The FAO( 1 ) defines food security as: when people, at all times, have physical, social and economic access to sufficient, nutritious food that meets their dietary needs and food preferences for an active and healthy life( 2 ). It outlines four interrelated elements for food security: availability, access, utilization and stability. Food security is an indicator of family and individual health; it can be a precursor of health and nutritional problems( Reference Behzadifar, Behzadifar and Abdi 3 ). Conversely, food insecurity is defined as a limited or uncertain access to sufficient amounts of safe and nutritious food for normal growth and healthy life( 1 ).
Currently, food security is a growing concern worldwide. The global measurement of food insecurity conducted in 2010 showed that more than 1 billion people were estimated to lack sufficient dietary energy availability, and at least twice that number suffered from micronutrient deficiencies( Reference Barrett 4 ). Food insecurity results in malnutrition, including Fe-deficiency anaemia (IDA)( 1 ). Anaemia is marked by reduced red blood cells and often comes with low blood Hb concentration. There are several forms of anaemia, including vitamin-deficiency anaemia, IDA and chronic disease anaemia. Studies show it affects all categories of countries and has negative impacts on health as well as social and economic development( Reference Stevens, Finucane and De-Regil 5 – 7 ). According to a WHO report( 8 ), anaemia affects 1·62 billion (95 % CI 1·50, 1·74 billion) people globally, representing 24·8 % (95 % CI 22·9, 26·7 %) of the world’s population. It also reports that the highest prevalence is in pre-school children (47·4 %; 95 % CI 45, 49·1 %) and the lowest prevalence is in men (12·7 %; 95 % CI 8·6, 16·9 %). Nevertheless, the population group with the greatest number of individuals affected is non-pregnant women (468·4 million, 95 % CI 446·2, 490·6 million)( 7 ). Anaemia can be caused by many factors. One study( 8 ) reported that 50 % of anaemia cases are caused by Fe deficiency. Other factors include one’s environment( Reference Stevens, Finucane and De-Regil 5 , Reference Horton and Levin 6 , Reference Stoltzfus, Mullany and Black 9 ), micronutrient deficiencies, infection, and disorders of Hb synthesis and red blood cell production( 10 , Reference Tolentino and Friedman 11 ). Kassebaum et al.( Reference Kassebaum, Jasrasaria and Naghavi 12 ) explain that surveillance for anaemia is challenging and therefore requires a simultaneous understanding of the epidemiology of its underlying causes.
A study by Metallinos-Katsaras et al.( Reference Metallinos-Katsaras, Colchamiro and Edelstein 13 ) concluded that anaemia develops among a substantial percentage of low-income WIC (Special Supplemental Nutrition Program for Woman, Infants, and Children) infants between the ages of 12 and 18 months, and low food security is a significant risk factor in developing anaemia during this time period. Among adult Mexican women, household food insecurity was associated with anaemia, and it was suggested that programmes to reduce household food insecurity may be effective at reducing the risk of anaemia among Mexican women( Reference Fischer, Shamah-Levy and Mundo-Rosas 14 ). Another study in Bangladesh suggested that household food insecurity is a significant predictor of anaemia among women of reproductive age( Reference Ghose, Tang and Yaya 15 ). Jones et al.( Reference Jones, Mundo-Rosas and Cantoral 16 ) did not find any association between household food insecurity and the co-occurrence of anaemia and obesity among female adolescents or women. In another study of children aged 6–24 months, no association was declared between household food insecurity and the occurrence of anaemia( Reference Salarkia, Neyestani and Omidvar 17 ).
The present study was undertaken to fill the gap created as a result of these inconsistent findings, by assessing the associations between food security and anaemia. It is intended that the findings of our systematic review and meta-analysis will contribute to the existing literature and help improve the health of all those at risk.
Methods
Literature search and selection
The present meta-analysis was performed in accordance with the MOOSE (Meta-Analysis of Observational Studies in Epidemiology) guidelines( Reference Stroup, Berlin and Morton 18 ). A systematic search of PubMed and MEDLINE was performed on 30 July 2017, followed by a search of EMBASE. The subsequent search was updated in August 2017. Search strategies used subject headings and keywords and did not use language and date restrictions. The following terms were used in the electronic search for the association between food insecurity and anaemia risk: (((‘Anemia’(MeSH) or ‘Anemia’(tiab) or ‘Anemias’(tiab)) OR ‘Iron Deficiency Anemia’(tiab))) AND (‘Food Supply’(MeSH) or ‘Food Supply’(tiab) or ‘Food Supplies’(tiab) or ‘Food Insecurity’(tiab) or ‘Food Insecurities’(tiab) or ‘Food Insecurities’(tiab) or ‘Food Security’(tiab) or ‘Food insufficiency’(tiab)))). The references cited in the retrieved review articles were also searched manually.
Eligibility criteria
Studies were included in the final analysis if they met the following criteria: (i) all longitudinal and cross-sectional studies which reported the association between food insecurity and anaemia risk; and (ii) studies that provided multivariable-adjusted OR with corresponding 95 % CI of food insecurity and anaemia risk. Studies were excluded if: (i) the information could not be extracted; (ii) they were reviews, case reports, conference reports or letters; (iii) they were non-English studies; (iv) they did not report the association between food insecurity and anaemia risk; and (v) they were abstracts with inadequate information and/or dissertations.
Study selection
The titles and abstracts of all articles retrieved in the initial search were evaluated independently by two reviewers. Articles not meeting the eligibility criteria were excluded using a screen form, with a hierarchical approach based on study design, population, or exposure and outcome. The reference lists of relevant review articles identified during this process were also examined to include additional studies. Full-text articles were retrieved if the citation was considered eligible and subjected to a second evaluation for relevance by the same reviewers. Any disagreements were discussed and resolved by consensus.
Data collection
For selected studies, two reviewers (S.M. and H.A.) extracted data separately using a standard data extraction form. They discussed any discrepancies in data extraction and sought the assessment of a third reviewer (Kh.M.) for resolution. Extracted information included relevant study details (name of the first author, year, database, geographical area, study design, sample size), population characteristics (age range or mean age, male/female sex), exposure (criteria for anaemia status, level of food insecurity measurement, method of food insecurity assessment, most fully adjusted OR estimate and the adjusted covariates for calculating OR), main findings and quality score.
