INTRODUCTION
The Tibetan Plateau (TP), located at the junction of East, South, and central Asia, with an average altitude of more than 4000 m above sea level (m asl), is one of the most challenging environments for human beings to live in. Recent archaeological research conducted on the TP has significantly improved our understanding regarding prehistoric human occupation of this high-altitude environment as well as the associated subsistence strategies and interactions with communities populating adjacent areas (Madsen et al., Reference Madsen, Haizhou, Brantingham, Xing, Rhode, Haiying and Olsen2006; Brantingham et al., Reference Brantingham, Gao, Olsen, Ma, Rhode, Zhang and Madsen2007; Aldenderfer, Reference Aldenderfer2011; Chen et al., Reference Chen, Dong, Zhang, Liu, Jia, An, Ma and Xie2015a, Reference Chen, Welker, Shen, Bailey, Bergmann, Davis, Xia, Wang, Fischer, Freidline and Yu2019; d'Alpoim Guedes et al., Reference d'Alpoim Guedes, Lu, Hein and Schmidt2015; Meyer et al., Reference Meyer, Aldenderfer, Wang, Hoffmann, Dahl, Degering, Haas and Schlütz2017; Zhang et al., Reference Zhang, Ha, Wang, Chen, Ge, Long and He2018; Zhang et al., Reference Zhang, Yan, Pan and Jin2019a; d'Alpoim Guedes and Aldenderfer, Reference d'Alpoim Guedes and Aldenderfer2019; Zhang et al., Reference Zhang, Bennett, Cheng, Wang, Zhang, Reynolds and Zhang2021). Although different opinions exist about the factors that enabled people to colonize this cold, harsh environment at such a large scale, research agrees that agriculture and animal husbandry played an essential role (Brantingham et al., Reference Brantingham, Gao, Olsen, Ma, Rhode, Zhang and Madsen2007; Chen et al., Reference Chen, Dong, Zhang, Liu, Jia, An, Ma and Xie2015a; Lu, Reference Lu2016; Zhang et al., Reference Zhang, Dong, Wang, Ren, Qiang and Chen2016). However, although the timing and mechanisms governing the process have been investigated from different perspectives, most conclusions until now have been based on archaeobotanical evidence, architecture, and associated archaeological finds (e.g., d'Alpoim Guedes et al., Reference d'Alpoim Guedes, Lu, Li, Spengler, Wu and Aldenderfer2014; Chen et al., Reference Chen, Dong, Zhang, Liu, Jia, An, Ma and Xie2015a; Liu et al., Reference Liu, Lister, Zhao, Petrie, Zeng, Jones and Staff2017; Tang et al., Reference Tang, Lu, Spengler, Boivin, Song, Wangdue, Chen, Liu and Zhang2021), while archaeofaunal evidence was of secondary importance. In fact, limited zooarchaeological data are available compared with the growing body of archaeological investigations in the TP, with most zooarchaeological research (including synthesis papers) focusing on the northeastern part of the TP (NETP). Conversely, for the southern TP (STP), published zooarchaeological work is limited to faunal reports dealing with single sites (e.g., Huang and Leng, Reference Huang and Leng1985; Zhou, Reference Zhou1999; Li, Reference Li2007; Zhang et al., Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b). This is unsatisfactory, in that the TP represents one of the largest pastoralist ecosystems in the world, and investigations into its formation using a zooarchaeological approach promise novel insights into the cultural and biological processes that have played crucial roles in shaping the region economically and socioculturally.
Until now, the lack of systematic zooarchaeological research in the STP impeded our understanding of animal exploitation patterns and hence human–environment dynamics in this region. Across the globe, the introduction of domestic crops and livestock resulted in major changes in human subsistence practices, land use and vegetation cover, human mobility, and population growth (Boyle et al., Reference Boyle, Levine and Renfrew2002; Fuller, Reference Fuller2006; Hunt et al., Reference Hunt, Campana, Lawes, PARK, Bower, Howe and Jones2011; Jones et al., Reference Jones, Hunt, Lightfoot, Lister, Liu and Motuzaite-Matuzeviciute2011; Boivin et al., Reference Boivin, Fuller and Crowther2012; Liu et al., Reference Liu, Jones, Matuzeviciute, Hunt, Lister, An, Przelomska, Kneale, Zhao and Jones2019a), phenomena that cannot be properly understood without studying bioarchaeological remains. In the course of the Holocene, animals domesticated in various parts of Eurasia, including pigs, sheep, goats, cattle, and horses, spread widely to new environments unlike those where domestic forms initially prospered (Meadow, Reference Meadow and Harris1996; Peters et al., Reference Peters, Helmer, Driesch and Saña Segui1999, Reference Peters, Dreisch, Helmer, Vigne, Peters and Helmer2005; Levine, Reference Levine, Mills and McDonnell2005; Flad et al., Reference Flad, Yuan and Li2007; Colledge et al., Reference Colledge, Conolly, Dobney, Manning and Shennan2013; Vigne, Reference Vigne2015; Librado et al., Reference Librado, Khan, Fages, Kusliy, Suchan, Tonasso-Calvière and Schiavinato2021), including the extreme harsh environments characterizing the STP (Miao et al., Reference Miao, Wang and Li2017; Hu et al., Reference Hu, Yang, Xie, Lv, Cao, Li and Liu2019; Liu et al., Reference Liu, Zhang, Li, Pan, Wang, Chen, Zheng, He, Zhao, Pu and Guan2019b; Wu et al., Reference Wu, Yang, Wang, Dong, Yan, Hao and Fan2020). Originating in southwestern Asia, economically productive herds of sheep, goat, and cattle dispersed across Asia and towards Europe starting some 10,000 years ago (Chessa et al., Reference Chessa, Pereira, Arnaud, Amorim, Goyache, Mainland and Kao2009; Peters et al., Reference Peters, Arbuckle, Pöllath, Özdoğan, Başgelen, Kuniholm and Galatasaray2014; Cai et al., Reference Cai, Sun, Tang, Hu, Li, Zhao, Xiang and Zhou2014, Reference Cai, Zhang, Shao, Sun, Zhu and Yang2018a; Lv et al., Reference Lv, Peng, Yang, Zhao, Li, Liu, Ma, Zhao, Yang, Wang and Li2015; Wang, Reference Wang2017; Hermes et al., Reference Hermes, Tishkin, Kosintsev, Stepanova, Krause-Kyora and Makarewicz2020; Yu, Reference Yu2020; Wilkin et al., Reference Wilkin, Miller, Taylor, Miller, Hagan, Bleasdale and Scott2020), reaching the TP in the mid-Holocene and becoming a mainstay of the local pastoral economy essential for human survival (Yang and Zheng, Reference Yang and Zheng2001). Previous archaeological and linguistic research has already revealed the key role played by the northern Eurasian steppe and the Proto-Indo-European language communities relative to the dispersal of pastoralist lifeways across Asia (Levine, Reference Levine, Mills and McDonnell2005; Frachetti, Reference Frachetti2012; Hermes et al., Reference Hermes, Frachetti, Doumani Dupuy, Mar'yashev, Nebel and Makarewicz2019; Wilkin et al., Reference Wilkin, Miller, Taylor, Miller, Hagan, Bleasdale and Scott2020). However, important questions regarding the adoption of livestock in the unique ecosystem of the STP need yet to be satisfactorily answered. Arguably, detailed zooarchaeological analyses and integration of the faunal spectra into the broader archaeological and palaeoclimatic picture available for the STP, and more generally East Asia, are essential.
One major limiting factor explaining the stagnation in zooarchaeological research addressing relevant questions is the poor foundation for comparative osteological research in a study area characterized by a highly diverse herbivorous mammalian fauna with many wild medium-sized ruminant taxa. Apart from several species of the family Cervidae, the study area witnesses the presence of two members of the subfamily Antilopinae, that is, Tibetan gazelle (Procapra picticaudata) and Tibetan antelope (Pantholops hodgsoni), as well as seven species of the subfamily Caprinae, that is, serow (Capricornis milneedwardsii), goral (Naemorhedus goral), blue sheep (Pseudois nayaur), Himalayan tahr (Hemitragus jemlahicus), argali (Ovis ammon), domestic sheep (Ovis aries), and domestic goat (Capra hircus) (Huang and Leng, Reference Huang and Leng1985; Feng et al., Reference Feng, Cai and Zheng1986; IUCN/SSC, 1997; Wang, Reference Wang2017; Zhang et al., Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b). These species overlap widely in size and exhibit a broadly similar osteomorphology, complicating classification of heavily fragmented archaeological specimens to the level of the genus, let alone to that of the species. Taxonomic identification of medium-sized bovids from this region poses a significant challenge, but progress has recently been made (Wang, Reference Wang2017; Wang et al., Reference Wang, Peters and Barker2020b).
In this study, with the aid of the rich comparative osteological collections of Tibetan fauna in the Institute of Zoology, Chinese Academy of Sciences (IZCAS), and the Institute of Tibetan Plateau, Chinese Academy of Sciences (ITPCAS), published reference works, osteological criteria published in Wang (Reference Wang2017) and Wang et al. (Reference Wang, Peters and Barker2020b), and additional novel features, we conducted taxonomic classification of medium-sized bovid remains recently excavated in Middle–Late Holocene assemblages in the STP and secured the ages of key domestic specimens through direct radiocarbon dating. The majority of the faunal materials come from archaeological sites situated on the riverbanks of the Yarlung Tsangpo, which flows some 1200 km east through the South Tibet Valley and likely served as a major corridor for the dispersal of goods, agricultural practices, and pastoralism. Combining our observations with the published zooarchaeological, archaeobotanical, and palaeoclimatic records, as well as radiocarbon dates from sites in the STP, our study addresses (1) the spatiotemporal developments of human subsistence practices in consecutive phases of occupation, (2) the origins and route(s) by which livestock populations were introduced, and (3) the natural and cultural circumstances triggering highland pastoralism in the region. In brief, our work deals with the adaptation of certain mammalian groups into high-altitude environments and the long-term trajectory of such nonlinear evolution of a very unique lifeway (so-called human–animal–environment dynamics) in the TP and provides new insights on methodological developments how to tackle such problems.
STUDY AREA
With an average altitude above 4000 m asl, the STP occupies the entire Tibetan Autonomous Region. Being located between 26°52′N and 36°32′N and 78°24′E and 99°06′E, it covers an area of more than 1,200,000 km2 (Guge, Reference Guge2013). Imposing mountain ranges—the Kunlun Mountains and Tangula Mountains in the north, the Henduan Mountains to the east, and the Himalayas to the south and west frame this elevated area. To the east and southeast, the plateau gives way to the forested gorge and ridge geography of mountainous headwaters. In the west, the rugged Karakoram range of the northern Kashmir embraces the plateau, and the Kailash Mountains give rise to, for example, the Indus (Sengge Zangbo in Tibet), Sutlej, and Yarlung Tsangpo Rivers. Together with their tributaries, these riverine landscapes offered suitable opportunities for extensive settlement and agriculture, and each of them housed important civilisations in former times, for example, the Indus civilisation (2600–1300 BC), the Shangshung civilization (ca. 500 BC–AD 625) (Aldenderfer, Reference Aldenderfer, Heller and Orofino2007), and the Tubo Kingdom (AD 618–AD 842).
Currently, the TP is characterized by a typically cold and dry alpine climate (Ding et al., Reference Ding, Yang, Zhao, Liu, Wang, Yao and Peng2018; Wang et al., Reference Wang, Pang and Yang2018). Being influenced by both the westerlies and the Asian monsoon, it in turn modifies the climate of neighbouring as well as more remote regions (Zhou et al., Reference Zhou, Zhao, Chen, Chen and Li2009). Because the surface topography of the plateau slopes from the northwest (average altitude > 5000 m in the Changtang region) to the southeast (average altitude of 3000 m in the Nyingchi region), precipitation and temperature show clear gradients. In northwest Tibet, the mean annual temperature is below 0°C, amounting to above 18°C in southeast Tibet. Regarding precipitation, an average of less than 30 mm characterizes the northwestern TP, amounting to 4495 mm in the southeastern TP (SETP) at the lower reaches of the Yarlung Tsangpo River. The vegetation distribution captures the overall gradient from subalpine forests in the southeast margin to alpine meadow and scrub, alpine/temperate steppes, and alpine/temperate deserts in the northwest TP (Hou, Reference Hou2001).
