Introduction
Mood fluctuates throughout the day as well as the lifespan, though overall most individuals feel ‘fine’ most of the time (Taquet et al. Reference Taquet, Quoidbach, de Montjoye, Desseilles and Gross2016). At one time or another, however, virtually everyone will experience low mood that is significant enough to endorse one or more symptoms of depression. In 24% of the cases, this will be severe enough to meet diagnostic criteria for major depressive disorder (Kessler et al. Reference Kessler, Berglund, Borges, Nock and Wang2005; Kessler & Bromet, Reference Kessler and Bromet2013). Much needed research has been dedicated to the affective, cognitive and neurobiological correlates of these major depressive episodes (Davidson et al. Reference Davidson, Pizzagalli, Nitschke and Putnam2002; Gotlib & Joormann, Reference Gotlib and Joormann2010; Menon, Reference Menon2011). Less however is known about the cognitive effects of depressive symptoms within the subclinical range commonly experienced by the general population. Here we explore the impact of depressive symptoms on memory, as experienced by a population cohort of over 2500 adults that was specifically selected for being currently free of neuropsychiatric disorders (the Cambridge Centre for Ageing and Neuroscience (Cam-CAN) cohort; http://www.cam-can.org). Importantly, these individuals were tested on a range of memory measures including subjective memory complaints, performance on a standardized measure of memory, and performance (in a subset of the cohort) on a task specifically designed to assess memory in affective contexts.
The findings from the clinical literature suggest the ability to recall relevant information over time is reduced in individuals who experience depression (Burt et al. Reference Burt, Zembar and Niederehe1995; Rock et al. Reference Rock, Roiser, Riedel and Blackwell2014). One possible neurobiological mechanism for these memory problems is prolonged exposure to elevated levels of corticosteroids, owing to the heightened psychological stress experienced in a depressive episode (Lamers et al. Reference Lamers, Vogelzangs, Merikangas, De Jonge, Beekman and Penninx2013; Baumeister et al. Reference Baumeister, Lightman and Pariante2014; Hammen, Reference Hammen2015). The animal literature shows robust, well-replicated associations between stress exposure, levels of corticosteroids and memory performance, specifically through the neurodegenerative effect of corticosteroids on the hippocampus (for a review see: Kim & Diamond, Reference Kim and Diamond2002), which is critical to the consolidation of information into long-term memory (Bird & Burgess, Reference Bird and Burgess2008). Studies in human depression yield more equivocal results, though evidence generally supports the theory of volumetric shrinkage of the hippocampal complex in individuals suffering from depression (MacQueen & Frodl, Reference MacQueen and Frodl2011; Fried & Kievit, Reference Fried and Kievit2015). However, the field faces methodological challenges, some of which are inherent to the population under investigation. For example, comparing brain abnormalities across studies entails comparing across subtypes of depression, different levels of chronicity, environmental factors and variations in exposure to psychotropic medication (Fried et al. Reference Fried, Tuerlinckx and Borsboom2014). Moreover, little is known about how the effects of clinical depression on memory relate to the effects of (subclinical) symptoms of depression experienced in the general population. Building on the clinical literature, the present study aims to address this gap. We predicted that symptoms of depression experienced in the general population would be related to memory impairments (as assessed by a standardized measure of memory), and that this relationship would be associated with smaller hippocampal volumes.
The association between depressive symptoms and subjective memory complaints has been the focus of considerable previous research, especially in late adulthood (Jorm et al. Reference Jorm, Christensen, Korten, Jacomb and Henderson2001; Minett et al. Reference Minett, Da Silva, Ortiz and Bertolucci2008; Kim et al. Reference Kim, Seo, Kim, Kim, Lee, Qiu and Na2013; Crumley et al. Reference Crumley, Stetler and Horhota2014; Yates et al. Reference Yates, Clare, Woods and Matthews2015). Research into the predictive utility of subjective memory complaints for objective memory performance and dementia diagnoses in older adults has revealed that subjective memory complaints may be better accounted for by individuals’ levels of depressive symptomatology than their actual memory performance (e.g. Schofield et al. Reference Schofield, Marder, Dooneief, Jacobs, Sano and Stern1997; Reid & MacLullich, Reference Reid and MacLullich2006; Hülür et al. Reference Hülür, Hertzog, Pearman, Ram and Gerstorf2014; Yates et al. Reference Yates, Clare and Woods2017). In line with these findings, we predicted that subjective memory complaints would increase as a function of symptoms of depression. Negative interpretative biases observed in those with clinical levels of depression, which increase as a function of symptoms of depression in non-clinical populations, may partially account for this finding (Mathews & MacLeod, Reference Mathews and MacLeod2005; Beck, Reference Beck2008). Alternatively the association between self-reported symptoms of depression and self-reported memory problems may simply reflect a response tendency on measures assessing neuropsychiatric health complaints. The current sample allowed a direct test of the latter hypothesis, by using the Hospital Anxiety and Depression Scale (HADS; Zigmond & Snaith, Reference Zigmond and Snaith1983) to assess symptoms of depression. The two subscales of the HADS assess symptoms of anxiety and depression, respectively. If increased memory complaints reflect a simple response tendency, then symptoms of depression and anxiety should show the same association with subjective memory complaints. In contrast, if the increase in self-reported memory complaints is specific to depressive symptomatology, then subjective memory complaints should be more reliably associated with symptoms of depression than symptoms of anxiety.