Quality assessment for individual studies
Two reviewers (S.M. and H.A.) assessed the quality of each selected study using the Newcastle–Ottawa scale( Reference Stang 19 ). This scale awards a maximum of nine stars to each study: four stars for the adequate selection of cases and controls, two stars for comparability of cases and controls based on the design and analysis, and three stars for the adequate ascertainment of exposure in both the case and control groups. Studies of high quality were defined as those that scored the maximum nine stars on the Newcastle–Ottawa scale; studies of medium quality scored seven or eight stars.
Statistical analysis
To assess the association between food insecurity and anaemia, the most fully adjusted risk estimates for anaemia and IDA risk were pooled. To accurately examine the association between food insecurity and anaemia, the study populations were grouped based on age as follows: group 1 was children under 3 years old; group 2 was children aged 3–5 years; group 3 was 6–18-year-olds (children and adolescents); and group 4 was above 18 years of age (adult women). Pooled OR and 95 % CI were estimated using a weighted random-effect model (the DerSimonian–Laird approach). Heterogeneity among the studies was assessed by the Cochran Q and I 2 statistics (I 2=(Q–df)/Q×100 %; I 2<25 %, no heterogeneity; I 2=25–50%, moderate heterogeneity; I 2=50–75 %, large heterogeneity, I 2>75 %, extreme heterogeneity). The heterogeneity was considered significant if either the Q statistic had P<0·1 or I 2>50 %. Visual inspection of asymmetry in funnel plots, Begg’s test and Egger’s test were conducted to evaluate publication bias (P<0·05 was considered representative of statistical significance). Also, the trim-and-fill approach was used to obtain an adjusted effect size which took publication bias into account. All statistical tests for the meta-analysis were performed with the statistical software packages Stata version 14.0 and IBM SPSS Statistics version 23.0.
Results
The systematic literature search produced a total of 295 publications, after the exclusion of duplicates from the different databases. From these, 235 publications were excluded because they did not meet study eligibility criteria, leaving sixty articles for full-text assessment (Fig. 1). A total of nineteen studies met the inclusion criteria to be included in the pooled analysis( Reference Metallinos-Katsaras, Colchamiro and Edelstein 13 – Reference Jones, Mundo-Rosas and Cantoral 16 , Reference Skalicky, Meyers and Adams 20 – Reference Weigel, Armijos and Hall 34 ). Among these nineteen studies, seventeen used a cross-sectional approach( Reference Fischer, Shamah-Levy and Mundo-Rosas 14 , Reference Ghose, Tang and Yaya 15 , Reference Jones, Mundo-Rosas and Cantoral 16 , Reference Skalicky, Meyers and Adams 20 – Reference Campbell, Akhter and Sun 24 , Reference Lisbôa, Oliveira and Lamounier 26 – Reference Weigel, Armijos and Hall 34 ), whereas the other two were longitudinal studies( Reference Metallinos-Katsaras, Colchamiro and Edelstein 13 , Reference Pirkle, Lucas and Dallaire 25 ). The risk ratios of 95993 individuals in these studies were pooled for the meta-analysis. These studies were published between 2006 and 2017, and performed in the USA( Reference Metallinos-Katsaras, Colchamiro and Edelstein 13 , Reference Skalicky, Meyers and Adams 20 – Reference Park, Kersey and Geppert 22 , Reference Weigel, Armijos and Hall 34 ), Brazil( Reference Lisbôa, Oliveira and Lamounier 26 , Reference Gubert, Spaniol and Bortolini 29 , Reference Demétrio, de Souza Teles-Santos and dos Santos 33 ), Mexico( Reference Fischer, Shamah-Levy and Mundo-Rosas 14 , Reference Jones, Mundo-Rosas and Cantoral 16 ), China( Reference Shen, Gao and Tang 28 ), India( Reference Nair, Fernandez-Rao and Nagalla 31 ), Nepal( Reference Osei, Pandey and Spiro 23 ), Indonesia( Reference Campbell, Akhter and Sun 24 ), Canada( Reference Pirkle, Lucas and Dallaire 25 ), Vietnam( Reference Nguyen, Gonzalez-Casanova and Nguyen 27 ), Ecuador( Reference Weigel, Armijos and Hall 34 ), Bangladesh( Reference Ghose, Tang and Yaya 15 ) and Pakistan( Reference Habib, Black and Soofi 30 ). Table 1 summarizes the characteristics of the studies included.