Most suitable for human inhabitation and agriculture from a climatic perspective is the SETP, where archaeological sites we examined located around 3000 m asl face comparatively warm and humid climates in conifer forest vegetation. A diverse range of crops (wheat, barley, naked barley, pea) and fauna (deer, pigs, wild boar, monkey, hare, fish, etc.) were exploited by humans in recent historic and prehistoric times (Feng et al., Reference Feng, Cai and Zheng1986; Wang et al., Reference Wang, Gao, Yang, Tan, Shargan, Zhang and Yang2021b). In the central part of the STP (CSTP), where most sites examined in this study are found between 3500 and 4000 m asl, the climate is cooler and drier, and the typical vegetation type is alpine steppe. Because of irrigation practices made possible by the Yarlung Tsangpo, it is one of the most productive areas for agriculture and pastoralism in Tibet. Currently, this region is characterised by wheat and barley cultivation and sheep and yak pastoralism (Guge, Reference Guge2013). In the Ali region of southwest Tibet (SWTP), where most sites are located above 4000 m asl, the climate is even cooler and drier, and the typical vegetation is alpine steppe. Here pastoralism dominates human subsistence, with limited agriculture. Because of its high elevation, solar radiation of the TP is strong and the air very thin (Yang and Zheng, Reference Yang and Zheng2001; Guge, Reference Guge2013).
The STP witnessed comparably humid conditions from the Early Holocene to the Middle Holocene (9.6–4.2 ka). After 4.2 ka, however, the climate generally turned more arid, and from ca. 3.0 ka until today, aridity increased and the climate became more variable (Nishimura et al., Reference Nishimura, Matsunaka, Morita, Watanabe, Nakamura, Zhu and Nara2014; Bird et al., Reference Bird, Polisar, Lei, Thompson, Yao, Finney, Bain, Pompeani and Steinman2014; Leipe et al., Reference Leipe, Demske, Tarasov and Members2014). The changes in the reconstructed palaeovegetation distribution correspond with climatic developments at the global scale and in the East Asian monsoon. In the mid-Holocene, the climatically most favourable and most suitable period for human inhabitation, subtropical vegetation, including forests, expanded northward. This process was reversed in the Late Holocene, with alpine meadow and steppe vegetation expanding southward, in line with the relatively cool and dry conditions after the climatic optimum (Qin et al., Reference Qin, Zhao and Cao2021; Li et al., Reference Li, Wang, Herzschuh, Cao, Ni and Zhao2022). It is reasonable to assume that these changes had a lasting impact on the livelihoods of early inhabitants and their livestock and crops.
MATERIALS AND METHODS
To elucidate human presence as well as early agricultural and pastoralist practices in the study area, systematic archaeological investigations were conducted between 2018 and 2019 in the frame of the Second Tibetan Plateau Scientific Expedition and Research Program (STEP) along the alluvial terraces of the Yarlung Tsangpo River and its tributaries. For sites yielding Neolithic material culture including pottery sherds, stone tools, charcoals, and animal bones, the archaeological layers as well as specific structures, such as ash pits, were sampled systematically, and the sediment was screened using the manual bucket flotation technique. The location of the sites is shown in Figure 1. All the sites reflect human occupation, although tomb and stone structures were also identified in two of the sites (Khog Gzung and Kha lding). The elevation of the sites ranges from 2800 m in the SETP to 4050 m in the SWTP.

Figure 1. Locations of the five studied sites. The numbers of the sites correspond to those in Fig. 3.
Most of the faunal remains considered in this study were hand-collected from the occupation layers. In addition, small amounts were retrieved using sieves with a mesh of 0.45 mm, through which carbonized plant and other remains were also obtained. Carbonized plant seeds were identified in the Archaeobotanical Laboratory of the ITPCAS, with selected specimens being directly radiocarbon dated.
Based on the results of the investigations in 2018, the site of Klu lding was found to be of particular interest, and a small-scale excavation was carried out in the winter of 2019. The site is located on the second terrace of the Nyang River, where it discharges into the Yarlung Tsangpo River. The terrace is composed of lacustrine sediments with horizontal stratigraphy, where the archaeological materials were discovered. Aeolian accumulation likely took place following the onset of the Holocene. At present, the site is located under the agricultural land of the village. The total area of the settlement is estimated to be ca. 20,000 m2. Excavation was restricted to three grids totalling 54 m2: two 5 × 5 m grids and one 2 × 2 m grid (Fig. 2a–d). Faunal remains were retrieved systematically from the subsequent occupation layers by hand and through 3 mm dry sieving of selected sediment samples.

Figure 2. Archaeological contexts of the sites in this study. Dating materials collected from features with an asterisk (*). (a) Plan view of Klu lding T2 and T3; (b) Klu lding-T2 western profile; (c) Klu lding-T3 eastern profile; (d) 18-Klu lding-P3; (e) Khog Gzung-P4; (f) Khog Gzung-P2; (g) Bos mandible from Kyamo-P1; (h) Kyamo -P1; (i) Kha lding-P1.
The Kha lding site is located on the terrace of the diluvial fan on the northern bank of the Bode Tsangpo. In the valley lowlands, wheat fields are found, and smooth-pit peach or Tibetan peach (Prunus mira) grows as well as drought-tolerant herbs such as Artemisia. James barberry (Berberis jamesiana) grows at lower altitudes in the mountains, while at higher altitudes the landscape is dominated by the drought- and high altitude–tolerant Quercus aquifolioides. The site's expanse has not been recorded.
The Khog Gzung site is located on the northern bank of the Yarlung Tsangpo River, at the front of a north-south alluvial-diluvial fan called Khog Gzung. The site was estimated to cover some 20,000 m2. From the vertical profiles of the gullies crossing the fan, it can be seen that the alluvial gravel layer is covered with an aeolian sediment layer (sandy loess layer) measuring 70 to 160 cm thick. The ash pits reported here all come from this layer, which is about 15 cm below the surface (Fig. 2e and f).
The Kyamo site is located on a secondary river terrace on the northern bank of a tributary flowing into the Gyelung Zangpo River. Prominent geomorphic features are a diluvial fan, the river terrace, and rocks. The newly discovered profile revealed a cultural layer rich in animal bones, charcoal debris, and a small amount of stone artefacts, located ca. 1.5 m above the ground surface (Fig. 2g and h) and extending nearly 8.7 m in length. Conceivably, we are dealing with an accumulation resulting from human activities, instead of the product of transportation of the river. At present, an estimation for the size of the site is missing.
Apart from the archaeofaunas retrieved during the STEP, the first author also had the opportunity to study in detail the remains of medium-sized bovids excavated from the site of Xiaoenda in 2012 and first reported by Zhengwei Zhang, who analysed the entire faunal assemblage excavated in an area of 170 m2 (Zhang et al., Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b). Xiaoenda is characterized by rectangular semi-subterranean household features; its material culture contained ceramics, bone tools, and lithics showing close resemblance to those from the Karuo site. Dated 5600 to 2900 cal yr BP, Karuo is a Neolithic settlement situated next to Xiaoenda and one of the earliest reported and best-studied Neolithic sites in the Tibetan Autonomous Region (BCRTAR and DHSU, 1985; d'Alpoim Guedes et al., Reference d'Alpoim Guedes, Lu, Li, Spengler, Wu and Aldenderfer2014). At Xiaoenda, collecting faunal remains combined hand-picking with systematic sieving of all sediments using 5 and 3 mm meshes. From a total of 7314 mainly mammalian specimens, 730 could be identified to the order level or below in the original faunal analysis (Zhang et al., Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b). The landscape of Xiaoenda exhibits contrasting vertical diversity in vegetation. Starting around 3400 m asl, scrub and meadow grasslands give way to alpine temperate coniferous forests.
The faunal assemblages retrieved by the STEP team were analysed at the Zooarchaeological Laboratory of the Institute of Tibetan Plateau (ITP). Focus has been on mammalian remains, which were identified with the aid of modern and ancient mammalian osteological collections housed at the IZCAS and ITPCAS. Identification manuals consulted by us include Olsen (Reference Olsen1964), Schmid (Reference Schmid1972), Chen (Reference Chen1995), and Hillson (Reference Hillson2005, Reference Hillson2016). Basic analysis comprised anatomical (body part) and taxonomic classification as well as the study of taphonomic markers (weathering, gnawing, burning, etc.) and traces of hunting and butchery indicative of past human behaviour.
Particular attention was paid to the study of dental and osseous remains of medium-sized bovids, which include nine species of Antilopinae and Caprinae that overlap in size and exhibit similar osteomorphologies. Being highly relevant in zooarchaeological research worldwide, diagnostic osteomorphological and osteometric criteria to distinguish sheep and goats have witnessed intense study since the 1950s (Gromova, Reference Gromova1953; Boessneck et al., Reference Boessneck, Müller and Teichert1964; Schramm, Reference Schramm1967; Boessneck, Reference Boessneck, Brothwell and Higgs1969; Payne, Reference Payne, Ucko and Dimbleby1969, Reference Payne1985; Kratochvil, Reference Kratochvil1969; Prummel and Frisch, Reference Prummel and Frisch1986; Clutton-Brock et al., Reference Clutton-Brock, Dennis-Bryan, Armitage and Jewell1990; Davis, Reference Davis1996, Reference Davis2000; Rowley-Conwy, Reference Rowley-Conwy1998; Helmer, Reference Helmer2000; Halstead et al., Reference Halstead, Collins and Isaakidou2002; Zeder and Lapham, Reference Zeder and Lapham2010; Zeder and Pilaar, Reference Zeder and Pilaar2010; Gillis et al., Reference Gillis, Chaix and Vigne2011; Gudea and Stan, Reference Gudea and Stan2011, Reference Gudea and Stan2012; Salvagno and Albarella, Reference Salvagno and Albarella2017; Zedda et al., Reference Zedda, Palombo, Brits, Carcupino, Sathé, Cacchioli and Farina2017). Conversely, diagnostic morphological features enabling identification of the other aforementioned taxa populating the TP and its piedmonts received less attention (Götze, Reference Götze1998; Tong et al., Reference Tong, Zhang, Li and Xu2008; Wang, Reference Wang2017; Wang et al., Reference Wang, Peters and Barker2020b). Not only the lack of criteria for accurately distinguishing between the relevant taxa mentioned earlier, but also the fact that modern comparative specimens of the genera Capricornis, Naemorhedus, Pseudois, or Hemitragus are quite rare in osteological reference collections worldwide, complicate establishing reliable osteomorphological keys. Recently, biomolecular techniques, including ancient DNA (aDNA) analysis and ZooMS, have proved helpful tools for taxonomic classification of archaeological specimens, but few institutions have access to such equipment. Moreover, sampling is destructive and analysis more expensive than morphological examination of bones, which can be done on the spot (Buckley et al., Reference Buckley, Whitcher Kansa, Howard, Campbell, Thomas-Oates and Collins2010; Rizzi et al., Reference Rizzi, Lari, Gigli, De Bellis and Caramelli2012; Dodson et al., Reference Dodson, Dodson, Banati, Li, Atahan, Hu, Middleton, Zhou and Nan2014).
Thus, starting points in our study were detailed morphological and morphometric analyses of the aforementioned medium-sized bovids. As a first step, the remains were sorted based on absolute size and morphological criteria described in the literature (Boessneck et al., Reference Boessneck, Müller and Teichert1964; Boessneck, Reference Boessneck, Brothwell and Higgs1969; Prummel and Frisch, Reference Prummel and Frisch1986; Helmer and Rocheteau, Reference Helmer and Rocheteau1994; Götze, Reference Götze1998; Halstead et al., Reference Halstead, Collins and Isaakidou2002; Hillson, Reference Hillson2005; Zeder and Lapham, Reference Zeder and Lapham2010; Zeder and Pilaar, Reference Zeder and Pilaar2010; Wang et al., Reference Wang, Peters and Barker2020b). Additional morphological features and morphometric data for the relevant species accumulated during their study of osteological reference collections in Europe, the United States, and ChinaFootnote 1 by Y Wang and JP (see Supplementary Material E) were helpful for specific identification. Second, where applicable, identification of specimens using morphometrics was performed. To this extent, measurements defined by von den Driesch (Reference Von Den Driesch1976) and complemented by additional ones defined by Y Wang and JP, were collected, and the data were evaluated by applying discriminant function analysis following the approach outlined in Wang (Reference Wang2017). This method obtains morphometric information on commonly preserved elements (i.e., distal humeri, distal metacarpals, distal metatarsals, etc.) and allowed quantitative analysis to sort out and identify several morphologically similar taxa, such as Ovis, Pseudois, Naemorhedus, and Capra. Osteometric data are provided in Supplementary Material A. Thus, taxonomic identification was achieved by merging multiple lines of evidence generated with the aid of modern reference specimens. In sum, combining the existing identification criteria and our novel osteomorphological and morphometric approaches, our analyses allow a systematic evaluation of the whole bulk of the skeletal elements of mammalian bodies preserved in archaeological sites, and specimens of domestic sheep can be clearly separated from medium-sized bovids of similar morphology, which is crucial for understanding sheep raising and domestication in this area.