Previous work additionally suggests that altered cognitive and affective processing in depression are associated with other changes in memory performance. For example, depressed individuals exhibit a mood-congruency bias, which makes them able to recall more negative memoranda compared with non-depressed individuals (Elliott et al. Reference Elliott, Rubinsztein, Sahakian and Dolan2002). Another memory phenomenon observed in individuals with depression is that their autobiographical memories lack specificity (i.e. depressed individuals memories are typically overgeneral; Dalgleish & Werner-Seidler, Reference Dalgleish and Werner-Seidler2014; Dritschel et al. Reference Dritschel, Beltsos and McClintock2014). These deviations from typical memory performance suggest an abnormality in basic memory operation and/or in the processing of affective information. Research on memory for affective stimuli and events more broadly shows that compared with neutral, affective information is better remembered (LaBar & Cabeza, Reference LaBar and Cabeza2006). What remains under-researched is the effect of affective context on memory performance. Henson et al. (Reference Henson, Campbell, Davis, Taylor, Emery, Erzinclioglu and Kievit2016) have recently shown that, in the same Cam-CAN cohort as is studied here, recognition memory for neutral objects varied as a function of the affective valence (negative, positive or neutral) of the background context against which those objects were originally presented. The increased affective significance (cf. Pessoa, Reference Pessoa2009) of negative information to individuals who currently experience symptoms of depression is likely to attract attentional resources towards negative backgrounds and away from neutral objects superimposed on those backgrounds, thereby impairing the encoding into memory of those objects. For this reason, memory for information presented in affective contexts may be more sensitive to the influence of subclinical depressive symptoms than the more commonly used, affect-neutral measures of memory.
In summary, the present study investigated the hypotheses that depressive symptoms are related to more subjective memory complaints (Hypothesis 1a) and worse objective memory performance (Hypothesis 1b). This first pair of hypotheses was investigated in all individuals from the Cam-CAN cohort who completed all measures of interest during an interview assessment in participants’ homes (N = 2544). The study further explored whether the relationship between memory performance and depressive symptoms is related to reductions in hippocampal volumes (Hypothesis 2). This was investigated in a subsample (n = 592) for whom volumetric data of the hippocampus were available from a more extensive neurocognitive assessment including the acquisition of T1- and T2-weighted MRI scans. The third prediction was that self-reported symptoms of depression would be more strongly related to a measure of memory in negative contexts compared with a standard measure of memory (Hypothesis 3). This hypothesis was investigated in a second subsample (n = 288) that completed a more specialized memory task.
The nature of the study's sample also allowed for a number of additional explorations: first, as outlined above, we tested whether H1a was specific to symptoms of depression. That is, whether the relationship between depressive symptoms and self-reported memory complaints reflected a general response tendency towards reporting more neuropsychiatric complaints and would therefore show the same relationship with symptoms of anxiety. Next, given the evidence suggesting that memory problems related to depressive symptoms may be a function of general impairments in cognitive ability observed in individuals experiencing symptoms of depression (Fossati et al. Reference Fossati, Coyette, Ergis and Allilaire2002; Elderkin-Thompson et al. Reference Elderkin-Thompson, Mintz, Haroon, Lavretsky and Kumar2007) the study investigated whether symptoms of depression remained significantly related to the various types of memory after controlling for general cognitive ability. Third, the study explored whether the relationships in hypotheses 1–3 would remain after accounting for variations in memory, hippocampal volume and depressive symptoms attributable to age (Jeste et al. Reference Jeste, Savla, Thompson, Vahia, Glorioso, Martin, Palmer, Rock, Golshan, Kraemer and Depp2013; Sutin et al. Reference Sutin, Terracciano, Milaneschi, An, Ferrucci and Zonderman2013; Schaakxs et al. Reference Schaakxs, Comijs, Lamers, Beekman and Penninx2017). And finally, because women tend to show better verbal recall performance and more symptoms of depression, we investigated whether sex differences contribute to the relationships predicted in hypotheses 1–3 (Piccinelli & Wilkinson, Reference Piccinelli and Wilkinson2000; Andreano & Cahill, Reference Andreano and Cahill2009).
Methods
Participants
The full sample included 2544 individuals from the CC3000 Cam-CAN sample (Shafto et al. Reference Shafto, Tyler, Dixon, Taylor, Rowe, Cusack, Calder, Marslen-Wilson, Duncan, Dalgleish, Henson, Baryne and Matthews2014). These participants (95% of the total Cam-CAN sample) were included because they had completed all measures pertaining to our first hypothesis. Structural imaging data was available for 592 participants from our overall sample. Hypothesis 2 was tested on this subsample. Finally, 288 participants from the total sample completed the valenced memory task and were included in the investigation of our third hypothesis. See online Supplementary Table S1 for participant characteristics.
Measures
Depressive symptoms
Symptoms of depression were assessed with the depression subscale of the HADS (Zigmond & Snaith, Reference Zigmond and Snaith1983). The subscale consists of seven items for which participants indicate how frequently they have felt them over the past week on a scale form ‘0’ = ‘Not at all’ to ‘3’ = ‘Most of the time’. The scales have been well validated for use in the general population (Olssøn et al. Reference Olssøn, Mykletun and Dahl2005). In the current sample Cronbach's α was acceptable–good, а = 0.79 and similarly McDonalds’ Ω hierarchical was 0.71.
Objective memory
Objective memory was assessed with a standard measure of memory, the delayed recall of a story taken from the Wechsler Memory Scale Third UK edition (Wechsler, Reference Wechsler1997).
Subjective memory
Participants were simply asked whether they experienced any memory problems or not: ‘Do you feel you have problems with your memory? Yes/No.’.