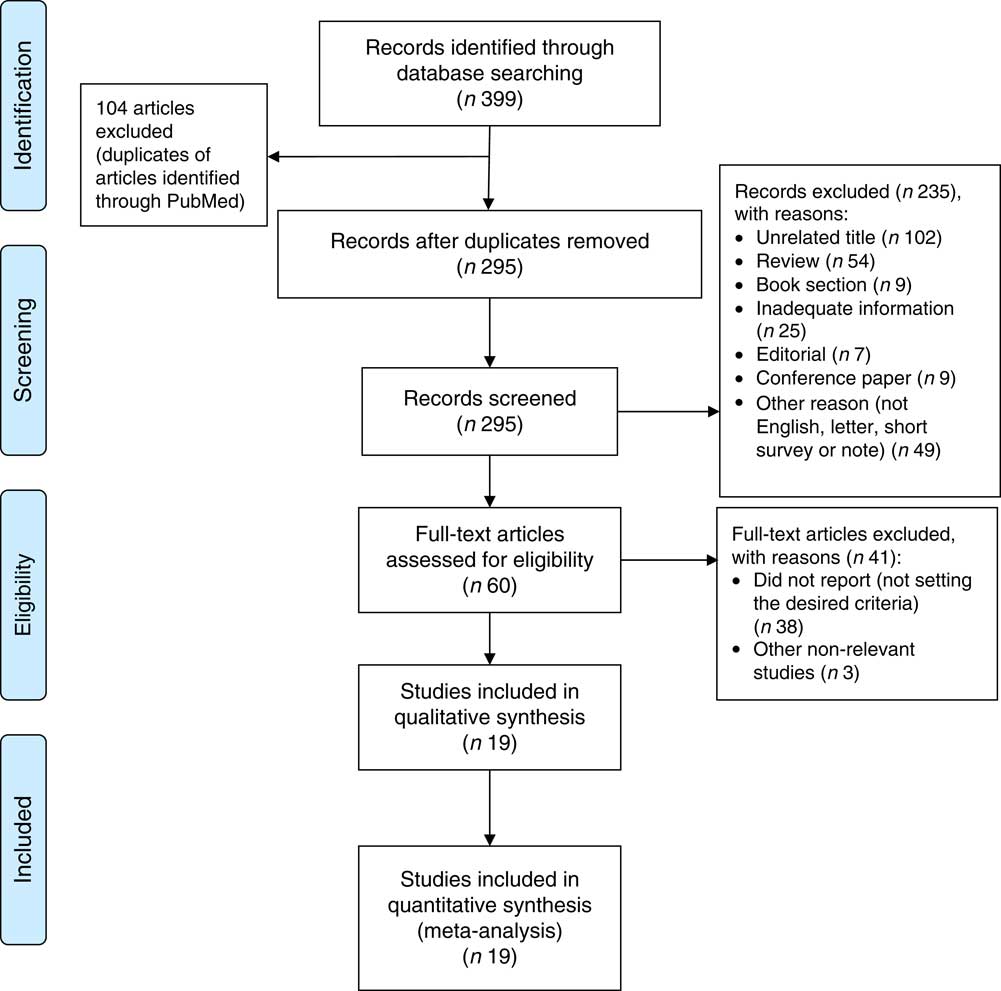
Fig. 1 (colour online) PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart( Reference Moher, Liberati and Tetzlaff 58 ) describing the systematic literature search and selection of studies on food insecurity and anaemia risk
Table 1 Description of the studies included in the present meta-analysis of the association of food insecurity with anaemia risk
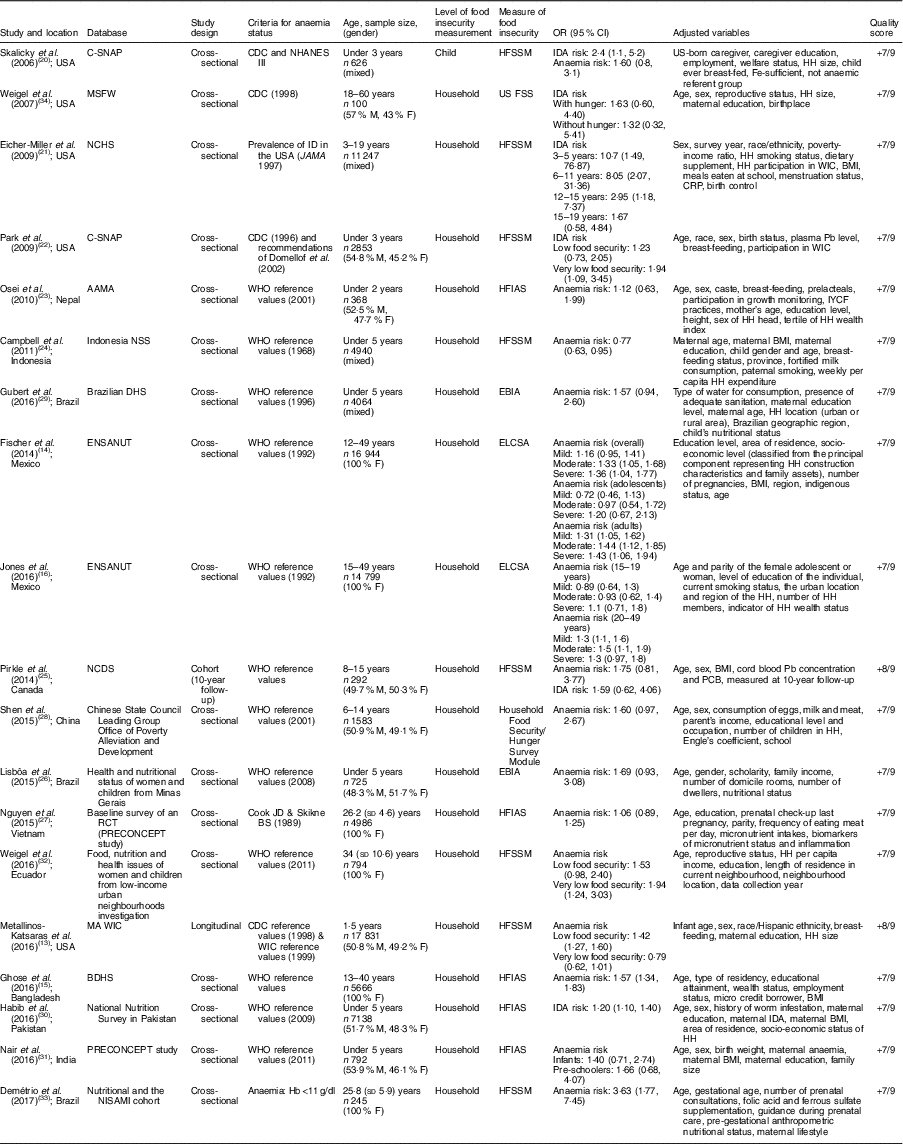
C-SNAP, Children’s Sentinel Nutrition Assessment Program; MSFW, Migrant and Seasonal Farmworker; NCHS, National Center for Health Statistics; AAMA, Action Against Malnutrition through Agriculture; NNS, Nutritional Surveillance System; DHS, Demographic and Health Survey; ENSANUT, Mexican National Health and Nutrition Survey; NCDS, Nunavik Child Development Study; RCT, randomized cotrolled trial; BDHS, Bangladesh Demographic and Health Survey; NISAMI, Nutritional and Genetic Risk Factors during Gestation Associated to Low Birth Weight/Prematurity; CDC, Centers for Disease Control and Prevention; NHANES III, Third National Health and Nutrition Examination Survey; ID, Fe deficiency; WIC, Special Supplemental Nutrition Program for Woman, Infants, and Children; M, male; F, female; HFSSM, Household Food Security Survey Module; US FSS, US Food Security Survey module; HFIAS, Household Food Insecurity Access Scale; EBIA: Escala Brasileira de Insegurança Alimentar; ELCSA, Latin American and Caribbean Food Security Scale; IDA, Fe-deficiency anaemia; HH, household; CRP, C-reactive protein; IYCF, infant and young child feeding; PCB, polychlorinated biphenyls.