Securing the age of the specimens under study combines radiocarbon dates from several sites. Radiocarbon dates were obtained on several key specimens from each of the sites. Eight dates from Xiaoenda published by Zhang et al. (Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b) showed that the site was occupied between 4900 and 4200 cal yr BP. The dated samples originate from distinct stratigraphic levels, likely representing the entire time range of site occupation. To these we can add two 14C dates each from the sites of Klu lding and Kha lding published by Wang et al. (Reference Wang, Gao, Yang, Tan, Shargan, Zhang and Yang2021b). Finally, in this study, we present the dates obtained for selected crop and livestock remains from each of the four sites investigated by the STEP team. Accelerator mass spectrometry (AMS) dating was conducted by Beta-Analytic. The 14C dates were calibrated using OxCal 4.3.2 (Ramsey, Reference Ramsey2017) and the IntCal 13 curve (Reimer et al., Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey and Buck2013), with ranges expressed at 2σ (95.4%) confidence level.
RESULTS
Stratigraphy, radiocarbon dates, and time frame for the occupation of the sites under study
Figure 2 offers insight into the stratigraphy of the four sites investigated by the STEP team. The top row presents relevant information for the excavations at Klu lding. Figure 2a offers a top view of the main excavation area. Excavated in 2019, Trenches 2 (T2) and 3 (T3) are separated by 1 m. Their stratigraphic sequences are depicted in Figure 2b and c. The blue parts that constitute much of layer ④ represent ditch G1 cutting through T2 and T3. Quite rich archaeological remains were recovered from G1. The layers below G1—k1, k3, ④g, and ④h—contain only pure sandy soils without archaeological remains. Recorded during the 2018 survey, profile 3 (18-P3) is situated immediately south of the eastern half of the southern profile of T2 (Fig. 2d). Not depicted in Figure 2 is Trench 1, which is located about 15 m north of T2 and T3. The bottom row offers insight into the stratigraphy of the three other sites of interest, more precisely of Khog Gzung-P4 (Fig. 2e), Khog Gzung-P2 (Fig. 2f), Kyamo-P1 (Fig. 2g and h), Kha lding-P1 (Fig. 2i). These profiles were exposed by modern earth moving. An ash pit (H1*) was identified in both Khog Gzung-P4 and Khog Gzung-P2. Kyamo and Kha lding both show layered stratigraphies.
Table 1 lists the published and new 14C dates of the plant and animal remains from the five sites under study. Information on the stratigraphic position of the radiocarbon-dated bone specimens from Xiaoenda can be found in Zhang et al. (Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b). Regarding Klu lding, 14C-dated samples include a pig molar (Beta592299), a sheep premolar (Beta593674), and a piece of charcoal (Beta513039) collected from the deeper stratigraphic section of Trench 3 (G1b*), Trench 2 (G1a*), and from Profile 3 (H1*), respectively. The H1 in P3 is likely to be of the same unit as G1 in T2. Within the stratigraphic sequence, the position of the pig specimen (Fig. 2c) is below that of the sheep. Another dated specimen is a nutshell (Beta513032) collected from layer P1② during the 2018 survey (Wang et al., Reference Wang, Gao, Yang, Tan, Shargan, Zhang and Yang2021b), which is on the opposite side of the southern profile of T1.
Table 1. The 14C dating results of the plant and animal remains from Xiaoenda, Klu lding, Khog Gzung, Kha lding, and Kyamo.

For Klu lding, all results point to a mid-fourth millennium BP occupation. In the excavated parts, the 14C results of the samples match their relative positions in the stratigraphy. In other words, the 2σ range obtained for the pig tooth (3569–3411 cal yr BP) corresponds to earlier occupation than that measured for the sheep tooth (3456–3364 cal yr BP), suggesting that the specimens remained in stratigraphic position after site abandonment. The 2σ range obtained with the charcoal specimen from the same layer is larger (3610–3458 cal yr BP) and could possibly be indicative of site occupation before 3.5 ka, provided we are not dealing with “old” wood (Schiffer, Reference Schiffer1986). With a 2σ range of 3544–3367 cal yr BP, the dating of the nutshell overlaps well with the ranges of the animal bones, suggesting that the sampled areas may have been occupied broadly contemporaneously. Based on the available 14C dates and considering the extent of the deposits separating the location of the samples from bedrock, it can be postulated that Klu lding was most likely occupied in the mid-fourth millennium BP.
At Khog Gzung, the faunal remains submitted to radiocarbon dating originated from two profiles, that is, P2 and P4, separated by ca. 100 m. The dated barley (Beta559278) and naked barley (Beta559279) were collected from the ash pits (H1*) visible in Profiles 2 (P2) and 4 (P4), respectively (Fig. 2e and f). The sheep molar dated by us (Beta592300) was found in the latter profile as well. Despite the fact that information about their exact positions is missing, the 2σ ranges suggest that the features in Profiles 2 and 4 may have been in use broadly contemporaneously. As such, the currently available dates frame site occupation at Khog Gzung between ca. 3300 and 2950 cal yr BP.
At Kha lding, faunal and crop remains submitted to radiocarbon dating were retrieved from P1② and P1③ (Fig. 2i). The 2σ ranges obtained for both the wheat grain (Beta514620) and the sheep bone (Beta592301) collected in Profile 1 match well. They date the formation of layer ③ to ca. 2700–2400 cal yr BP. Another wheat grain (Beta514619) collected from overlying layer ② provided a 2σ range of 2140–1952 cal yr BP, thus confirming human presence at the end of the third millennium BP. Taken together, the dates suggest that the Kha lding specimens remained in stratigraphic position after people had abandoned the site.
At Kyamo, two faunal specimens retrieved from layer P1② (Fig. 2h), more precisely a sheep bone (Beta592302) and an unidentified mammal bone (Beta536111), were dated. Based on these, it can be argued that the formation of this layer took place between ca. 1400 and 1200 cal yr BP.
Summing up, the time frame suggested by the available radiocarbon dates covers several millennia. It allows us to address human–animal interactions in the study area from the early fifth until the late second millennium BP.
Taxonomic composition of the faunal assemblages
General considerations
The faunal assemblages evaluated by us comprise the remains of two excavated and three surveyed sites. Table 2 presents the taxonomic identifications, together with the number of identified specimens (NISP). Understandably, applying hand-picking and selected flotation during surveys resulted in a bias against small-sized bone and tooth specimens, explaining the absence of vertebrate taxa the size of pika (Ochotona) or even fox (Vulpes). In contrast, sieving, flotation, and hand-picking during a meticulous excavation at Klu lding produced a significant number of tiny bone specimens that, due to their high degree of fragmentation, could not be assigned to the taxonomic level of the order essential for further assessment. Thus, while the faunal assemblage from Klu lding totalled 984 specimens, mainly of mammals, with some birds and fishes, only 73 of these (or 7.4%) could be classified taxonomically (Table 2).
Table 2. Taxonomic representation of identifiable specimens from Klu lding, Khog Gzung, Kha lding, and Kyamo.a

a NISP, number of identified specimens.
Among the identified taxa, livestock is represented by pig (Sus domesticus), sheep (Ovis aries), and cattle and/or yak (Bos sp.). Comparison of the Sus tooth specimens from Klu lding with modern reference skulls confirmed that we are most likely dealing with domestic pigs based on visual assessment of their size. The teeth turned out comparably small, a criterion separating them from wild boar (Sus scrofa). We are aware of the fact that age, sex, state of wear, and geographic region can affect the size of Sus molars (Zeder and Lemoine, Reference Zeder and Lemoine2020), but the small size of the specimens and the species’ relatively high frequency (10.5–14.4%) in the assemblage favour their classification as domesticates. In addition, the site's altitude renders the presence of wild boar rare, as it is at the upper limit of its altitudinal distribution (Wilson and Reeder, Reference Wilson and Reeder1993), although the animals’ occasional presence above this altitude seems not impossible.
At Klu lding, only a single tooth specimen could be identified as Bos sp. It can be assumed that amongst the remains classified as large artiodactyls, additional fragmented Bos specimens are present, but their taxonomic status should be confirmed by ancient DNA analysis or protein fingerprinting. At Khog Gzung and Kyamo, Bos has been recorded as well. As such, both domestic cattle (Bos taurus) and/or domestic yak (Bos grunniens) may have occurred, but morphological criteria enabling us to distinguish between fragmented osseous remains of the two species are not presently available.
Other mammalian taxa identified by us include the Sichuan vole (Microtus millicens), pika (Ochotona princeps), a small felid (Felis sp.), a medium felid, the red fox (Vulpes vulpes), a musk deer species (Moschus sp.), and barking deer (Muntiacus sp.). The medium felid bone may represent either a clouded leopard (Neofelis nebulosa) or a snow leopard (Panthera uncia). The small felid represented by an ulna is enigmatic. Its size and morphology fit with two wild cat taxa—Pallas's cat (Otocolobus manul) and leopard cat (Prionailurus bengalensis)—distributed across the SETP in the Middle–Late Holocene (Yamaguchi et al., Reference Yamaguchi, Driscoll, Kitchener, Ward and MacDonald2004). However, close morphological resemblance to the domestic cat (Felis catus) has been noted as well, which complicates addressing the taxonomic status of this specimen using morphological and morphometric criteria alone. Nevertheless, such early presence of a domestic cat would be surprising in view of its domestication history as revealed by ancient DNA (Ottoni et al., Reference Ottoni, Van Neer, De Cupere, Daligault, Guimaraes, Peters and Spassov2017). Besides mammals, remains of fishes and birds were identified from Klu lding as well. Finally, the presence of either red deer (Cervus elaphus) or white-lipped deer (Cervus albirostris) was noted at Khog Gzung. Because all deer bones pertain to similar-sized animals, the possibility that they represent a single species cannot be excluded.
An assessment of the unidentifiable mammalian specimens shows that the relative abundance of the animals of different size categories of each site are similar to those reflected by the identifiable specimens. This suggests that regardless of the comparably small number of specimens classified to genus and/or species, the relative species frequencies reflected in the identified assemblages can be considered representative of overall animal exploitation at the site.
Identification of medium-sized bovids
Table 3 shows the Caprinae remains identified by us from each of the sites studied. As can be seen, apart from Xiaoenda, domestic sheep (and probably goats) could be identified at each of the other sites. Relative to Xiaoenda, four out of five medium bovid remains available for re-analysis could be attributed to blue sheep (Pseudois nayaur), a wild taxon widely distributed across the TP. In the case of the distal humeri, the sagittal ridge in particular and other discrete morphological features allowed distinguishing Pseudois from similar-sized Ovis and Capra. As to the proximal radius, shape and size of the medial and dorsal margins as well as the lateral protuberance and the lip at the medial edge allow excluding Ovis, Capra, and Naemorhedus (see also Appendix S5, Fig. S5, Fig. 2A,B in Wang et al., Reference Wang, Peters and Barker2020b). Morphometric analyses of two sufficiently well-preserved humerus specimens confirmed morphological diagnosis (Supplementary Tables A.1 and C.1). Because only part of the medium-sized bovid remains were examined by us, our study cannot entirely exclude the possibility of the presence of domestic Caprinea at the site, but just indicates that the majority of the medium bovids represent wild rather than domestic taxa. This corroborates the results of the previous work by Zhang et al. (Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b).
Table 3. Caprinae remains identification results from Xiaoenda, Klu lding, Khog Gzung, and Kha lding.