Valenced memory
Memory for objects in positively and negatively valenced as well as valence neutral contexts was assessed with a newly designed measure (Henson et al. Reference Henson, Campbell, Davis, Taylor, Emery, Erzinclioglu and Kievit2016). The task consisted of a study and a test phase. The study phase was divided into two 10 min blocks with a short break in between. In each block participants were presented with 60 background images selected from the International Affective Picture System (Lang et al. Reference Lang, Bradley and Cuthbert2008) that appeared on the screen for 2 s before an object was superimposed on the background image. The object and background stayed on the screen for 7.5 s. Participants were asked to press the button as soon as they had come up with a story to help them link the object and background together. They were asked to keep elaborating on that story until the object and background disappeared. There was a 0.5 interval second before the next trial started.
Participants were advised that some images would be pleasant and others unpleasant, but they were not informed that their memory for the items and their background would be tested later. After participants completed the second block they were given a 10 min break before the test phase. In the test phase they saw 160 trials that were split into four 20 min blocks. The trials included 120 studied objects and 40 new objects. Each test trial first presented participants with a masked (pixel noise) picture of an object and participants had to name the object or respond ‘I don't know’ before pressing the key to reveal the object. Memory for the object was then tested by asking participants whether or not the object had been presented in the study phase. Participants then indicated how confident they were of their response by pressing one of four keys: ‘sure new’, ‘think new’, ‘sure studied’ or ‘think studied’. For trials on which participants indicated ‘studied sure’ or ‘studied think’ their associative memory was tested. That is, participants were asked to say out loud whether the object had been presented over a positive, neutral or negative background or to respond ‘I don't know’, if they could not remember the valence of the background. Finally, they were asked to describe the background image. The priming, associative memory and qualitative data are not reported as part of this study.
Memory accuracy was computed as the d′ measure of discriminability (Green & Swets, Reference Green and Swets1966): d′ = Φ−1 (pH) − Φ−1 (pFA). The pH denotes the proportion of hits, pFA the proportion of false alarms and Φ−1 the inverse cumulative distribution function of the Normal distribution (d′ = 0 for chance performance; extreme values of 0 or 1 for d′ were adjusted using a log-linear approach).
General cognitive functioning
In the overall cohort, cognitive ability was assessed with the Addenbrooke's Cognitive Examination – Revised assessment (ACE-R; Mioshi et al. Reference Mioshi, Dawson, Mitchell, Arnold and Hodges2006). The screening measure was devised to detect signs of dementia and cognitive impairment assessing five domains of cognitive functioning: orientation/attention, memory, verbal fluency, language and visuospatial ability. The memory domain assess both immediate and delayed recall. As with our assessment of objective memory, participants had to recall verbal information after a delay interval. We therefore used the ACE-R sum score of all domains except memory. For the neuroimaging and affective subsamples, the ACE-R was not an informative test of cognitive ability due to participants scoring at ceiling. We therefore included the Cattell's culture-free test of intelligence (Cattell, Reference Cattell1971), which was available in both subsamples (not the overall cohort). The test requires participants to complete complex pattern matrices, and has previously shown strong associations with behavioural and neural domains within the Cam-CAN cohort (Kievit et al. Reference Kievit, Davis, Mitchell, Taylor, Duncan and Henson2014).
Structural MRI
Grey matter was estimated from the combined segmentation of 1 mm3, T1- and T2-weighted MR images, followed by diffeomorphic registration of grey-matter segments from all participants in Stage 2 of the Cam-CAN study in order to create a sample-specific template. This template was then transformed into Montreal Neurological Institute (MNI) space, and every participant's gray-matter image resliced to the same space, while being modulated by the warping entailed. These stages were done in SPM12 (http://www.fil.ion.ucl.ac.uk/spm). For details of the MRI sequences, see (Shafto et al. Reference Shafto, Tyler, Dixon, Taylor, Rowe, Cusack, Calder, Marslen-Wilson, Duncan, Dalgleish, Henson, Baryne and Matthews2014) for further details of the MRI preprocessing, see (Taylor et al. Reference Taylor, Williams, Cusack, Auer, Aguilar, Dixon, Tyler and Henson2017). We estimated the mean grey matter volume across voxels within the hippocampus, by modulating the grey matter density in each voxel by the Jacobean of the warps used to transform to MNI space within the left and right Hippocampal ROIs (regions of interest) from the Harvard-Oxford atlas (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases), as is standard in many previous studies.
Statistical analyses
Given the non-normal distribution of depressive symptoms in the cohort (Fig. 1), we ran all analyses as non-parametric tests. More specifically, we entered the HADS-scores into a non-parametric logistic regression analyses based on ranks for the dichotomous outcome (i.e. subjective memory complaints) and non-parametric regression for the continuous outcomes (i.e. standard and affective memory measures). Given the directionality of our hypotheses, all significance testing of our a priori hypotheses was one-tailed, whereas significance level for all exploratory tests was two-tailed.
