We have read with interest the article entitled ‘Neuroendocrine markers of high risk of psychosis: salivary testosterone in adolescent boys with prodromal symptoms’ by van Rijn et al. (Reference van Rijn, Aleman, de Sonneville, Sprong, Ziermans, Schothorst, van Engeland and Swaab2011), published in Psychological Medicine. The authors concluded that levels of testosterone were significantly lower in adolescents with prodromal symptoms as compared with non-clinical controls and that there were no statistically significant differences in oestradiol between groups.
A possible mechanism linking low testosterone and psychosis is overweight/obesity, which was not reported in the study. In fact, a robust association between the metabolic syndrome and low testosterone levels has been found (Kupelian et al. Reference Kupelian, Hayes, Link, Rosen and McKinlay2008), with hypogonadism being associated with obesity and type 2 diabetes mellitus (Dandona & Rosenberg, Reference Dandona and Rosenberg2010). The relationship between schizophrenia and metabolic abnormalities is also a well-replicated finding, being reported even in the early stages of the disease (Verma et al. Reference Verma, Subramaniam, Liew and Poon2009). A possible explanation for the relationship between obesity and reduced testosterone is an increase in the activity of the enzyme aromatase, present in adipose tissue (Loves et al. Reference Loves, Ruinemans-Koerts and de Boer2008). Aromatase is a member of the cytochrome P450 superfamily that catalyses the conversion of C-19 androgens (testosterone and androstenedione) into C-18 oestrogens (oestradiol and oestrone) (Williams, Reference Williams2010). Therefore, it is possible to hypothesize that adipose aromatase hyperactivity may be a contributor to reductions in testosterone levels. As the authors did not mention the weight or body mass index (BMI) of the sample, it is possible to investigate whether higher BMI in the ultra-high-risk (UHR) group is mediating the relationship between prodromal symptoms and low levels of testosterone (Niskanen et al. Reference Niskanen, Laaksonen, Punnonen, Mustajoki, Kaukua and Rissanen2004).
The results of the study by van Rijn et al. (Reference van Rijn, Aleman, de Sonneville, Sprong, Ziermans, Schothorst, van Engeland and Swaab2011) together with the possible interaction of overweight/obesity and hyperactivity of aromatase with consequent low levels of testosterone offer an exciting and innovative avenue of investigation. Since gonadal hormones dramatically change in puberty and the majority of cases of psychosis have their onset at this age, it seems plausible that these substances can mediate cellular and molecular processes related to neuroplasticity, interconnectivity and cell resilience occurring during adolescence (Galea et al. Reference Galea, Uban, Epp, Brummelte, Barha, Wilson, Lieblich and Pawluski2008). If subsequent studies determine that aromatase hyperactivity is the cause of hormonal dysfunction in UHR individuals, it will be reasonable to investigate if manipulation of this pathway with an aromatase inhibitor might be a beneficial effect as a preventive strategy in UHR individuals with low testosterone levels.
Declaration of Interest
E.B. has received support from CAPES and CNPq (Brazil). R.A.B. has received support from CAPES, CNPq and FAFESP (Brazil) and has been a speaker for Janssen-Cilag, Astra-Zeneca, Novartis and Lilly.
The authors reply
In response to our study showing reduced levels of testosterone in adolescent boys with prodromal symptoms, Brietzke & Bressan have put forward the hypothesis that adiposity may be a mediating factor contributing to reduced levels of testosterone and related vulnerability for psychosis. This is an interesting hypothesis, particularly considering implications for treatment and improving outcome of those at risk for psychosis. Although we did not include information with regard to overweight/obesity in our report, data on body mass index (BMI) have been collected alongside the collection of saliva samples in our study. Hence, we were able to test their hypothesis in our sample using empirical data.
We used a digital Tanita Body Composition Analyzer (Tanita Corp., USA), which is a reliable method for measuring BMI. For comparison with other studies, we included BMI category distributions derived from the Centers for Disease Control and Prevention (CDC) growth charts for children and adolescents (aged 2–20 years) (Ogden & Flegal, Reference Ogden and Flegal2010). These charts provide age-specific norms for BMI scores in the general population. According to CDC definitions, ‘overweight’ corresponds to a BMI score between the 85th and 95th percentile whereas ‘obese’ corresponds to a BMI score above the 95th percentile. Using these criteria we assessed the number of adolescents with overweight or obesity in our control sample and clinical sample. Results are shown in Table 1.
Table 1. Mean BMI score and number of overweight/obese adolescents in the UHR group and control group
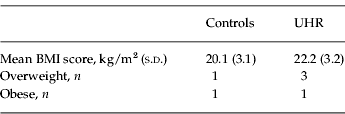
BMI, Body mass index; UHR, ultra-high-risk; s.d., standard deviation.
In order to assess whether the subjects with overweight or obesity were driving the group differences in salivary levels of testosterone, we reanalysed our data with obese and overweight subjects excluded. Results of this new analysis showed that mean levels of testosterone were 20.9 (s.d.=14.2) pg/ml for the UHR group and 35.6 (s.d.=29.7) pg/ml for controls, as compared with 20.0 (s.d.=13.6) pg/ml in the UHR group and 33.6 (s.d.=28.2) pg/ml for controls in our initial analysis. The degree to which testosterone levels were lower in the UHR group as compared with controls did not change, as the effect size, Cohen's d, remained 0.7. Our sample size was too small to compare frequency of overweight/obesity in both groups, so we were not able to assess whether adolescent boys with prodromal symptoms were at increased risk for overweight/obesity.
In addition to analysing these group differences, we also used a more dimensional approach and assessed the correlation between BMI score and level of testosterone across all subjects (UHR and control groups collapsed). Pearson correlational analysis resulted in r=−0.27, p=0.16, Spearman correlational analysis in r=−0.05, p=0.78.
Although the increased risk for the metabolic syndrome in individuals with schizophrenia and the relationship between the metabolic syndrome and lower testosterone do suggest that obesity may help explain reduced levels of testosterone in adolescents with prodromal symptoms, data in our sample do not convincingly support this hypothesis. Exclusion of those with overweight or obesity did not substantially change our findings and no significant relationship between BMI score and levels of testosterone was found. Based on data in our study, we have to conclude that reduced levels of testosterone in our sample of adolescents cannot be attributed to adiposity. However, more studies, with larger sample sizes and a wider range of measures, are needed to further test the hypothesis put forward by Brietzke & Bressan.
Declaration of Interest
None.