a Dated samples are noted with an asterisk (*).
b Confidence refers to the degree of certainty of our identification, as archaeological specimens often bear traces of damage complicating taxonomic classification. 1 = identification secure; 2 = identification likely.
In the assemblage studied, gazelles are missing, which may relate to the altitude of the sites investigated by us. At higher altitudes, however, we expect the subfamily Antilopinae to contribute to the fauna as well.
At Klu lding, two specimens (LD001 and LD004) were identified as domestic sheep, O. aries. The identification of LD001, a complete astragalus, was achieved through both morphological and morphometric analyses. As such, the specimen's small size excludes Capricornis, O. ammon, Pseudois, and Naemorhedus as possible identifications (Supplementary Tables A.3 and C.3). From a morphological perspective, all four characters combined (the medial articular ridge from dorsal view, distal articular surface at lateral aspect, proximo-plantar projection of the medial articular ridge, and the proximo-plantar projection of medial articular ridge of the trochlea in medial view) point to Ovis and eliminate similar-sized Capra, Pseudois, and large (male) Antilopinae (Supplementary Table C.3). Morphometric discriminant analysis also classified this specimen with high posterior probability (99.2%) as Ovis (Supplementary Table A.3). Specimen LD004, a right lower premolar (P3) just beyond the “mature” wear stage (12S; Fig. 2 in Payne, Reference Payne1987), was determined to be from O. aries too. Its morphology distinguishes it from its homolog in similar-sized Capra and Pseudois, characterized by a well-developed “step” in the middle of the lingual face; the mesio-buccal quarter of the tooth tends towards a right angle, while its overall shape tends to be short and broad (which is, however, somewhat difficult to judge due to the slightly damaged mesio-buccal corner). These features can be considered typical for O. aries (Halstead et al., Reference Halstead, Collins and Isaakidou2002; Supplementary Fig E.11). In addition, we noted that the size of the P3 is below that observed in a series of mandibles from Pseudois and O. ammon (Supplementary Table E.2). In summary, both LD001 and LD004 exhibit close morphological and morphometric similarities with domestic sheep (O. aries), while features typical of argali (O. ammon) including large size could not be observed.
At Khog Gzung site, two lower molar teeth, an M1 (KX013) and an M2 (KX012), can be classified as Ovis. These specimens can be morphologically separated from Capra and Pseudois based on: (1) the gently convex mesial part of the buccal edge, (2) the absence of a marked posterior orientation in the disto-buccal cusp, (3) the overall shape of the buccal edge tending to a rounded “arcaded” appearance, and (4), the quite broad flange on the mesial face. Taken together and independent of tooth wear, these traits strongly suggest we are dealing with molars of sheep. In this respect, identification criteria are particularly reliable when used in suites (Halstead et al., Reference Halstead, Collins and Isaakidou2002; Zeder and Pilaar, Reference Zeder and Pilaar2010). Despite the fact that classification as Ovis is secure, we are not able to separate domestic sheep (O. aries) from wild argali (O. ammon), because the dimensions of the teeth fit the range of size overlap of both taxa. Having said that, it is noteworthy to mention that genetic analysis of the sediments from the same layer revealed DNA sequences of both O. aries and O. ammon (Gu et al., Reference Gu, Gao, Wang, Yang, Ran, Yang and Shargan2023), which corroborated the identification made by the morphological analyses.
At Kha lding site, domestic sheep are represented by a first phalanx (KD003) and two third phalanges (KD001, KD002). While based on morphology alone, difficulties arise deciding if one is dealing with, O. ammon, O. aries, or Pseudois (Supplementary Table C.5), morphometric comparison confirms that the specimens are smaller in size than the lower range recorded in adult O. ammon and Pseudois (Wang et al., Reference Wang, Peters and Barker2020b: Appendix S5, Table S5.5). For the other Caprinae specimens, taxonomic classification proved difficult. A small caprine maxilla with dentition (KD006) implies either domestic sheep (O. aries) or goat (C. hircus).
At Kyamo, one scapula specimen (KM005) was identified as probably/likely to be O. aries. The certainty of this diagnosis is not very high, though, due to surface erosion and parts being damaged, including the tuberculum supraglenoidale and the coracoid process, two diagnostic parts that are helpful for separating Nemorhaedus and juvenile Pseudois from O. aries. Nevertheless, there is still a higher probability for this specimen to be O. aries, as the remaining diagnostic features are typical for O. aries (Wang, Reference Wang2017, vol. 2: Figs. H3, H4). Like that observed at Kha lding, an incomplete maxilla with dentition (KM002) from a small-sized individual most likely pertains to either O. aries or C. hircus (Table 3).
Apart from the specimens discussed, no other medium-sized caprine specimen could be classified to the genus or species level.
Bone taphonomy
Expectedly, the use of 3 mm sieves at Klu lding produced a faunal assemblage with many small-sized specimens, usually splinters of long bones. The average specimen weight is 0.61 g. More than 60% of the identified specimens measure between 1 and 3 cm in length; fragments measuring >9 cm are lacking. Such size distribution mirrors careful recovery by the excavating team. From our observations of the bone specimens, which displayed percussion, scratching marks, sawing, root etching, carnivore gnawing, and erosion, among other markings (Supplementary Fig. B.1a, c, d, and f), human beings and other agents as well as natural processes contributed to fragmentation. Moreover, one-third of the specimens in the assemblage have either a blackish-greyish or whitish appearance, illustrating that discarded bone refuse witnessed significant heat exposure, with temperatures surpassing 700°C. Although our results do not conclusively prove the use of animal bones as fuel, the fact that some 33% of the finds showed traces of direct heat exposure implies that the use of fire on-site was another major factor contributing to bone fragmentation.
At Klu lding, animal bones also served as a source of raw material. This is illustrated by the presence of four entirely polished bone needles (Supplementary Fig. B.1b). Such finds suggest that during the processing of the carcass for consumption, certain elements were selected in order to modify them into tools (Supplementary Fig. B.1c). The metapodials of medium- and large-sized bovids and cervids were particularly preferred as raw material for manufacturing diverse objects of daily use.
Turning to Khog Gzung, 126 specimens were retrieved during the site's survey. The fact that collecting occurred through hand-picking is reflected in the average weight of 5.7 g per specimen, which is 10 times the value noted at Klu lding. In addition, more than 60% of the identified specimens are clearly larger, with lengths between 4 and 10 cm. The negative bias against smaller bone fragments in assemblages collected by hand-picking versus sieving has been addressed repeatedly in zooarchaeology (e.g., Payne, Reference Payne and Higgs1972). Percussion marks on long bone shafts imply efforts to open the medullary cavity in order to access the bone marrow (Supplementary Fig. B.1e). Of the identified specimens, 5% testify to exposure to heat (black colour; ca. 400°C), implying postdepositional contact with a source of intense heat, for instance fireplaces.
The faunal assemblage of Kha lding totals 90 specimens. Their average weight is 1.5 g, and more than 60% of the specimens identified by us surpass 9 cm. This survey material did not contain burnt bones at all, which is in marked contrast to the archaeofauna from Klu lding.
The Kyamo site produced an assemblage of 99 fragments with an average weight of 14.4 g. The assemblage lacks both burnt pieces and specimens with cutmarks; the latter—if present—being hardly visible due to the poor preservation of the bone surface. However, percussion marks were observed on two medium-sized artiodactyl specimens, one of which being a caprine.
DISCUSSION
Fourth millennium BP human–animal dynamics in the SETP
Faunal research into human–animal dynamics and early food production in the STP is still in its infancy. New faunal data from the Klu lding site illustrate that among the specimens classified into the subfamily Caprinae, two could be identified as domestic sheep (O. aries). Conversely, the presence of wild Caprinae could not be confirmed in the assemblage studied (Table 2). Despite limitations of sample size, positive identification of the domestic form and lack of unequivocal evidence for wild Caprinae suggest that domestic sheep likely figured prominently in the non-identified Caprinae assemblage as well (n = 15; i.e., 19.7% of NISP). Thus, on the assumption that all taxa identified to the species level contributed significantly to the non-identified assemblage, it seems reasonable to postulate that sheep husbandry was essential in the livestock economy of Klu lding.
The relative abundance of pig remains (10.5–14.4%) indicates that swine husbandry contributed to human subsistence as well, possibly equivalent to sheep. The only Bos specimen identified by us suggests that large bovines, be it domestic cattle, yak, or their hybrid (termed “dzo”), were present in small numbers as well. For reasons of economic efficiency, however, the keeping of large ruminants often goes hand in hand with their use as draught, dairy, or pack animals, but unequivocal evidence for this is lacking. Nevertheless, the economic importance of domestic bovines may be underestimated in view of the pronounced fragmentation of the bone material at Klu lding.
During excavation of Klu lding, a square-corner stone enclosure (Q1) made of large gravels was partially uncovered in layer ③ of T1, with a length of 450 cm exposed inside the trench. The radiocarbon date of layer ② (Table 1) suggests its construction before 3400 cal yr BP. Similar stone enclosures were found in numbers at Bangga in the south-central TP, with early-phase structures dating to 3000–2800 cal yr BP (Lu et al., Reference Lu, Chen, Zhang, Tang, Lemoine, Wangdue, Chen, Liu and Frachetti2021). In comparison to the findings from stone enclosures F2/F5 at Bangga yielding abundant animal dung and interpreted by Lu et al. (Reference Lu, Chen, Zhang, Tang, Lemoine, Wangdue, Chen, Liu and Frachetti2021, see Fig. 3 in this reference) as animal corrals, the possibility of Q1 at Klu lding serving the same purpose cannot be excluded. However, stone structures are prevalent in the prehistoric and historic TP, so without additional evidence, any statement regarding their exact function remains speculative.
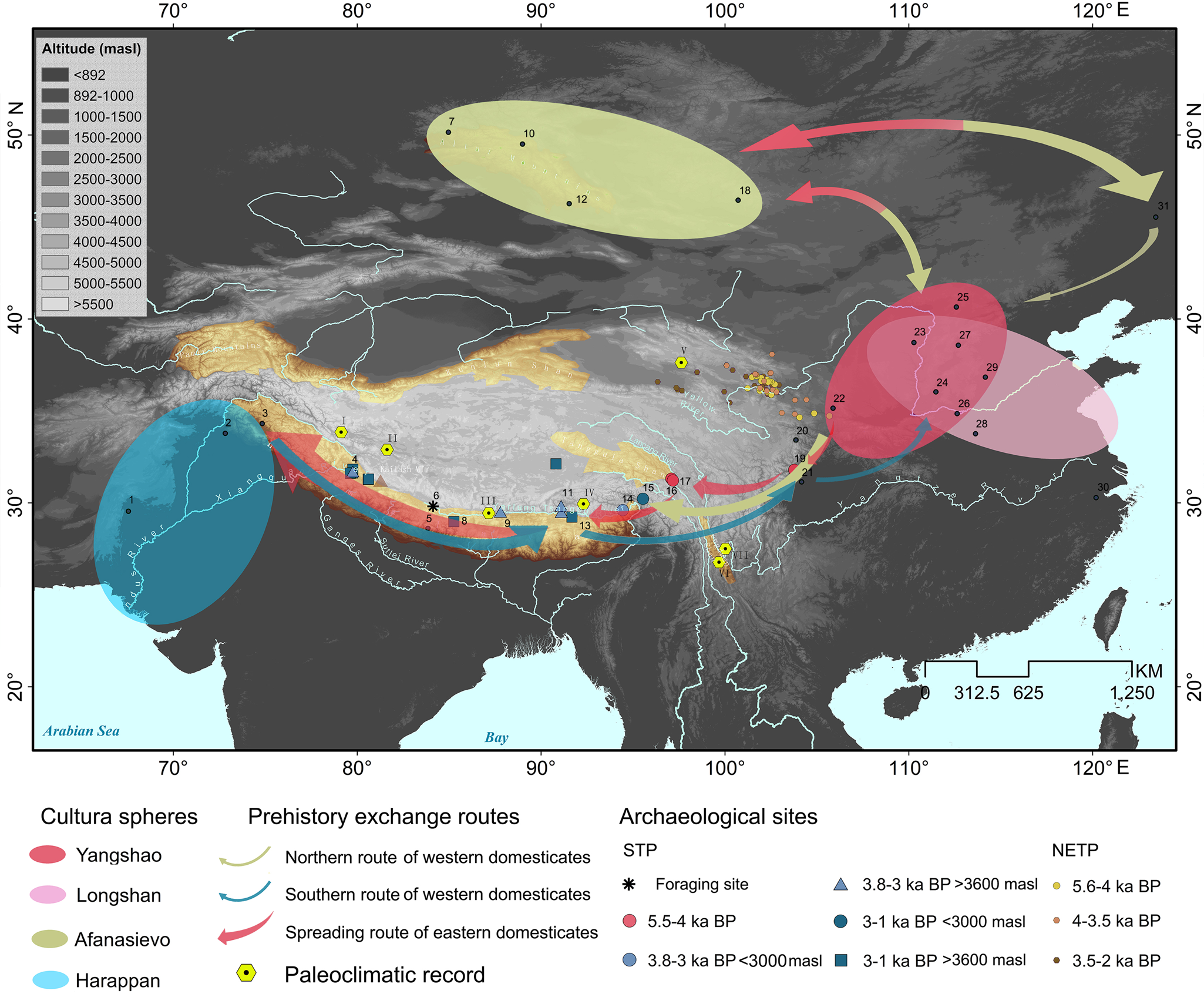
Figure 3. Proposed pathways of cultural exchange in Middle–Late Holocene, distribution of prehistoric culture groups in central and East Asia, archaeological sites mentioned in the text, location where palaeoclimatic records were obtained. 1: Mehrgarh; 2: Harappa; 3: Burzahom; 4: Gepa Serual; 5: Mebrak/Phudzeling; 6: Zhongba; 7: Nizhnaya; 8: Kyamo; 9: Khog Gzung; 10: Khuurai Gobi2; 11: Qugong; 12: Yagshiin Huduu2; 13: Bangga; 14: Klu lding; 15: Kha lding; 16: Xiaoenda; 17: Karuo; 18: Shatar Chuluu1; 19: Yingpanshan; 20: Ashaonao; 21: Sanxingdui; 22: Dadiwan; 23: Shimao; 24: Taosi; 25: Shihushan; 26: Erlitou; 27: Youyao; 28: Jiahu; 29: Cishan; 30: Kuahuqiao; 31: Houtaomuga. Hexagons for palaeoclimate records: I: Bangong Co; II: Aweng Co; III: Ngamring Tso; IV: Paru Co; V: Delingha; VI: Tiancai Lake; VII: Heihai Lake. The red box indicates the area of the studied archaeological sites.