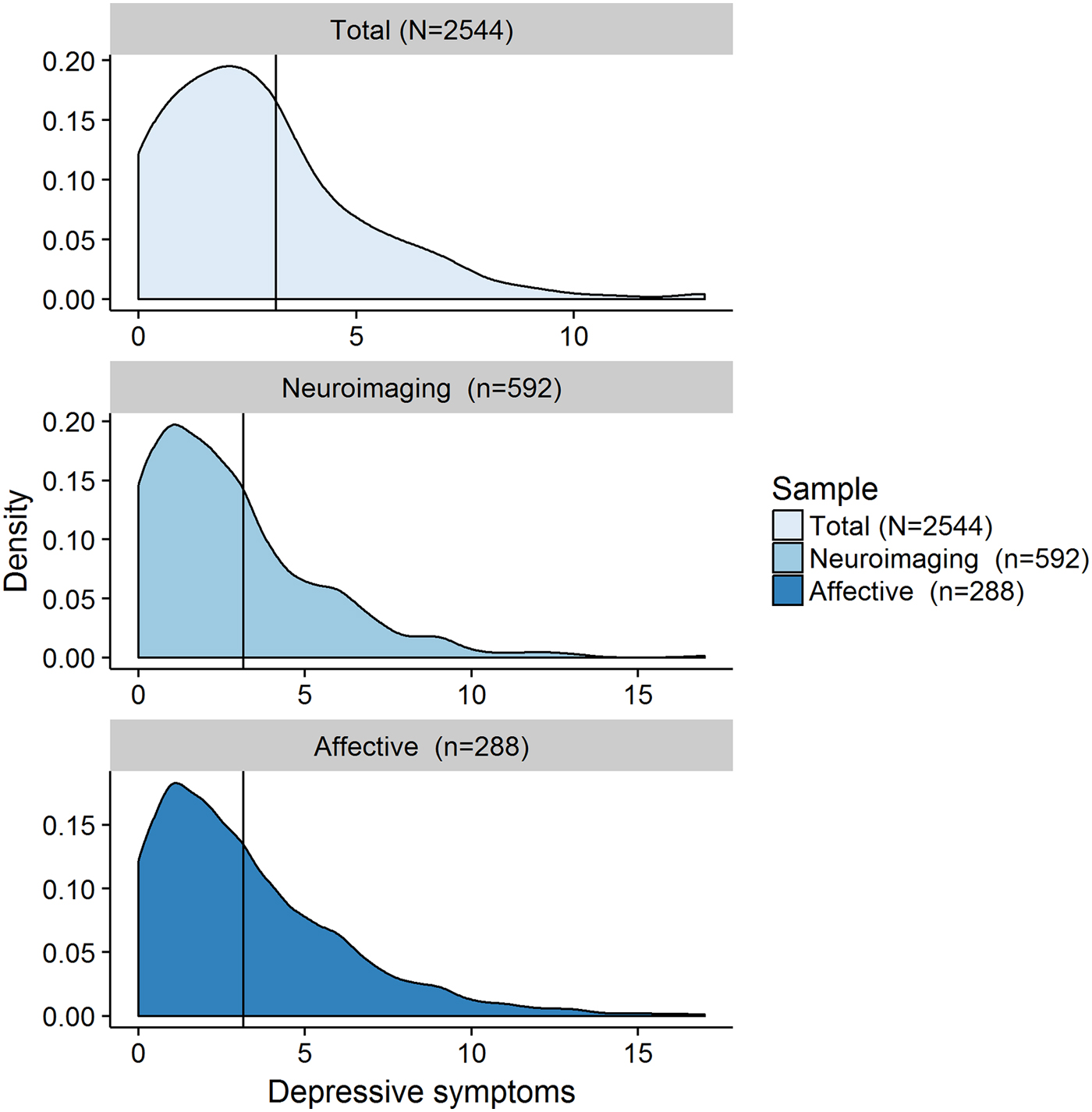
Fig. 1. Neuroimaging cohort = individuals from the overall cohort for whom structural neuroimaging data is available; Affective cohort = individuals from the overall cohort who completed the valenced memory measure; N/n = number of participants; Depressive symptoms = number of self-reported symptoms of depression on the Hospital Anxiety and Depression Scale (HADS) depression subscale (range 0–21, Zigmond & Snaith, Reference Zigmond and Snaith1983).
Procedure
After providing informed written consent, participants completed the ‘Stage 1 – Interview’ of the Cam-CAN study including computerised health and lifestyle questionnaires as well as a core cognitive assessment (Shafto et al. Reference Shafto, Tyler, Dixon, Taylor, Rowe, Cusack, Calder, Marslen-Wilson, Duncan, Dalgleish, Henson, Baryne and Matthews2014). The subset of measures included in the present study are described below. A subset of the sample (subsamples selected to investigate hypotheses 2 and 3, see above) were included in the ‘Stage 2 – Core Cognitive Neuroscience’ phase of the Cam-CAN study. As part of the second stage of the study, participants completed a series of cognitive task across three sessions. One of these sessions also included core structural and functional MRI measures. This second stage also included the valenced memory task. Given the time constraints across multiple sessions, the Cam-CAN protocol included a subset of tasks, including the valenced memory tasks, administered to only a randomized subset (50%) of participants. This study complied with the Helsinki Declaration, and was approved by the Cambridgeshire 2 Research Ethics Committee (reference: 10/H0308/50).
Results
Figure 1 shows the distribution of scores from the depression subscale of the HADS, while online Supplementary Table S1 provides a comprehensive overview of the characteristics of all three samples. We found that 87% (n = 2211) of participants reported at least one symptom of depression, and over 91% scored a total of seven points or less on the depression subscale (Zigmond & Snaith, Reference Zigmond and Snaith1983), which is below the commonly reported clinical cutoff of eight points for this subscale (Bjelland et al. Reference Bjelland, Dahl, Haug and Neckelmann2002). This shows that symptoms of depression are common in the general population, but typically do not reach clinically significant levelsFootnote † Footnote 1 .