Quantitative synthesis
The study-specific maximally adjusted OR results were pooled to examine the association between food insecurity and anaemia risk. As shown in Fig. 2, there was a significant overall association between food insecurity and risk of anaemia (OR=1·27; 95 % CI 1·13, 1·40) when all OR were combined with the random-effects model. Heterogeneity existed among the studies (P<0·000, I 2=66·1 %). Moreover, the results showed that food insecurity increased the risk of IDA (OR=1·45; 95 % CI 1·04, 1·86; Fig. 3). The results, stratified by categorized food insecurity, are shown in Fig. 4. These results revealed that food insecurity at two levels, namely mild food insecurity (OR=1·15; 95 % CI 1·00, 1·31) and moderate food insecurity (OR=1·36; 95 % CI 1·23, 1·48), increased the risk of anaemia, but severe food insecurity did not (OR=1·27; 95 % CI 0·89, 1·64; Fig. 4).
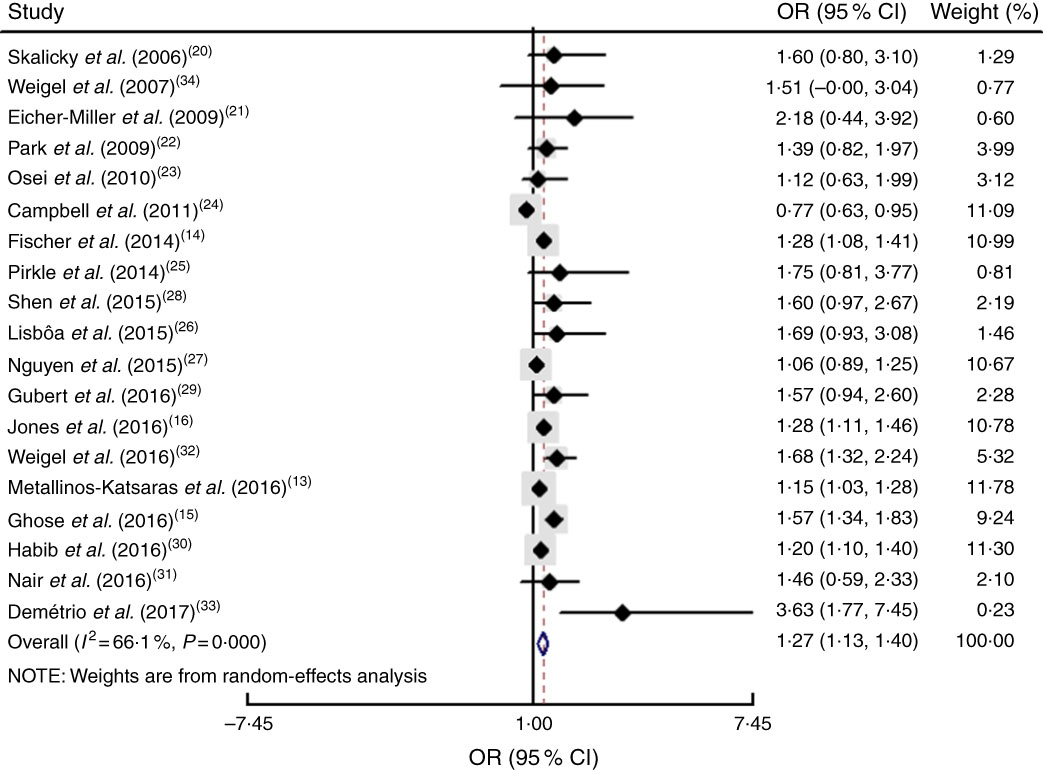
Fig. 2 (colour online) Forest plot showing the pooled OR and 95 % CI of the association of food insecurity with anaemia risk in all included studies. The study-specific OR and 95 % CI are represented by the black diamond and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled OR, and its width represents the pooled 95 % CI
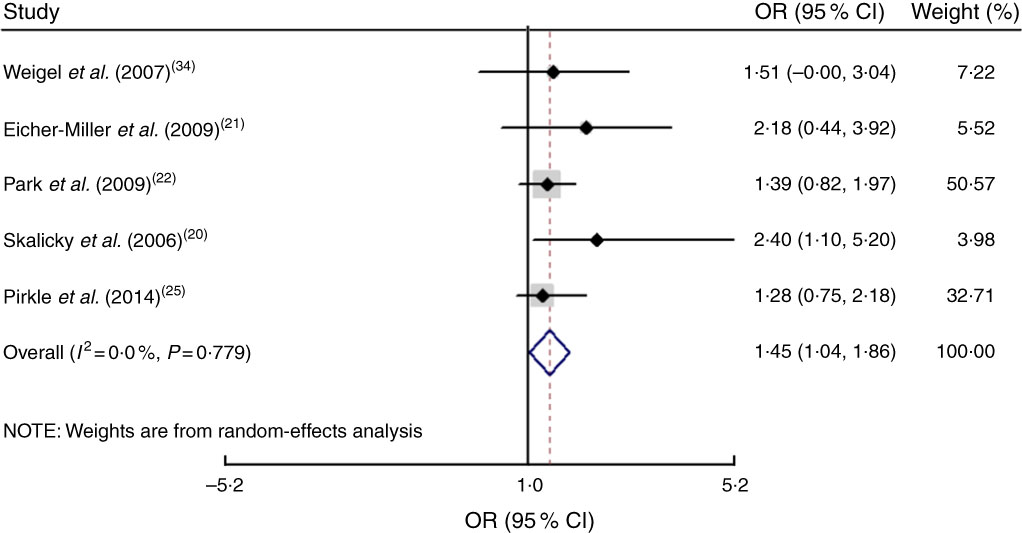
Fig. 3 (colour online) Forest plots showing the pooled OR and 95 % CI of the association of food insecurity with iron-deficiency anaemia risk. The study-specific OR and 95 % CI are represented by the black diamond and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled OR, and its width represents the pooled 95 % CI
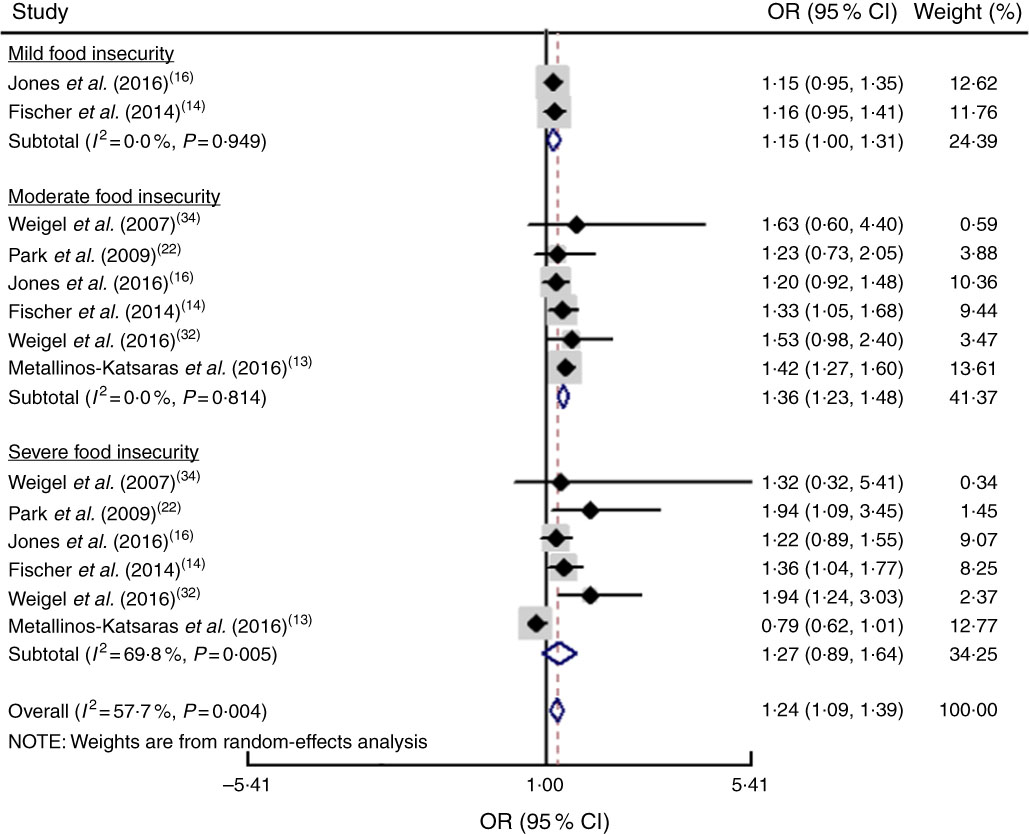