Apart from domestic livestock, a diverse archaeofauna comprising remains of Sichuan vole, a small cat, a fox, a leopard, musk deer, barking deer, birds, and fishes was recovered at Klu lding. These taxa formed part of the local fauna populating the natural environment surrounding the site. As such, the vole and small cat most likely represent commensal species frequenting human habitats in search of food. With the onset of sedentism and cereal cultivation in SW Asia at the transition of the Pleistocene to the Holocene, the human niche witnessed increasing numbers of seed-eating rodents (Tchernov, Reference Tchernov1984; Willcox and Stordeur, Reference Willcox and Stordeur2012). Higher rodent densities certainly attracted the species’ natural enemies, such as (wild) cats (Vigne et al., Reference Vigne, Guilaine, Debue, Haye and Gérard2004, Reference Vigne, Briois, Zazzo, Willcox, Cucchi, Thiébault and Carrère2012) and foxes (Peters et al., Reference Peters, Arbuckle, Pöllath, Özdoğan, Başgelen, Kuniholm and Galatasaray2014), and the fact that crop seeds including millet have been found in numbers in the archaeobotanical samples of Klu lding (Wang, Y., personal communication, August 2021) makes commensalism a likely explanation. Elsewhere in China, the presence of commensal carnivores as a means of reducing crop losses has already been postulated for Neolithic farming villages of the Yangshao culture dating to the sixth millennium BP (Guan et al., Reference Guan, Hu, Hu, Sun and Wang2008; Hu et al., Reference Hu, Hu, Wang, Wu, Marshall, Chen, Hou and Wang2014; Vigne et al., Reference Vigne, Evin, Cucchi, Dai, Yu, Hu and Soulages2016). Just as in the ancient Near East (e.g., Weissbrod, Reference Weissbrod and Dean2010), the relative abundance of cereal finds and the presence of rodents and their predators testify to the sedentary agricultural nature of the Klu lding settlement.
With regard to the other vertebrate taxa, hunting or trapping of pika, leopard, musk deer, barking deer, birds, and freshwater fishes primarily served food purposes. Similar faunal spectra have been reported from other SETP sites (Huang and Leng, Reference Huang and Leng1985; Li, Reference Li2007; Zhang et al., Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b). Besides meat and fat, the furs of pikas, foxes, and leopards were certainly valued for clothing (Feng et al., Reference Feng, Cai and Zheng1986; Monchot and Gendron, Reference Monchot and Gendron2011), while the hides of deer and Caprinae may have been used as seat carpets. (Feng et al., Reference Feng, Cai and Zheng1986). Deer musk obtained from the species’ caudal glands may have been appreciated for fragrance or as admixture to medicine, among other uses (e.g., Green, Reference Green1986; Yang et al., Reference Yang, Meng, Xia and Feng2003). As wild taxa account for almost 28% of the assemblage identified, hunting, fowling, and fishing still represented essential subsistence activities at Klu lding, even if the relative importance of these wild taxa had already decreased compared with the earlier settlement sites of Xiaoenda and Karuo (Li, Reference Li2007; Zhang et al., Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b).
In summary, the fauna from Klu lding illustrates the dynamics of human–animal–environment dynamics in the SETP during the mid-fourth millennium BP. In this warm-humid and most suitable area for human habitation in Tibet, the site inhabitants explored a diverse range of food resources. Their subsistence combined husbandry of pigs, sheep, and cattle with millet agriculture, the latter also attracting crop-eating rodents and their enemies, more precisely wild small felids. Meanwhile, a large part of the resources were obtained through the hunting of leopards, foxes, different species of deer, and pikas, as well as fowling and fishing.
Spread of millet–pig agriculture in the STP
Pig domestication and early husbandry can be evidenced in the Central Plain of northern China (i.e., Jiahu site, Jiahu culture) and the Lower Yangtze of southern China (i.e., Kuahuqiao site, Kuahuqiao culture) some 9000–8000 years ago (Luo and Zhang, Reference Luo and Zhang2008; Cucchi et al., Reference Cucchi, Hulme-Beaman, Yuan and Dobney2011). Millet was domesticated in northern China ca. 10,000 ago (Cishan site, Cishan culture), and by the sixth millennium BP, millet agriculture had already dispersed across much of northern China (Lu et al., Reference Lu, Zhang, Liu, Wu, Li, Zhou and Ye2009; Yang et al., Reference Yang, Wan, Perry, Lu, Wang, Zhao and Li2012; Zhao, Reference Zhao2014). As such, millet–pig farming developed into the main mode of subsistence of the Yangshao (7–5 ka) and Majiayao (5.3–4 ka) farming communities inhabiting the Yellow River basin (Yuan, Reference Yuan1999; Wang et al., Reference Wang, Martin, Hu and Wang2012, Reference Wang, Martin, Wang and Hu2015). Millet–pig farming has also been observed in Dadiwan, situated at 1593 m asl (6.5–4.9 ka; Barton et al, Reference Barton, Newsome, Chen, Wang, Guilderson and Bettinger2009) close to the NETP, and along the upper Min River at the eastern margin of the TP in NW Sichuan province, as exemplified at the Yingpanshan site (5.3–4.6 ka; 1650 m asl; Zhao and Chen, Reference Zhao and Chen2011; He, Reference He2015).
In view of the geographic origins of pig husbandry in China, domestic pigs were introduced to the TP from the east, along with millet cultivation. Of interest is the fact that in the STP, Sus remains have been identified in sites located above 3000 m asl, for instance at 3700 m asl Qugong (3.8–3 ka, Qugong culture; Zhou, Reference Zhou1999) or at the earlier sites of Xiaoenda (3140 m asl; Zhang et al., Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b), Karuo (3100 m asl; Huang and Leng, Reference Huang and Leng1985), though in quite low numbers. Debate continues regarding whether these Sus remains represent domestic pigs or wild boars (Zhou, Reference Zhou1999; Li, Reference Li2007; Zhang et al., Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b). Conceivably, the species’ successful association with millet-cultivating Neolithic communities inhabiting the Central Plain (Han, Reference Han2012) allows arguing in favour of domestic pigs spreading along with millet farming Yangshao and Majiayao communities that migrated into the TP from lowland eastern China. As the aforementioned Tibetan sites are all close to or above the altitude limit of the natural distribution usually accepted for Eurasian wild boar (~3000 m asl; Feng et al., Reference Feng, Cai and Zheng1986; Groves and Grubb, Reference Groves, Grubb and Oliver1993; Wilson and Reeder, Reference Wilson and Reeder1993; Keuling and Leus, Reference Keuling and Leus2019), the arrival of pig husbandry offers the most plausible explanation for the species’ presence in the fifth millennium BP contexts of the study region.
Although the site inhabitants obviously managed to provide suitable fodder and indoor space so that pigs could endure the harsh Tibetan winter, numbers may have been limited by the area of available space and the amounts of food the community could spare (e.g., Mitchell, Reference Mitchell2002; Albarella et al., Reference Albarella, Dobney, Ervynck and Rowley-Conwy2007). Investigation of the correlation between the intensity of millet farming and the rather low frequency of Sus in archaeological contexts of the TP needs further exploration, particularly when the climate turned colder in the Middle–Late Holocene TP and millet cultivation was at low-level production due to ecological constraints (d'Alpoim Guedes, Reference d'Alpoim Guedes2015). Besides millet, other cereals, including barley, naked barley, wheat, and Tartary buckwheat identified in STEP sites served as food for humans and perhaps animals as well (Gao et al., Reference Gao, Yang, Ma, Tong and Yang2021; Song et al., Reference Song, Gao, Tang, Zhang, Tang, Xu and Wangyal2021). With more reliable numbers at hand, a closer look at the demographic profiles of pigs could be helpful to test the hypothesis that people removed surplus young and old animals from the herd to save fodder for valuable breeding stock over the winter. Finally, thriving well in a temperate habitat, domestic lineages had to adapt to the conditions of living at significantly higher altitudes. Future ancient DNA studies will have to clarify whether this involved deliberate selection by pig breeders or even hybridization with local wild boar.
Origins of bovid pastoralism in the STP
At present, none of the sites located in the STP and predating 4 ka yielded unequivocal evidence for human exploitation of domestic bovids. This assumption draws upon the faunal records from Xiaoenda (Zhang et al., Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b; this study), Karuo (Huang and Leng, Reference Huang and Leng1985; Li, Reference Li2007), and Mabu Co (located at the very southern edge of the TP; fauna analysed by the first author and colleagues), although in the course of the fifth millennium BP, SETP witnessed the introduction of agricultural practices including millet cultivation and pig husbandry, as discussed earlier.
Relative to the first half of the fourth millennium BP, earlier work proposed that the inhabitants of Qugong in the central TP kept domestic sheep and yak (Zhou, Reference Zhou1999). Though livestock husbandry at ~3700 cal yr BP cannot be excluded, taxonomic identification of the specimens and their 14C dating need verification. Recent archaeobotanical work at Qugong by Gao et al. (Reference Gao, Yang, Ma, Tong and Yang2021) pointed out issues with 14C dating of contexts likely due to the old wood effect and/or the use of lab instrumentation generating dates with wide analytical errors (Schiffer, Reference Schiffer1986; Dong et al., Reference Dong, Wang and Ren2014). In addition, the Ovis skull found in Pit 5 has been classified taxonomically as a domestic sheep of the “Tibetan breed” due to its large horns and very large size (Zhou, Reference Zhou1999, p. 238). However, given the widespread occurrence of argali in the region, criteria separating O. ammon from domestic “Tibetan sheep” must be detailed. By analogy, morphological separation of domestic yak from its wild relative and from cattle × yak hybrids is essential to postulate the early presence of the first.