In the full cohort (N = 2544), more symptoms of depression were related to more frequent self-report of memory complaints, β = 6.99−4, s.d. = 5.94−5, [95% confidence interval (CI) 5.83−4–8.16−4], z = 11.76, p ⩽ 0.001, R 2 Nagelkerke = 0.08 (H1a) and poorer performance on a standardized measure of memory, β = −1.00−3, s.d. = 1.32−4, (95% CI −1.00−3 to −8.58−4), t (2542) = −8.44, p ⩽ 0.001, R 2 = 0.03 (H1b, Fig. 2 a). However, only the relationship between depressive symptoms and subjective memory survived adjustment for age, cognitive abilityFootnote 2 and sexFootnote 3 , β = 5.19−4, s.d. = 6.37−5, (95% CI 3.94−4–6.44−4), z = 8.15, p ⩽ 0.001, R 2 Nagelkerke = 0.20; the same was not true for the relationship with standardized memory performance, β = −1.31−4, s.d. = 1.14−4, (95% CI −1.00−3 to −8.58−4), t (2539) = −1.15, p = 0.125, R 2 = 0.33, R 2 adj = 0.33 (Fig. 2 b). The absence of a relationship was confirmed with Bayesian analysis (JASP Team, 2016), BF01 = 10.85. Moreover, exploratory analyses showed that the relationship between symptoms of depression and subjective memory complaints did not appear to be due to individuals who suffer from symptoms of depression simply reporting more neuropsychiatric health complaints. That is, while symptoms of anxiety were related to subjective memory complaints, β = 2.35−4, s.d. = 5.63−5, (95% CI 1.24−4–3.45−4), z = 4.16, p ⩽ 0.001, R 2 Nagelkerke = 0.01, the relationship was no longer significant when accounting for depressive symptoms in the same analyses, β = −5.63−5, s.d. = 6.30−5, (95% CI −1.80−4 to 6.71−4), z = −0.89, p = 0.372, R 2 Nagelkerke = 0.00, whereas the relationship between depression and subjective memory complaints remained significant after adjusting for symptoms of anxiety, β = 7.23−4, s.d. = 6.52−5, (95% CI 5.96−4–8.51−4), z = 11.09, p ⩽ 0.001, R 2 Nagelkerke = 0.08.
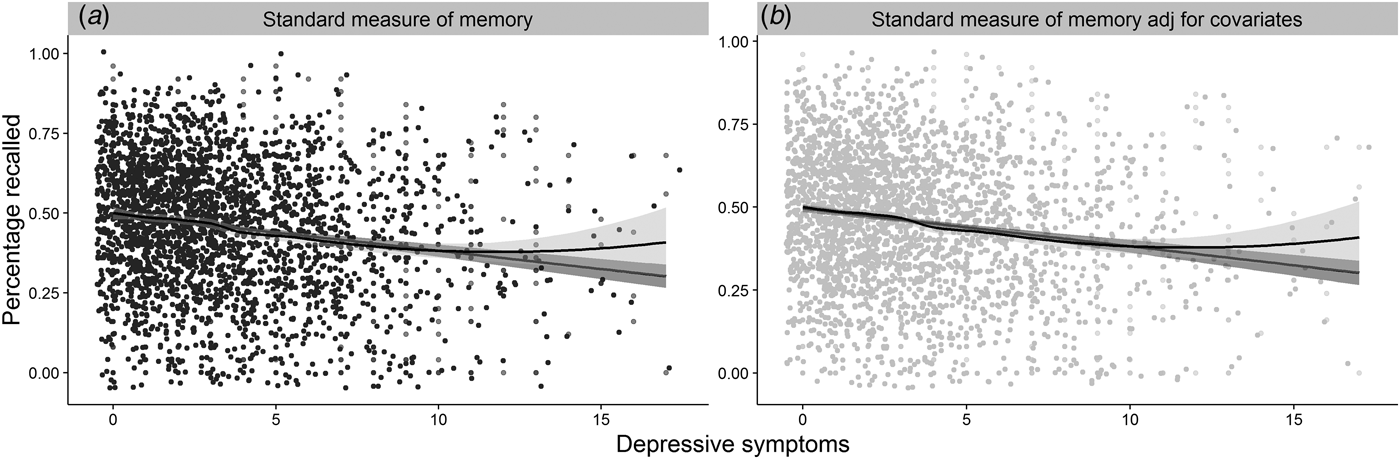
Fig. 2. The figure represents the relationships between: (a) depressive symptoms and performance on the standard measure of objective memory; (b) 2a, after adjustment for age, cognitive ability and sex; jitter was added to the distribution for illustration purposes.
In the neuroimaging cohort (n = 592), multiple regression including total intracranial volume (TIV) as a covariate showed that hippocampal volume was related to both subjective memory, β = −4.64, s.d. = 1.71, (95% CI −8.02 to −1.23), z = −2.71, p = 0.004, R 2 Nagelkerke = 0.02, and the standardized objective measure, β = 17.94, s.d. = 3.29, (95% CI 11.47–24.41), t (589) = 5.45, p ⩽ 0.001, R 2 = 0.05, R 2 adj = 0.04 (Fig. 3 a). However, neither the relationship between hippocampal volume and subjective memory complaints, β = 0.43, s.d. = 2.00, (95% CI −3.50 to −4.36), z = 0.21, p = 0.416, R 2 Nagelkerke = 0.07, nor relationship between hippocampal volume and objective memory β = 0.22, s.d. = 3.40, (95% CI −6.45 to 6.90), t (586) = 0.07, p = 0.474, R 2 = 0.25, R 2 adj = 0.24, survived adjustment for age, cognitive abilityFootnote 4 and sex. Contrary to our expectations, there was no significant association between depressive symptoms and hippocampal volume, β = −2.57, s.d. = 2.01, (95% CI −6.51 to 1.38), t (589) = 1.28, p = 0.100, R 2 = 0.00 (Fig. 3 b), which was again supported by a Bayes factor of 6.87 in favour of the null hypothesis. Unsurprisingly therefore, the relationships between depressive symptoms and both subjective β = 3.00−1, s.d. = 5.59−4, (95% CI 2.00−1–4.00−1), z = 5.04, p ⩽ 0.001, R 2 Nagelkerke = 0.08 and objective β = −2.00−1, s.d. = 1.00−1, (95% CI −4.00−1 to 1.03−4), t (588) = −1.86, p = 0.032, R 2 = 0.05, R 2 adj = 0.05 memory remained significant after adjusting for hippocampal volume (and TIV). That is, there was no support for the hypothesis that hippocampal volumes account in part for the relationship between depressive symptoms and memory performance in this non-clinical population (H2).