Fig. 4 (colour online) Forest plot showing the pooled OR and 95 % CI of the association of specific levels of food insecurity with anaemia risk. The study-specific OR and 95 % CI are represented by the black diamond and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled OR, and its width represents the pooled 95 % CI
To accurately examine the association between food insecurity and anaemia risk, the study populations were grouped based on age as explained above. Subgroup analysis indicated that age had a significant impact on the association between food insecurity and anaemia risk (OR=1·22; 95 % CI 1·09, 1·36). As shown in Fig. 5, food insecurity significantly increased the risk of anaemia among children under 3 years (OR=1·17; 95 % CI 1·05, 1·29) and among adult women (OR = 1·35; 95 % CI 1·16, 1·54). However, there was no significant relationship between food insecurity and risk of anaemia among 3–5-year-old children (OR=1·15; 95 % CI 0·79, 1·51) or 6–18-year-old children and adolescents (OR=1·08; 95 % CI 0·71, 1·44).
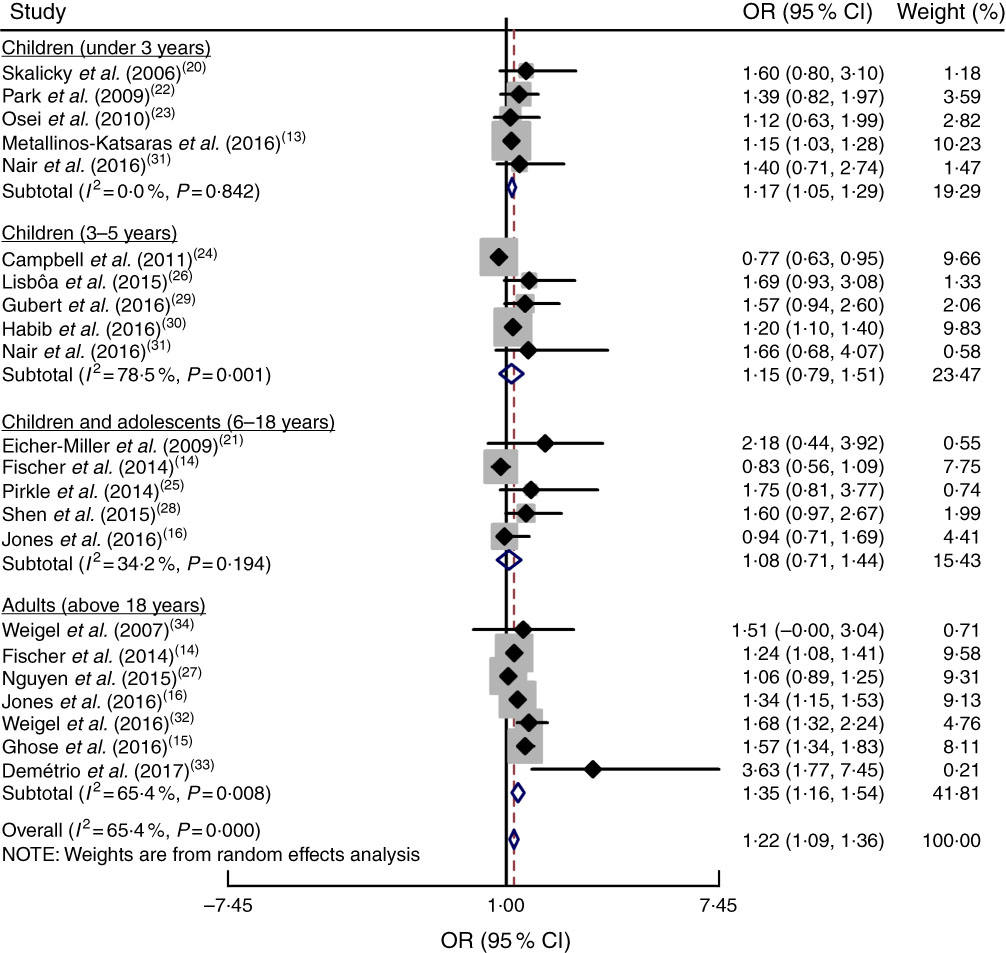
Fig. 5 (colour online) Forest plot showing the pooled OR and 95 % CI of the association of food insecurity with anaemia risk according to age subgroup. The study-specific OR and 95 % CI are represented by the black diamond and horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled OR, and its width represents the pooled 95 % CI
Sensitivity analysis
Sensitivity analysis was performed by omitting each of the included studies. The results showed that the OR was not significantly altered by omitting any of the studies. This indicated that the meta-analysis results were stable and not sensitive to any one of the nineteen studies (Fig. 6).