After 3.5 ka, domestic sheep and cattle/yak gain visibility in the archaeological record of the STP, with remains now being identified in every site located above 3000 m asl (Supplementary Material D). Current evidence thus suggests that bovid husbandry gained a foothold around the middle of the fourth millennium BP. Following initial introduction of the domestic bovids, STP witnessed the establishment of an agro-pastoral system combining wheat and barley cultivation with livestock husbandry (Gao et al., Reference Gao, Yang, Ma, Tong and Yang2021). From the early third millennium BP onward, however, barley becomes the dominant crop staple at the expense of millet in pastoral communities inhabiting high-elevations in the NETP (Qinghai) and central STP (d'Alpoim Guedes et al., Reference d'Alpoim Guedes, Manning and Bocinsky2016; Tang et al., Reference Tang, Lu, Spengler, Boivin, Song, Wangdue, Chen, Liu and Zhang2021). Interestingly, this shift in cereal exploitation seems restricted to those regions where mobile pastoralism became an essential feature of local economies.
Like elsewhere in alpine Eurasia, the adoption and widespread use of livestock in the STP relates to the species’ adaptation to high-altitude environments and pasture opportunities (Schaller, Reference Schaller1977; Felius, Reference Felius1985; IUCN/SSC, 1997) on the one hand, and their returns in terms of lifetime (secondary) products, such as milk, hair/wool, dung, and labor on the other (Greenfield, Reference Greenfield2010). Perhaps noteworthy as well is the fact that in mountainous terrain, small ruminants also can be used for transporting goods—for instance, bags containing salt (Yang and Zheng, Reference Yang and Zheng2001). Moreover, caprines and cattle/yak can be pastured in the valleys during wintertime to avoid harsh alpine conditions and periods of food shortage, but will return to higher altitudes to enjoy summer pastures without too much need of human attention for feeding and shelter (Cai, Reference Cai1981; Ryder, Reference Ryder1983). Conceivably, lowland lineages of sheep and cattle translocated into the Himalayas at altitudes well above 3000 m asl needed specific adaptations in order to thrive well under such conditions (Zhang et al., Reference Zhang, Dong, Wang, Ren, Qiang and Chen2016; Wang, Reference Wang2017; Wang et al., Reference Wang, Ju, Jiang, Zhong, Liu, Wang and Hoff2021a). Deliberate selection by breeders seems one option, but with respect to sheep and cattle, hybridization respectively with local wild argali (Ovis ammon) (Aniwashi et al., Reference Aniwashi, Ali Kaleri, Sulaiman, Li and Zhuang2011; Wang, Reference Wang2017) and yak (Bos mutus) (Liu et al., Reference Liu, Li, Yan, Li, Wu, Pei, Yan, Yang, Guo and Lan2020; Wang et al., Reference Wang, Ju, Jiang, Zhong, Liu, Wang and Hoff2021a) could have accelerated acquisition of vital adaptive and in the meantime genetically fixed traits to deal with conditions of hypoxia and ultraviolet signaling. As these traits have been identified in the chromosomal DNA of modern representatives of Tibetan sheep (Hu et al., Reference Hu, Yang, Xie, Lv, Cao, Li and Liu2019) and cattle (Chen et al., Reference Chen, Cai, Chen, Li, Wang, Huang and Hu2018; Wu et al., Reference Wu, Ding, Wang, Wójcik, Zhang, Tokarska and Li2018b), it raises the question whether hybridization of domestic lineages with local wild forms had a much deeper history. Estimates using the molecular clock offer a timeframe of 2400–1400 years ago for introgression events between cattle and yak (Chen et al., Reference Chen, Cai, Chen, Li, Wang, Huang and Hu2018). That said, analysis of the genetic makeup of prehistoric sheep and cattle is essential to refine the picture.
If, as already said, the northern Fertile Crescent witnessed the domestication of wild sheep, goat, and cattle based on zooarchaeological (Peters et al., Reference Peters, Helmer, Driesch and Saña Segui1999, Reference Peters, Dreisch, Helmer, Vigne, Peters and Helmer2005, Reference Peters, Arbuckle, Pöllath, Özdoğan, Başgelen, Kuniholm and Galatasaray2014; Zeder and Hesse, Reference Zeder and Hesse2000; Helmer et al., Reference Helmer, Gourichon, Monchot, Peters, Sana, Vigne, Peters and Helmer2005) and ancient DNA studies (Meadows et al., Reference Meadows, Hiendleder and Kijas2011; Bollongino et al., Reference Bollongino, Burger, Powell, Mashkour, Vigne and Thomas2012; Demirci, Reference Demirci2012, Reference Demirci, Baştanlar, Dağtaş, Pişkin, Engin, Özer, Yüncü, Doğan and Togan2013; Lv et al., Reference Lv, Peng, Yang, Zhao, Li, Liu, Ma, Zhao, Yang, Wang and Li2015; Scheu et al., Reference Scheu, Powell, Bollongino, Vigne, Tresset, Çakırlar, Benecke and Burger2015), the routes by which these domestic ungulates spread across central, South, and East Asia can only be addressed in broad outline for reasons of poor archaeological coverage in many parts of this vast region. Migratory routes of sheep pastoralism across Europe and Asia have been proposed based on the study of endogenous retroviruses (Chessa et al., Reference Chessa, Pereira, Arnaud, Amorim, Goyache, Mainland and Kao2009) and mitogenomes (Lv et al., Reference Lv, Peng, Yang, Zhao, Li, Liu, Ma, Zhao, Yang, Wang and Li2015) of modern Eurasian sheep breeds and wild Ovis. The Mongolian Plateau region was postulated as acting as a main “transportation hub” that witnessed two major migratory waves by the fifth millennium BP (Lv et al., Reference Lv, Peng, Yang, Zhao, Li, Liu, Ma, Zhao, Yang, Wang and Li2015). According to direct radiocarbon dating and proteomics analysis, sheep were exploited by Afanasievo communities of the Altai Mountains and Mongolian Plateau ca. 5300–4800 cal yr BP (Hermes et al., Reference Hermes, Frachetti, Doumani Dupuy, Mar'yashev, Nebel and Makarewicz2019; Wilkin et al., Reference Wilkin, Miller, Taylor, Miller, Hagan, Bleasdale and Scott2020). In NE China, direct radiocarbon dating and aDNA analyses confirmed that cattle (Bos taurus) of Near Eastern origin was exploited at Jilin by ca. 5300 cal yr BP (Cai et al., Reference Cai, Zhang, Zhu, Chen, Wang, Zhao, Ma, Royle, Zhou and Yang2018b). Furthermore, domestic sheep were exploited in the upper and middle Yellow River basin (including Qinghai, Gansu, southern Shaanxi, and Shanxi) by the late fifth/early fourth millennium BP (IACASS and SPLCRB, 2005; Yan and He, Reference Yan and He2005; Cai et al., Reference Cai, Tang, Yu, Han, Ren, Zhao, Zhu and Zhou2011; Li, Reference Li2012; Wang, Reference Wang2017; Brunson et al., Reference Brunson, Lele, Xin, Xiaoling, Hui, Jing and Flad2020), while at sites located at higher latitudes in northern Shaanxi and Shanxi, the species may have been present a few centuries earlier (Dodson et al., Reference Dodson, Dodson, Banati, Li, Atahan, Hu, Middleton, Zhou and Nan2014; Hu et al., Reference Hu, Yang, Sun and Shao2016, Reference Hu, Yang, Yang, Shao and Di2022; Yang et al., Reference Yang, Hu, Guo and Wang2017; Sun et al., Reference Sun, Shao and Di2020; Hu, Reference Hu2021b). Zooarchaeological and ancient DNA analyses moreover confirm that cattle appeared in the middle Yellow River Valley at broadly the same time ca. 4.5–4 ka (Cai et al., Reference Cai, Sun, Tang, Hu, Li, Zhao, Xiang and Zhou2014, Reference Cai, Zhang, Shao, Sun, Zhu and Yang2018a; Yu, Reference Yu2020), suggesting that their spatiotemporal dispersal may have coincided with that of domestic sheep, possibly even along the same route(s) of dispersal. In the same region, goats likely occurred a few centuries later than sheep.
Thus, by 5.5–5 ka, sheep and cattle were raised by Neolithic communities inhabiting the Mongolian Plateau and NE China. From there, livestock husbandry progressed south into the northern Shanxi and Shaanxi Provinces at around 4.5 ka and further into the Yellow River basin to reach the NETP around 4 ka. In this respect, the spatiotemporal pattern proposed for the onset of livestock farming in NE China and Gansu/Qinghai seems paralleled by routes of dispersal and timing of arrival of wheat and barley (Dodson et al., Reference Dodson, Li, Zhou, Zhao, Sun and Atahan2013; Chen et al., Reference Chen, Dong, Zhang, Liu, Jia, An, Ma and Xie2015a; Stevens et al., Reference Stevens, Murphy, Roberts, Lucas, Silva and Fuller2016; Liu et al., Reference Liu, Lister, Zhao, Petrie, Zeng, Jones and Staff2017). By the same route but in the opposite direction, broomcorn millet spread into central Asia, with evidence for its cultivation 4.2 ka in Begash, south Kazakhstan (Stevens et al., Reference Stevens, Murphy, Roberts, Lucas, Silva and Fuller2016).
Current opinion agrees that in China, livestock husbandry dispersed south from the Yellow River basin. To reach the SWTP, the most likely route taken (>1500 km) crossed the Tao River basin in Gansu to continue through the mountain and gorge regions of northwest Sichuan and across the Hengduan Mountains. Being adapted to cold-temperate environmental conditions prevailing in NE China, dispersal must have been challenging for livestock populations, because herds faced new climate and vegetation conditions when crossing distinct eco-geographic zones. Nevertheless, the recent evidence from early occupations in Ashaonao, Jiuzhaigou, may exemplify such north-south dispersal during the fourth millennium BP (d'Alpoim Guedes et al., Reference d'Alpoim Guedes, Lu, Hein and Han2017; Lv et al., Reference Lv, Fan, Yang and Li2017; Zhang et al., Reference Zhang, Li, Ma and Lv2017b).
As shown for the SW Asian domestic crops wheat and barley, there might be different routes and episodes for domestic bovids spreading into China in prehistory. At present, four routes into China have been proposed for the spread of wheat and barley, including the Eurasian steppe route, the sea route, the Silk Road, and possibly the South Asia route (Zhao, Reference Zhao2009; Flad et al., Reference Flad, Li, Wu and Zhao2010; Barton and An, Reference Barton and An2014; Betts et al., Reference Betts, Jia and Dodson2014; Liu et al., Reference Liu, Lister, Zhao, Petrie, Zeng, Jones and Staff2017; Lister et al., Reference Lister, Jones, Oliveira, Petrie, Liu, Cockram, Kneale, Kovaleva and Jones2018; Long et al., Reference Long, Leipe, Jin, Wagner, Guo, Schröder and Tarasov2018). The wheat–barley–pea combination and the round-bottomed pottery basins discovered at Qugong (~3.5 ka) in the central STP (Tang, Reference Tang2014; Gao et al., Reference Gao, Yang, Ma, Tong and Yang2021), and the cowrie shells from Karuo (Tang, Reference Tang2014) in the SETP were most likely from northern South Asia, and the archaeobotanical assemblage including barley, pea, millet, and rice found in Mebrak/Phudzeling (3–2.1 ka) in Nepal illustrates an important trade route connecting the Indian subcontinent with the TP (Knörzer, Reference Knörzer2000). Given that domestic sheep and goats appeared in the NW Indian subcontinent comparably early, that is, at Neolithic Mehrgarh (8.3–6 ka), and with caprine husbandry spreading across much of South Asia in the subsequent millennia (Meadow, Reference Meadow, Crabtree, Ryan and Campana1989, Reference Meadow, Clutton-Brock J. and S1993, Reference Meadow and Harris1996; Thomas, Reference Thomas2002; Miller, Reference Miller2004; Joglekar et al., Reference Joglekar, Sharada and Abhayan2013; Chase, Reference Chase2014), the possibility of a southern, sub-Himalayan route of introduction into Tibet must be considered. Evidence for cultural exchange between NW South Asia, Kashmir, and the STP since the late fifth millennium BP (Mughal and Halim, Reference Mughal and Halim1972; Huo, Reference Huo1990; Han, Reference Han2012; Cao et al., Reference Cao, Wen, Yu, Shargan, Tash and Wang2021) underscores the possibility of a livestock transfer. According to this scenario, sheep and goats may have travelled along the hilly flanks from the sites at the foothills in NW subcontinent, through Kashmir, and then along the Sengge Zangbo River valley into the SWTP. Alternatively, they could have followed the mountain ranges along the Sutlej River valley from the Indus plain into the CSTP. Considering the nearly synchronous presence of sheep in the SWTP (represented by Gepa Serual), CSTP (represented by Qugong), and SETP (represented by Klu lding), several trajectories of introduction into the Himalayas seem to be a scenario worth considering, even if definitive proof of this still needs to be provided. The genetic contributions of Pamir O. ammon to Tibetan domestic sheep (Hu et al., Reference Hu, Yang, Xie, Lv, Cao, Li and Liu2019) and of South Asian zebu cattle (Bos indicus) (Chen et al., Reference Chen, Lin, Baig, Mitra, Lopes, Santos and Magee2010) in ancient central plain Chinese cattle (Cai et al., Reference Cai, Sun, Tang, Hu, Li, Zhao, Xiang and Zhou2014) equally suggest the possible existence of a “southern route” of livestock translocation. It agrees with previous archaeological studies suggesting that the formation of a “highland silk road” has deep prehistoric roots (Huo, Reference Huo2017). Hence, regardless of its formidable altitude and varying climates, the STP was likely a dynamic arena of cultural activity spanning distant regions of central Asia, South Asia, and East Asia as early as 5 ka (Tang, Reference Tang2014; d'Alpoim Guedes, Reference d'Alpoim Guedes2015; Huo, Reference Huo2017; Liu et al., Reference Liu, Jones, Matuzeviciute, Hunt, Lister, An, Przelomska, Kneale, Zhao and Jones2019a; Gao et al., Reference Gao, Yang, Ma, Tong and Yang2021).