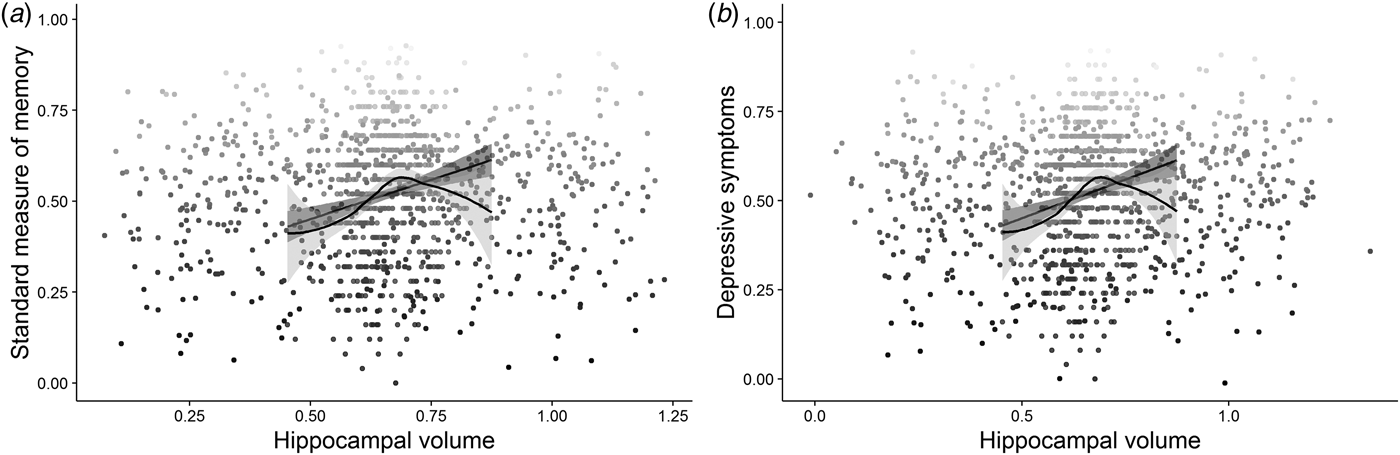
Fig. 3. The figure represents the relationships between: (a) hippocampal volume and performance on the standard measure of memory; (b) hippocampal volume and symptoms of depression; jitter was added to the distribution for illustration purposes.
In the cohort that completed the measure of memory in affective contexts (n = 288), we investigated whether depressive symptoms showed a differential association with memory performance in affective contexts compared with a standardized measure of memory (H3). As in the overall sample, the relationship between depressive symptoms and performance on a standardized memory measure, β = −5.00−1, s.d. = 3.00−1, (95% CI −1.10−1 to 6.91−4), t (286) = −1.78, p = 0.043, R 2 = 0.01, did not survive adjustment for age, cognitive abilityFootnote 5 , and sex, β = −2.00−1, s.d. = 3.00−1, (95% CI −7.00−1 to 3.00−1), t (283) = −0.66, p = 0.509, R 2 = 0.23, R 2 adj = 0.21, BF01 = 4.69. Symptoms of depression were, however, significantly related to poorer memory performance in negative, β = −4.00−2, s.d. = 1.00−2, (95% CI −6.00−2 to −1.00−2), t (286) = −3.48, p ⩽ 0.001, R 2 = 0.04 and positive β = −2.00−2, s.d. = 1.00−2, (95% CI −5.00−2 to −6.39−5), t (286) = −2.52, p = 0.006, R 2 = 0.02 contexts (Fig. 4). However, when adjusting for performance in neutral contexts only the relationship between depressive symptoms and negative context remained significant, β = −1.45−4, s.d. = 5.93−5, (95% CI −2.61−4 to −2.79−5), t (285) = −2.44, p = 0.015, R 2 = 0.76, R 2 adj = 0.76, even after adjusting for the same covariates, β = −1.38−4, s.d. = 5.78−5, (95% CI −2.51−4 to −2.40−5), t (285) = −2.38, p = 0.018, R 2 = 0.78, R 2 adj = 0.78., but depressive symptoms were not related to memory in positive contexts after adjusting for performance in neutral contexts, β = −3.22−5, s.d. = 5.85−5, (95% CI −1.47−4 to 8.32−5), t (285) = −0.55, p = 0.584, R 2 = 0.76, R 2 adj = 0.75 and covariates, β = −2.15−5, s.d. = 5.76−5, (95% CI −1.00−3 to 9.19−5), t (282) = −0.37, p = 0.709, R 2 = 0.77, R 2 adj = 0.76. Moreover, when controlling for memory in positive contexts, depressive symptoms remained a significant predictor of memory in negative contexts, β = −1.46−4, s.d. = 5.52−5, (95% CI −2.54−4 to −3.75−5), t (285) = −2.58, p = 0.010, R 2 = 0.79, R 2 adj = 0.79, even after adjusting for the covariates, β = −1.39−4, s.d. = 5.39−5, (95% CI −2.45−4 to −3.29−5), t (285) = −2.58, p = 0.009, R 2 = 0.81, R 2 adj = 0.81.