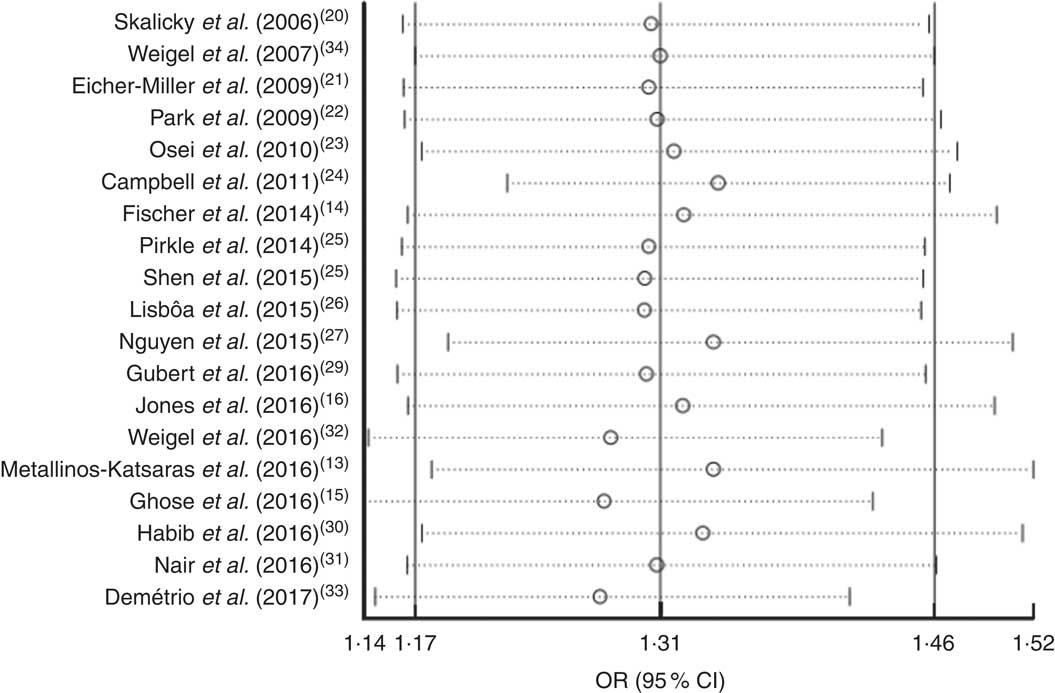
Fig. 6 Forest plot showing the pooled OR and 95 % CI of the association of food insecurity with anaemia risk from sensitivity analyses in which the given named study is omitted. Meta-analysis estimates of the pooled OR and 95 % CI with the given named study omitted are represented by the open circle and the dotted horizontal line, respectively; the solid vertical lines (from left to right) represent the lower 95 % CI, the pooled OR and the upper 95 % CI for all included studies
Publication bias
The funnel plot for anaemia risk was slightly skewed to the right (Fig. 7), indicating that smaller studies with negative results or reverse associations might not have been published (Fig. 8). This notwithstanding, no evidence of publication bias among studies related to food insecurity and anaemia risk was observed according to the result of Begg’s (P=0·10) and Egger’s (P=0·10) linear regression tests.