On the other hand, there are also arguments against a sub-Himalayan route of dispersal (Witzel, Reference Witzel and Osada2009; Stevens et al., Reference Stevens, Murphy, Roberts, Lucas, Silva and Fuller2016). To test the hypothesis of a transfer of domestic bovids via a sub-Himalayan corridor, additional excavations and zooarchaeological studies involving ancient DNA analysis examining phylogenetic relationships with either northern Eurasian or southern Asian livestock lineages is needed for STP archaeological sites along potential trajectories.
Three-phase subsistence development and formation of Tibetan pastoralism
The TP hosts one of the world's largest pastoral systems, the formation of which is still poorly understood (Miehe et al., Reference Miehe, Miehe, Kaiser, Reudenbach, Behrendes, Duo and Schlütz2009; Wei et al., Reference Wei, Hou, Fan, Madsen, Qin, Du, Sun, Gao and Shan2020; Huang et al., Reference Huang, Zhang, Storozum, Liu, Gill, Xiang and Ren2020). Against the background of Holocene climate archives in the wider region and by contrasting this information with zooarchaeological and archaeobotanical findings, this study evaluated and integrated ecological and biocultural developments in the study area in order to trace the dynamics of the human–animal–environment relationship and the emergence of pastoralism on the TP.
Precipitation and temperature records have been obtained in different regions of the TP (see yellow hexagons in Fig. 3). Most Holocene archives illustrate that towards the end of the Holocene thermal maximum, the climate became cooler and drier (Fig. 4a–d). Figure 4b and d show the decline in precipitation in the SWTP (Fig. 3, hexagon I) and CSTP (Fig. 3, hexagon III) respectively. The hydrogen data from Delingha in the NETP (Fig. 3, hexagon V; Yang et al., Reference Yang, Qin, Bräuning, Osborn, Trouet, Ljungqvist and Esper2021) and the temperature data from Lakes Tiancai and Heihai in the SETP (Fig. 3, hexagons VI and VII; Chang et al., Reference Chang, Zhang, Liu and Shulmeister2017; Zhang et al., Reference Zhang, Chang, Cao, Sun, Shulmeister, Tang, Langdon, Yang and Shen2017a) show similar patterns. Two other climate archives seem to diverge, though, namely the ones from Paru Co in the STP (Fig. 3, hexagon IV; Bird et al., Reference Bird, Polisar, Lei, Thompson, Yao, Finney, Bain, Pompeani and Steinman2014) and Aweng Co in the SETP (Fig. 3, hexagon II; Li et al., Reference Li, Wang, Zhang, Lei and Hou2017), most likely for reasons of low resolution of the data and the effects of complex factors other than climate, such as vegetation (Liang et al., Reference Liang, Russell, Xie, Lupien, Si, Wang, Hou and Zhang2019; Wang et al., Reference Wang, An, Lu, Zhao and Liu2020a). Based on pollen, alkenone, and chironomid records from the TP, Chen et al. (Reference Chen, Zhang, Liu, Cao, Hou, Zhu and Xu2020) were able to confirm a drop in both temperature and precipitation in Middle–Late Holocene times following the Holocene thermal maximum. Alongside this, it was also concluded that in the course of this development, the extent of forested habitat decreased (Fig. 4e).

Figure 4. Spatiotemporal development of climate and fauna in the southern Tibetan Plateau (STP) during the Middle–Late Holocene. (a) Reconstructed annual temperature at midlatitude (30–50°N) based on marine and terrestrial archives (red line; Routson et al., Reference Routson, McKay, Kaufman, Erb, Goosse, Shuman, Rodysill and Ault2019). (b) Effective precipitation reconstructed based on leaf wax δD records of Bangong Co (blue line; Hou et al., Reference Hou, D'Andrea, Wang, He and Liang2017). (c) Mean annual temperature reconstructed by fossil pollen from the TP (red line; Fig. 4D in Chen et al., Reference Chen, Zhang, Liu, Cao, Hou, Zhu and Xu2020). (d) Summer monsoon precipitation reconstructed based on sediment records of Ngamring Tso (light blue line; Conroy et al., Reference Conroy, Hudson, Overpeck, Liu, Wang and Cole2017). (e) Reconstructed forest extent based on arboreal pollen records in the TP (green line; Fig. 9A in Chen et al., Reference Chen, Zhang, Liu, Cao, Hou, Zhu and Xu2020). (f) Variations in the percentage of number of identified specimens (NISP) of wild game in the STP from 5000 to 1000 BP (Supplementary Table D.2). (g) Spatiotemporal distribution of archaeological sites with major exploited animals in the STP (Supplementary Table D.1). Symbols for STP sites correspond to those in Fig. 3.
Despite notable differences in the quality of hitherto recorded faunal data (Supplementary Table D.2), exploitation of animal resources in the STP postdating the Holocene climatic optimum reveal some interesting trends. Figure 4f thus illustrates that relative to human subsistence, the contribution of game species declined over time. Even smaller faunal collections hand-picked during surveys reveal this pattern. Parallel to this, an increase in the number of archaeological sites and the relative contribution of domestic animals to the archaeofaunas is noted (Fig. 4f, g). While climate deterioration undoubtedly affected the natural environment and its vegetation, we postulate that the growing human presence in the study area reflected by increased settlement density and livestock numbers had a lasting impact on natural woodland habitats as well, given that people cut wood for building (houses, fences), cooking, heating, and other purposes. In addition, shepherds pasturing increasingly larger herds at different altitudes and in all vegetation types including forests, certainly contributed over time to the decline of wildlife in local catchment areas.
Interestingly, diachronic analysis of the archaeological record clearly shows that over time, people started exploiting higher elevations more systematically, which would have hardly been possible without adjustments to their subsistence strategy (Fig. 4g). Based on (bio)cultural developments in the STP, three main phases can be distinguished (Fig. 4g). During the first phase (~5.5–4 ka), CSTP and SWTP human groups obviously went on forays above 4400 m asl in search of game. However, communities continued residing at lower altitudes, that is, below 3000 to 3200 m asl in the SETP. Here subsistence relied primarily on foraging and low-level millet and pig farming, as exemplified by Karuo (3100 m asl; BCRTAR&DHSU, 1985) and Xiaoenda (3140 m asl; Zhang et al., Reference Zhang, Chen, Marshall, Lü, Lemoine, Wangyal, Dorje and Liu2019b). Conceivably, the agricultural component of the subsistence dispersed via the Upper Yellow River into the TP, gaining foothold in the SETP ca. 5–4 ka. Though situated at the upper altitudinal and temperature limits for cultivating foxtail millet and raising pigs (d'Alpoim Guedes, Reference d'Alpoim Guedes2015; Keuling and Leus, Reference Keuling and Leus2019), the Karuo villagers obviously participated in small-scale farming alongside a good deal of hunting and gathering. We assume that this represented a viable strategy, because some 5000 years ago, local temperature and precipitation were significantly higher than today (Chen et al., Reference Chen, Zhang, Liu, Cao, Hou, Zhu and Xu2020), by an estimated ca. 1.2°C and 400 mm, respectively (Chen et al., Reference Chen, Dong, Zhang, Liu, Jia, An, Ma and Xie2015a; Wu et al., Reference Wu, Chen, Lv, Brenner, Curtis, Zhou, Chen, Abbott, Yu and Chen2018a). The site's material culture moreover yielded stone tools for harvesting millet, while archaeobotanical analysis confirmed the presence of weeds commonly encountered in millet fields (Lu, Reference Lu2016). Finally, architectural features indicate facilities for millet storage and the stabling of pigs during the cold season.
From the second phase onwards at ~3.8 ka, we observe a significant decline in the role of game in the diet of the TP inhabitants (Fig. 4f). Domestic crops and animals other than millet and pigs become increasingly important in archaeological assemblages, more precisely, wheat, barley, sheep, and cattle. Against the background of shifting climatic conditions, this cultural adaptation makes sense, given the higher ecological tolerance of these crops and livestock species towards colder and drier conditions. Apart from being a reliable food source under these shifting climatic conditions, the mastery of agro-pastoral techniques, including the cultivation of barley at higher altitudes or the hybridization of cattle and wild yak or domestic sheep and wild argali to obtain offspring better adapted to higher altitudes, seems essential in order to significantly advance human exploitation in the TP above 3500 m asl. Archaeological traces bear testimony to these dynamics during the second (4.2–2.7 ka) and third phases (2.7–1 ka), though evidence for village life at altitudes above 3500 m asl remains limited, for example, at Bangga (3–1.8 ka; 3673 m asl; Lu et al., Reference Lu, Chen, Zhang, Tang, Lemoine, Wangdue, Chen, Liu and Frachetti2021) and Dingdong (2.7–2.2 ka; 4100 m asl; CTSSU et al., 2007). Rather, most high-altitude sites discovered and excavated until now represent cemeteries, such as Qugong (3.7–3 ka; 3680 m asl; IACASS and BCRTAR, 1999), Gepa Serual (3.6–2.1 ka; 3780 m asl; Hu, Reference Hu2021a), Butaxiongqu (2.7–2.4 ka; 4650 m asl; Zhang et al., Reference Zhang, Shargan, Lv and Suolang2015); and Gurujia (1.9–1.7 ka; 4300 m asl; Tong et al., Reference Tong, Li and Huang2014). Presumably, the custom of burying the deceased at higher elevations in order to strictly separate them from the living likely has deep roots in Tibetan culture, which believed in preventing evil spirits from threatening the living (Zhu, Reference Zhu1989).
Thus, despite the fact that the current archaeological record does not confirm extensive permanent inhabitation at high-altitude locations, the location of cemeteries nonetheless testifies to regular human presence in this alpine zone, possibly in the context of pastoralism. As observed in montane ecosystems worldwide, vertical transhumance involving upland pasturing in summer and feeding at lower elevation in the valleys during winter is to be expected in the TP as well. In vertical transhumance, usually relatively few people and their herds are involved, with most villagers remaining permanently in the valleys. In the SETP, the sites of Klu lding and Kha lding (Nyingchi area) provide archaeological evidence for such permanent inhabitation at an altitude below 3000 m asl. Bioarchaeological studies at these sites confirm exploitation of pigs, wheat, and sheep, with commensal rodents benefiting from feeding opportunities provided by the human niche.
During the second phase, “eastern” domesticates and crops, that is, pigs and millet, were still exploited in both low-elevation SETP (2900 m asl) and high-elevation CSTP sites (3700 m asl). However, we noted that pigs were a minor source of meat supply in the CSTP (e.g., Qugong) compared with contemporaneous SETP sites (e.g., Klu lding). At Qugong, the remains of “western” crops (wheat and barley) were almost four times (300:83) as abundant as “eastern” ones (foxtail millet and broomcorn millet) (Gao et al., Reference Gao, Yang, Ma, Tong and Yang2021). The low relative abundance of millet and pigs in the assemblages would make it difficult to compensate for losses under fluctuating climatic conditions. In parallel with this, farmers over time may have faced difficulties to cultivate millet and breed pigs at an elevation of 3700 m asl when temperatures dropped. Taken together, regional subsistence patterns characterizing the second phase illustrate an economic emphasis on “eastern” domesticates and crops in the lower SETP and on “western” foods in the higher CSTP and SWTP.