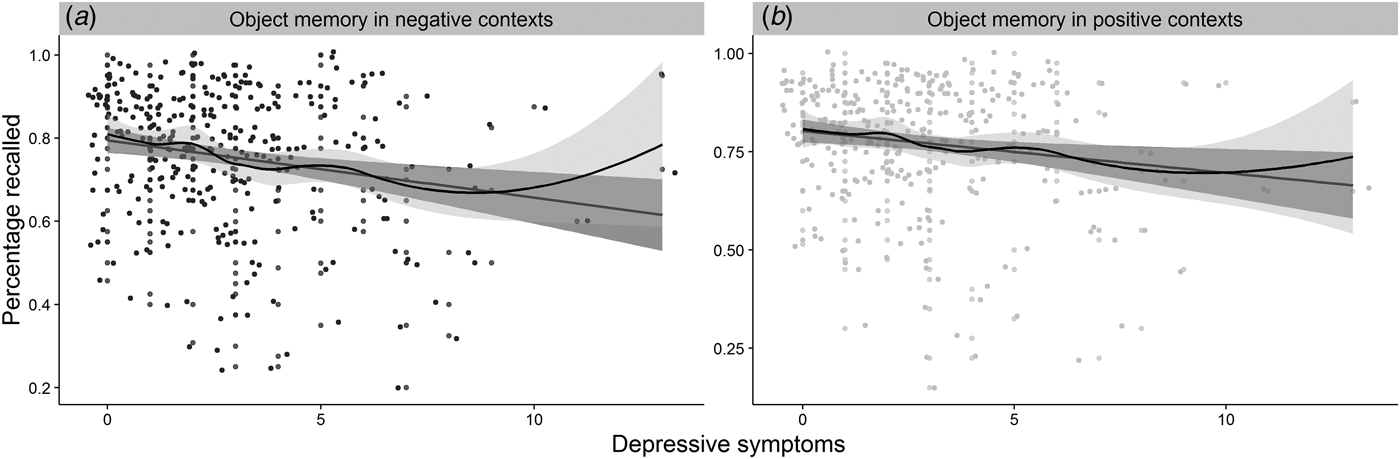
Fig. 4. The figure represents the relationships between: (a) depressive symptoms and memory for objects presented in negative contexts; (b) depressive symptoms and memory for objects presented in positive contexts; jitter was added to the distribution for illustration purposes.
Furthermore, when directly comparing the size of the relationship of depressive symptoms with memory for objects in negative contexts with that for objects in positive contexts, the former was significantly higher, Williams’ t (285) = 3.07, p = 0.002. There was a marginal relationship between symptoms of depression and memory for objects presented in negative contexts to be stronger than their relationship with memory on a standardized measure of memory, Williams’ t (285) = −1.70, p = 0.045 (H3).
Finally, we explored whether self-reported history of depression moderated any of the associations between current symptoms of depression and memory performance (H1), memory performance accounting for hippocampal volume (H2) or memory in affective contexts (H3). Self-reported history of depression that required treatment did not moderate any of the associations significantly, ts < 1, ps ⩾ 0.40.
Discussion
The present study examined the memory correlates of depressive symptoms in a large, population-derived cohort. First, we showed that depressive symptoms were related to self-reported memory problems, even after controlling for variations in age, cognitive ability and sex. Moreover, the relationship was not simply due to individuals’ tendency to report more mental health problems, as the relationship between depressive symptoms and subjective memory remained after adjusting for symptoms of anxiety. One possibility is that the association between symptoms of depression and subjective memory reflects a negative interpretative bias. This notion, known as ‘depressive realism’, suggests that individuals who report symptoms of depression show less positivity bias (Mezulis et al. Reference Mezulis, Abramson, Hyde and Hankin2004; Watson et al. Reference Watson, Dritschel, Obonsawin and Jentzsch2007). Future research should therefore investigate whether other types of self-reported cognitive functioning problems, for example attentional control problems (Derryberry & Reed, Reference Derryberry and Reed2002), are also selectively associated with symptoms of depression but not other measures of mental health functioning.
A second set of findings showed that depressive symptoms were also related to performance on a standardized test of memory, but in this case, we could not rule out the possibility that this relationship was due to variations in memory as a function of age, cognitive ability and/or sex (which were all significantly related to objective memory; see online Supplementary Table S2). In line with Fried & Kievit (Reference Fried and Kievit2015), we also found no evidence for a significant relationship between depressive symptoms and hippocampal volume in this non-clinical cohort. Future research could investigate alternative sources of brain alterations associated with commonly experienced symptoms of depression (Hamilton et al. Reference Hamilton, Siemer and Gotlib2008). Alternatively, the lack of an association between depressive symptoms and hippocampal volume may be because the current sample was a non-clinical sample (i.e. participants reported no functional impairment form their depressive symptoms). To date, hippocampal volume has been studied in the context of clinical depression, and its association with subclinical symptoms of depression remains under researched. There is some evidence pointing towards possible gender differences, with men showing an association between subclinical symptoms of depression and hippocampal volumes but not women (Hayakawa et al. Reference Hayakawa, Sasaki, Takao, Mori, Hayashi, Kunimatsu, Aoki and Ohtomo2013; Samplin et al. Reference Samplin, Ikuta, Malhotra, Szeszko and DeRosse2013; Spalletta et al. Reference Spalletta, Piras, Caltagirone and Fagioli2014)Footnote 6 .