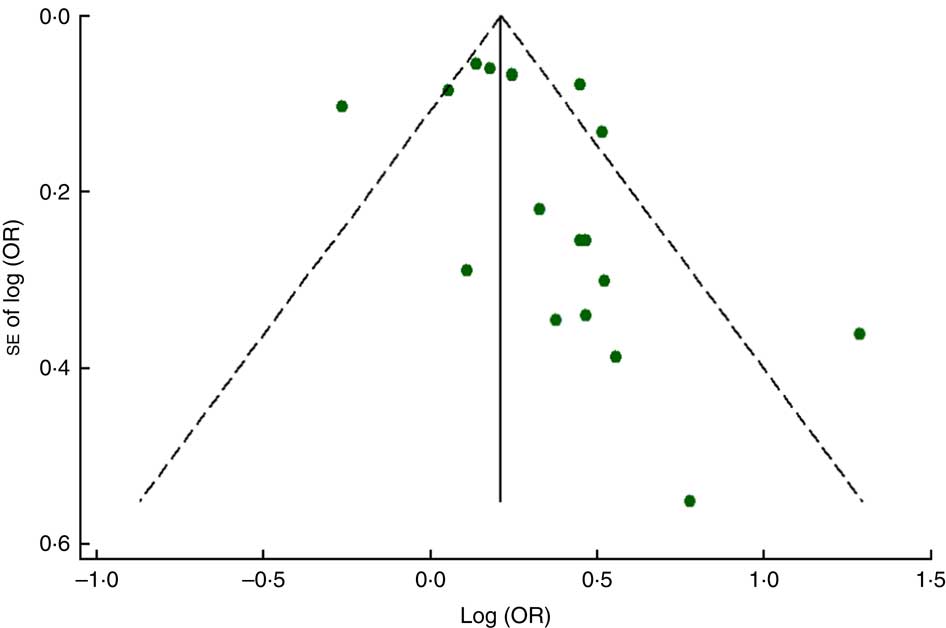
Fig. 7 (colour online) Funnel plot with pseudo 95 % CI (– – –) for evaluation of publication bias in studies (![]() ) included in the meta-analysis of the association of food insecurity with anaemia risk
) included in the meta-analysis of the association of food insecurity with anaemia risk
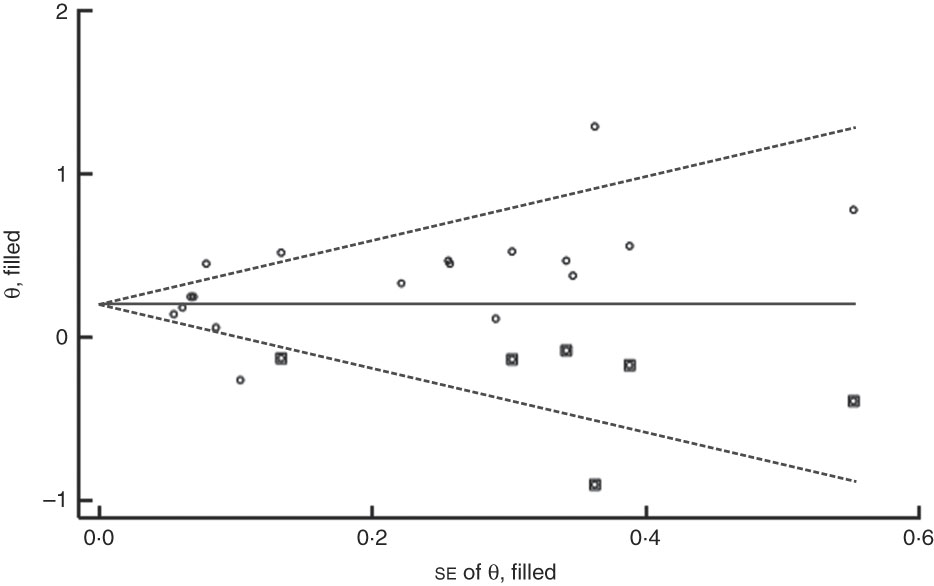
Fig. 8 Filled funnel plot with pseudo 95 % CI (– – –) accounting for publication bias in studies (○, included studies; ◘, filled studies) included in the meta-analysis of the association of food insecurity with anaemia risk
Discussion
It is estimated that more than 1 billion people around the world are food insecure( Reference Barrett 4 ). Food-insecure individuals have an increased risk of: overweight/obesity( Reference Holben and Taylor 35 , Reference Casey, Simpson and Gossett 36 ); underweight( Reference Casey, Simpson and Gossett 36 ); developmental risks( Reference Cook, Frank and Casey 37 ); behavioural problems( Reference Whitaker, Phillips and Orzol 38 ); depression/anxiety and hyperactivity/inattention( Reference Melchior, Chastang and Falissard 39 ); diabetes mellitus( Reference Marjerrison, Cummings and Glanville 40 ); and anaemia( Reference Jones, Mundo-Rosas and Cantoral 16 , Reference Skalicky, Meyers and Adams 20 , Reference Park, Kersey and Geppert 22 , Reference Osei, Pandey and Spiro 23 ). Currently, anaemia is considered the most common nutritional problem in the world( Reference Rakesh 41 ). Food insecurity, through the causation of anaemia( Reference Metallinos-Katsaras, Colchamiro and Edelstein 13 , Reference Eicher-Miller, Mason and Weaver 21 , Reference Habib, Black and Soofi 30 ), can lead to poor scholastic performance and cognitive impairment( Reference Rakesh 41 ) and increase susceptibility to infection, morbidity and mortality in children( Reference Jonker, Te Poel and Bates 42 ). Also, maternal anaemia can lead to poor pregnancy outcomes, including low infant birth weight( Reference Rahmati, Delpishe and Azami 43 ), preterm birth, perinatal mortality and neonatal mortality( Reference Rahman, Abe and Rahman 44 ). Moreover, IDA increases the risk of thyroid dysfunction( Reference Morchiladze, Tkeshelashvili and Gagua 45 , Reference Li, Gao and Wei 46 ) and reduces choroid thickness( Reference Yumusak, Ciftci and Yalcin 47 ). Therefore, because of the important role of anaemia in health, the present study undertook a systematic review and meta-analysis of the literature, providing quantitative estimates of the association between food insecurity and the risk of anaemia in population-based studies for the first time.
The present results agree with previous studies which showed that there was an overall positive relationship between food insecurity and anaemia risk( Reference Fischer, Shamah-Levy and Mundo-Rosas 14 , Reference Ghose, Tang and Yaya 15 , Reference Eicher-Miller, Mason and Weaver 21 , Reference Demétrio, de Souza Teles-Santos and dos Santos 33 ). Similar results were observed for IDA( Reference Skalicky, Meyers and Adams 20 , Reference Habib, Black and Soofi 30 ). Previous studies had reported that deficiencies of several nutrients could link food insecurity and increase anaemia risk, namely Fe( Reference Karp, Kersey and Cutts 48 ), folate, vitamin B12, vitamin A( Reference Nguyen, Gonzalez-Casanova and Nguyen 27 ) and vitamin D( Reference Lucisano, Di Mauro and Montalto 49 , Reference Tse, Weiler and Kovesi 50 ). Furthermore, previous studies had also separated food insecurity into different levels in order to accurately examine the association between food insecurity and risk of anaemia( Reference Metallinos-Katsaras, Colchamiro and Edelstein 13 , Reference Fischer, Shamah-Levy and Mundo-Rosas 14 , Reference Jones, Mundo-Rosas and Cantoral 16 , Reference Weigel, Armijos and Hall 34 ).
