury2002) reported that wheat (Triticum aestivum L.) plants grown in continuous light had higher leaf ABA levels than plants grown using in an 18 or 21 h photoperiod. In quinoa, Bendevis et al. (Reference Bendevis, Sun, Shabala, Rosenqvist, Liu and Jacobsen2014) cultivated two differently adapted cultivars (Achachino and Titicaca) under long day length (17.5 h) and short day length (10.0 h) after the reproductive stages to clarify the effects of day length on the concentration of ABA and soluble sugars. This study suggested that both ABA and soluble sugars were regulators of the photoperiodic response leading to pronounced differences in reproductive developmental patterns between cultivars. The application of Paclobutrazol (GA-synthesis inhibitor), lead to an increase in seed number, from 308,000 to 432,000 seeds per m2 and in seed yield, from 517 to 791 g per m2, in the quinoa cultivar 2-Want (Gomez et al., Reference Gomez, Castro, Mignone and Bertero2011). Therefore, we would hypothesize that plant hormones or carbohydrate balance might affect embryo and seed growth in Amarilla de Marangani under specific day length regimes.
Conclusions
Pollen tube elongation and the formation of an early embryo in Amarilla de Marangani and NL-6 were not inhibited under the 11 h and the 15 h day length conditions after flowering. Embryo development after flowering in NL-6 was not inhibited under the 15 h day length condition. However, in Amarilla de Marangani, embryo development was inhibited under the 15 h day length condition. Thus, the cause of the decrease in seed number under the 15 h day length condition was the suspension of embryo development after fertilization.