Finally, a third investigation showed that, while performance on a standard memory test may be unaffected in individuals experiencing subclinical symptoms of depression, objective memory impairments are found when the memoranda are encountered in negatively valenced settings. More specifically, depressive symptoms were related to worse recognition memory for visual objects presented against negative backgrounds, even when adjusting for age, cognitive ability and sex. Importantly, this relationship remained even when further adjusting for recognition memory for the same types of objects presented against neutral backgrounds. This suggests that the relationship was specific to the valenced context, rather than differences between the visual object recognition memory test and the standardized verbal recall test, in terms of, for example, the nature of the memoranda or retrieval demands. Furthermore, the relationship between depressive symptoms and recognition memory for objects in positive contexts was no longer significant after the same adjustment for memory in neutral contexts, and the size of the relationship between depressive symptoms and recognition memory for objects in negative contexts was significantly greater than that between depressive symptoms and memory in positive contexts. In other words, the sensitivity of memory to depressive symptoms was selective to memory in negative contexts.
The implication of this finding is that measures of memory in negatively valenced contexts (e.g. Henson et al. Reference Henson, Campbell, Davis, Taylor, Emery, Erzinclioglu and Kievit2016) may be particularly sensitive to subtle differences in memory performance caused by current affective state. Importantly, the study demonstrates that even subclinical depressive symptoms appear to have an impact on both self-perceived and objective measures of memory functioning. Again it remains an open question as to whether the impact of subclinical depressive symptoms in the general population is limited to memory performance in negative contexts, or whether this extends to other types of higher cognitive functions in negatively laden environments.
When considering the implications of the findings from this large-scale population-based study, a few limitations should be noted. First, subjective memory impairments were only assessed with a single item, which may not be a sensitive measure of subjective concerns about memory functioning. Moreover, inferences about subclinical levels of depression and self-reported history of clinical episodes of major depression need to be drawn with caution because they were assessed through a self-report questionnaire and retrospective recall, respectively. These assessments may differ from clinician-rated levels of past and present depressive symptomatology in the current sample.
Future research should explore whether the association between depressive symptoms and the different memory measures observed in the current sample will replicate in currently depressed individuals, or whether they exhibit different memory profiles. Given the impairment in autobiographical memory specificity that is characteristic of individuals in a major depressive episode, the relation between performance on the affective memory measure and the specificity of autobiographical memory should be assessed in healthy and currently depressed individuals. Future research should also investigate whether the strength of the association between individuals’ memory performance in negative contexts and their symptoms of depression has predictive utility for the development of more severe clinical forms of depressive disorders. That is, whether memory for neutral information in negative context fits within a larger pattern of cognitive vulnerabilities to depression (Gotlib & Joormann, Reference Gotlib and Joormann2010).
In conclusion, these findings show that the frequency of self-reported memory problems increases as a function of subclinical depressive symptoms. However, depressive symptoms are not associated with memory performance on a standard objective memory measure, when controlling for age, general cognitive ability and sex. Rather, depressive symptoms are associated with poorer memory for objects presented in negative contexts. These results suggest that memory for objects presented in negative contexts may be particularly sensitive to the memory problems reported by those experiencing symptoms of depression.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717001519.
Acknowledgements
The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) research was supported by the Biotechnology and Biological Sciences Research Council (grant number BB/H008217/1). SS is supported by UK Medical Research Council Programme MC-A060-5PQ60; RNH and TE are supported by MC-A060-5PR10; RAK is supported by MC-A060-5PR60 and a Sir Henry Wellcome Trust Fellowship (grant number 107392/Z/15/Z). The authors are grateful to the Cam-CAN respondents and their primary care teams in Cambridge for their participation in this study. They also thank colleagues at the MRC Cognition and Brain Sciences Unit MEG and MRI facilities for their assistance. The Cam-CAN corporate author consists of the project principal personnel: Lorraine K Tyler, Carol Brayne, Edward T Bullmore, Andrew C Calder, Rhodri Cusack, Tim Dalgleish, John Duncan, Fiona E Matthews, William D Marslen-Wilson, James B Rowe, Meredith A Shafto; Research Associates: Karen Campbell, Teresa Cheung, Simon Davis, Linda Geerligs, Anna McCarrey, Abdur Mustafa, Darren Price, David Samu, Jason R Taylor, Matthias Treder, Kamen Tsvetanov, Janna van Belle, Nitin Williams; Research Assistants: Lauren Bates, Tina Emery, Sharon Erzinçlioglu, Andrew Gadie, Sofia Gerbase, Stanimira Georgieva, Claire Hanley, Beth Parkin, David Troy; Affiliated Personnel: Tibor Auer, Marta Correia, Lu Gao, Emma Green, Rafael Henriques; Research Interviewers: Jodie Allen, Gillian Amery, Liana Amunts, Anne Barcroft, Amanda Castle, Cheryl Dias, Jonathan Dowrick, Melissa Fair, Hayley Fisher, Anna Goulding, Adarsh Grewal, Geoff Hale, Andrew Hilton, Frances Johnson, Patricia Johnston, Thea Kavanagh-Williamson, Magdalena Kwasniewska, Alison McMinn, Kim Norman, Jessica Penrose, Fiona Roby, Diane Rowland, John Sargeant, Maggie Squire, Beth Stevens, Aldabra Stoddart, Cheryl Stone, Tracy Thompson, Ozlem Yazlik; and Administrative Staff: Dan Barnes, Marie Dixon, Jaya Hillman, Joanne Mitchell, Laura Villis.