Another important finding revealed that food insecurity levels, including mild food insecurity and moderate food insecurity, increased the risk of anaemia. However, severe food insecurity did not show any association with anaemia risk. These results may be because participants with mild and moderate food insecurity, in addition to having inadequate intakes of essential micronutrients, have greater access to and intakes of dietary components that inhibit mineral absorption, contributing to anaemia( Reference Jones, Mundo-Rosas and Cantoral 16 ). The existence of different methods and assumptions in categorizing food insecurity status can alter the classification of food-insecure households and thus may potentially explain the lack of association between very severe food insecurity and anaemia risk( Reference Maxwell, Coates and Vaitla 51 ). Also, the low number of subjects in the severe food insecurity category in some studies may have led to its wide confidence interval, which could have affected the overall results( Reference du Prel, Hommel and Röhrig 52 ). For example, just three patients were in the severe food insecurity category of Weigel et al.’s34 study. In total, due to the different cut-off points for the classification of food insecurity, and the very low number of subjects in the higher category of food insecurity in some studies, this part of the results should be interpreted with caution.
In addition, it was found that age has an impact on the association between food insecurity and anaemia risk. The results of the age subgroup analysis suggested that food insecurity in children under 3 years of age increases the risk of anaemia. Infants and toddlers are at especially high risk of Fe deficiency, because of their rapid growth rates and common inadequate intakes of dietary Fe, and increased risk of IDA( Reference Park, Kersey and Geppert 22 ). Moreover, certain feeding behaviours in infants and toddlers are associated with higher rates of IDA in children; these include prolonged bottle-feeding and excessive intake of milk, both of which have been found to displace Fe-rich foods and reduce the bioavailability of Fe from complementary foods( Reference Metallinos-Katsaras, Colchamiro and Edelstein 13 ). Also, extended breast-feeding could result in lower consumption of recommended minimum dietary diversity, meal frequency or Fe-rich foods( Reference Hipgrave, Fu and Zhou 53 ).
However, in other children and adolescents, food insecurity did not show any effect on anaemia risk. It is possible that mothers provide full meals for their children by cutting their own intake( Reference Speirs, Fiese and Team 54 ). Children may also have access to higher-quality food than their mothers or households( Reference Speirs, Fiese and Team 54 ). Therefore, maternal care and support for children and adolescents can be considered an important factor for the prevention of anaemia in food-insecure households. In addition, studies regarding the prevalence of anaemia among school-aged children and adolescents indicate that anaemia appears considerably less for this age group than in younger children, which may be due to a lower prevalence of Fe deficiency and infection( Reference Leenstra, Kariuki and Kurtis 55 , Reference Lwambo, Brooker and Siza 56 ). Hence, the null association between food insecurity and anaemia among school-aged children and adolescents could be explained by the small number of subjects with anaemia in this age group.
The findings among adult women indicated that food insecurity increases their risk of anaemia. Food insecurity can lead to anaemia in adult women through inadequate consumption of Fe, insufficient consumption of micronutrients that facilitate the bioavailability of Fe (e.g. vitamin A vitamin C, folate and carotenoids) and the consumption of foods rich in phytic acid, which may decrease the absorption of Fe. Moreover, adult women need double the Fe intake of men due to their menstrual cycles and pregnancy, which may make them susceptible to higher anaemia risk during food insecurity( Reference Ghose, Tang and Yaya 15 , Reference Jones, Mundo-Rosas and Cantoral 16 , Reference Demétrio, de Souza Teles-Santos and dos Santos 33 ).
Limitations
Some limitations of our meta-analysis should be discussed. First, significant statistical heterogeneity was observed in the overall comparisons as well as some subgroup analyses, although various subgroup and sensitivity analyses were used. Second, despite the existence of several studies related to the associations between food security and anaemia, small numbers of studies reported IDA. Third, one article reported mixed subgroups (men and women) in terms of anaemia risk( Reference Weigel, Armijos and Hall 34 ). However, the other studies reported anaemia risk for women separately. Lastly, the studies included used different tools for assessing food insecurity, notably the HFIAS (Household Food Insecurity Access Scale) and the HFSSM (Household Food Security Survey Module). Although the different measures of food insecurity in studies have validity and reliability, the comparability of these measures is an important gap that should be addressed. It has been documented that there are generally quite strong correlations (P<0·01) between different measures of food insecurity, which suggests that they all represent certain dimensions of food insecurity( Reference Maxwell, Coates and Vaitla 51 ).
Conclusion
In conclusion, the present meta-analysis suggested that there is an overall positive relationship of food insecurity with anaemia and IDA risk. Furthermore, it was found that age has an impact on the association between food insecurity and anaemia risk. In summary, food insecurity among infants, toddlers and adult women increases their risk of anaemia. However, the analysis of the evidence of the selected studies implied that food insecurity is not associated with anaemia risk in children and adolescents. These results showed that essential nutrients and fortified foods (especially with Fe) are highly important for food-insecure households with infants, toddlers and adult female members. Also, implementing strategies to reduce the risk of anaemia, especially in food-insecure regions, through improving the bioavailability of Fe from complementary foods and following dietary guidelines, as well as improving infant and young child feeding practices( Reference Balarajan, Ramakrishnan and Özaltin 57 ), should be integrated into poverty alleviation programmes. Further cohort studies with longer follow-up periods are required to support the possible relationship between food insecurity and anaemia risk.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: The authors declare no potential competing interests. Authorship: S.M. and Kh.M. designed the research; S.M., H.A. and Kh.M. conducted the research; Kh.M. and S.M. performed the statistical analysis; S.M., Kh.M. and A.I. wrote the paper; Kh.M. had primary responsibility for the final content. All authors read and approved the final manuscript. Ethics of human subject participation: Not applicable.