In our three-stage process, the third phase lasting 2.7–1 ka is characterized by the fact that in the archaeological record, two novel large-sized pastoral animals, that is, domestic yak and horses, gain growing visibility (Fig. 4g). By that time, farming communities living at altitudes above 3600 m asl had already shifted crop cultivation in favor of barley at the expense of the less cold-tolerant millet and wheat (d'Alpoim Guedes et al., Reference d'Alpoim Guedes, Manning and Bocinsky2016; Tang et al., Reference Tang, Lu, Spengler, Boivin, Song, Wangdue, Chen, Liu and Zhang2021). Parallel to this, pork had disappeared from the menu as well. Among the newly adopted domestic animals, yak appears locally domesticated (Qiu et al., Reference Qiu, Wang, Wang, Yang, Ma, Wang and Zhang2015), a process likely elicited by hybridization with domestic cattle adapted to montane environments (Medugorac et al., Reference Medugorac, Graf, Grohs, Rothammer, Zagdsuren, Gladyr and Zinovieva2017; Wu et al., Reference Wu, Ding, Wang, Wójcik, Zhang, Tokarska and Li2018b). Conversely, horses, the typical steppe pastoralism animals domesticated first in the Black Sea region (Librado et al., Reference Librado, Khan, Fages, Kusliy, Suchan, Tonasso-Calvière and Schiavinato2021), likely underwent considerable selection during dispersal into high-altitude environments of central and East Asia as well, including improved adaption to conditions of hypoxia (Xu et al., Reference Xu, Luosang, Hua, He, Ciren, Wang, Tong, Liang, Wang and Zheng2007; Liu et al., Reference Liu, Zhang, Li, Pan, Wang, Chen, Zheng, He, Zhao, Pu and Guan2019b). Expectedly, at this stage game did not play a significant role in human food economies anymore. That said, the third phase witnessed the emergence of high-altitude Tibetan pastoralism as we know it today, featuring mainly yaks, horses, and sheep, and the cultivation of barley. Overall, our synopsis fits the three-stage faunal exploitation pattern previously proposed by Zhang (Reference Zhang2016).
Compared with the model proposed by d'Alpoim Guedes et al. (Reference d'Alpoim Guedes, Lu, Li, Spengler, Wu and Aldenderfer2014, Reference d'Alpoim Guedes, Manning and Bocinsky2016) and Chen et al. (Reference Chen, Xu, Chen, Birks, Liu, Zhang and Jin2015b), which shows how shifts in palaeoclimate led to the changes in the crops and land use by Tibetan people, our data suggest palaeoclimate, human activities, and faunal distributions are interrelated in the STP. The cooler and drier climate characterizing the Late Holocene period and continuous exploitation of game during the late Neolithic period (5–4 ka) resulted in a decline of both forest habitat and wildlife, two factors that forced humans to adopt and engage in breeding new livestock species and crops able to better cope with cool and dry conditions. This in turn facilitated the more systematic use of higher altitudes in the STP through pastoralism. In fact, sheep and cattle had already spread to Mongolia and northeast China from the north Eurasian steppe by 5.3 ka (Cai et al., Reference Cai, Zhang, Zhu, Chen, Wang, Zhao, Ma, Royle, Zhou and Yang2018b; Wilkin et al., Reference Wilkin, Miller, Taylor, Miller, Hagan, Bleasdale and Scott2020), but they only appeared in the Yellow River and TP around 1000 years later, as we discussed earlier. The reason why there is such a long time gap before people adopted them was most likely because the agriculturalists and foragers inhabiting the Yellow River Range and TP were still well sustained by their own traditional systems during the sixth and early fifth millennium BP. Humans only started to adopt the new livestock and crops when their own systems were challenged as climate changed and ecological conditions deteriorated. In other words, climatic and ecological change paved the way for a new system exploiting different animals and plants.
The climate change, especially the significant reduction in precipitation, has demonstrably always been directly related to the southward migration of pastoralists during historic times (Bai and Kung, Reference Bai and Kung2011; Pei and Zhang, Reference Pei and Zhang2014). In the STP, we argue that Late Holocene climate change and ecological degradation triggered the adoption and/or development of the new cool-and-dry-tolerant herds and crops. Through introduction of suitable animals and crops, and in case of sheep and cattle improvement through intentional selection and breeding (e.g., hybridizing sheep and cattle with local wild argali and yak respectively), highland domestic herds adapted to the Tibetan alpine ecology and environment were developed, thus contributing to the formation of the highland pastoral system in Tibet.
From the overall data set, we also note that the transitions between the three phases occurred earlier in the NETP than in the STP—in the NETP, the first phase dates to ca. 5.6–4.3 ka and is represented by Majiayao and Zongri culture settlements (Fig. 3, yellow dots), the second phase dates to ca. 4.3–3.5 ka, and is represented by Qijia culture (Fig. 3, orange dots), and the third phase (3.5–2 ka) is represented by Xindian, Kayue, and Nuomuhong culture sites (Fig. 3, purple dots) (Dong et al., Reference Dong, Du and Wei2021; Ren et al., Reference Ren, Yang, Wang, Zhang, Chen, Cui, Wang, Liang and Dong2021). This time lag relates to the fact that sheep and cattle appeared later in the catchment area of the STP. Arguably, once they were incorporated into livestock economies, time was needed for breeding and developing populations adapted to pasturage above 3500 m asl in the STP, which was accomplished by hybridizing domestic lineages of sheep and cattle with argali and wild yak, respectively, giving rise to cold-resistant and hypoxia-tolerant Tibetan sheep, yak, and dzos.
Despite the fact that the large majority of STP sites dating to the later phases exhibit agro-pastoral features, discovery of microlithic assemblages at sites like Zhongba (occupation intervals dated to 6.6–2.6 ka and 3.4–1.3 ka) shows that in the STP, pre-pastoral lithic technology and associated modes of subsistence continued to persist for some time (Hudson et al., Reference Hudson, Olsen and Quade2014). Obviously, communities of foragers and agro-pastoralists coexisted in parts of the TP before the latter displaced the first, as observed elsewhere across the globe (Stephens et al., Reference Stephens, Fuller, Boivin, Rick, Gauthier, Kay and Marwick2019).
CONCLUSIONS AND OUTLOOK
In this study, zooarchaeological analysis and AMS 14C dating were carried out on the faunal remains from five Middle–Late Holocene sites located in the southern Tibetan Plateau (STP). Integrating our results into the existing body of faunal data, our study addresses the objectives raised at the beginning of the paper.
The spatiotemporal developments of human subsistence practices in consecutive phases of occupation can be described as a three-stage process: first, during 5.5–4 ka, permanent inhabitation was restricted to the low-altitude southeast Tibetan Plateau, and subsistence was mainly based on hunting, fowling, fishing, and gathering combined with low-level millet and pig farming. This first stage likely reflects a more sedentary way of life. Second, after ca. 3.8 ka, the use of wild resources declined, while sheep, cattle, barley, and wheat were adopted in the TP. Traces of human presence in the alpine zone above 3500 m asl increased, and in the central part of the STP and southwest TP, a heavier reliance on barley–pastoralism is noted. Third, to ca. 2.7 ka, domestic horse and yak were added, and an economy solely based on barley cultivation and pastoralism was developed. Since that time, a more mobile pastoralism–barley economy dominated the subsistence pattern in the high-elevation central part of the STP and southwest TP.
Regarding the origins and route(s) by which livestock populations were introduced, before 4 ka, domestic pigs were the only livestock in the STP, having been introduced along with millet farming in communities that originated in the Neolithic Yellow River basin. Since 3.8 ka, domestic bovines and caprines were adopted in the STP, likely through both northern and southern routes. These animals were first domesticated in the Fertile Crescent. The northern route was probably through the northern Eurasian landmass and Mongolian Plateau, and the southern route probably involved the NW Indian subcontinent and sub-Himalayan dispersal. After ca. 2.7 ka, domestic horse and yak were added to TP livestock, while pigs disappeared. Yak appears locally domesticated, and horse was domesticated in the Black Sea region and dispersed to the TP through west and central Asia.
The natural and cultural circumstances triggering highland pastoralism in the region involved increased human settlement density and continuous exploitation of wild game during the late Neolithic on the one hand, and the cooler and drier climate characterizing the Late Holocene period on the other, resulted in a decline of both forest habitat and wild game. Alongside the global dispersal of the livestock and crops first domesticated in the Fertile Crescent and Black Sea region—cattle, sheep, goats, horse, wheat, and barley, which are more ecologically tolerant to colder and drier conditions—prehistoric humans in the TP started to adopt and breed a new set of livestock and crops. Arguably, sheep, goats, cattle, and horses had undergone considerable selection during the dispersal into Tibetan highland, which probably involved the hybridization of domestic cattle and sheep lineages with local wild yak and argali, and the herds’ resilience to colder, drier, and hypoxic conditions were improved. Yak domestication was likely elicited by hybridization with domestic cattle adapted to montane environments. In turn, the mastery of the new livestock and crops facilitated highland pastoralism in the STP above 3500 m asl in the Middle–Late Holocene that has lasted up until today.
From a methodological viewpoint, our study illustrates the usefulness of morphological and morphometric approaches for identifying the medium sized bovid species inhabiting the TP (Wang, Reference Wang2017; Wang et al., Reference Wang, Peters and Barker2020b). Many archaeofaunal assemblages excavated in the TP and adjacent areas still await detailed analysis of this taxonomic group essential for our understanding of human–livestock dynamics in East Asia. In recent years, genetic and proteomic analyses have been increasingly applied to archaeofaunal remains and have been valued for their accuracy in taxonomic identification, but the critical role of morphological and morphometric approaches should not be overlooked, considering that it is the base for sorting large quantities of archaeofaunal remains and understanding past human–animal relationships. Ancient DNA analysis, proteomics, and 3D geometric morphometrics will help advance our knowledge regarding the developments outlined above, and breeding issues in particular. We thus call for a broad morphological and biomolecular approach for elucidating biological and cultural processes involving livestock exploitation at high altitudes. In addition, alignment with palaeoclimate archives proved beneficial for data interpretation in our research.
In sum, our study broadens our understanding of human–animal–environment dynamics in the prehistoric STP, supporting that the Middle–Late Holocene climatic shifts might have been one of the triggers for Tibetan human communities to adopt domestic ruminants through both cultural exchanges and local breeding. Over time, the newly developed “highland” herds and crops enabled people to use the land above 3500 m asl more systematically.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2023.6.
Acknowledgments
For collecting and analysing the faunal remains, funding was provided by Natural Science Foundation of China (42001082, 41930323), Second Tibetan Plateau Scientific Expedition and Research Program (2019QZKK0601), and China Posdoctoral Scientific Foundation (302172). For developing the methodology, the CSC Cambridge Overseas Trust (2010601248) and Wenner-Gren Dissertation Fieldwork Grants kindly provided the necessary financial support. We acknowledge the assistance of Xichao Zhu (Institute of Zoology, CAS), Songmei Hu (Shaanxi Provincial Institute of Archaeology), Yimin Yang (Department of Archaeology and Anthropology, University of Chinese Academy of Science), Hongliang Lv (Department of Archaeology, Sichuan University), Yuchao Zhao (Department of Anthropology, University of Michigan), Linyao Du (College of Earth and Environmental Sciences, Lanzhou University), Xiaokang Liu (Institute of Tibetan Plateau, CAS), Naifan Zhang (Research Center for Chinese Frontier Archaeology of Jilin University), and Ningbo Chen (College of Animal Science and Technology, Northwest A&F University) for kindly offering important information and advice. Martin Gray (author/writer Penguin Random House and Pan Macmillan) and Christian Austin helped with language checking and proofreading. Yang Yu (Nanjing University) provided necessary support in revision of the maps. Two anonymous reviewers and the editors of the journal Quaternary Research provided valuable advice that contributed significantly to the improvement of the article.










