Article contents
Crystal structure of the ternary semiconductor Cu2In14/3□4/3Se8 determined by X-ray powder diffraction data
Published online by Cambridge University Press: 19 September 2018
Abstract
The crystal structure of the partially ordered vacancy compound Cu2In14/3□4/3Se8, belonging to the system I3-III7-□2-VI12, was analyzed using X-ray powder diffraction data. Several structural models were derived from the structure of the selenium-rich phase β-Cu0.39In1.2Se2 by permuting the cations in the available Wyckoff positions. The refinement of the best model by the Rietveld method in the tetragonal space group P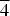 $\overline 4 $ 2c (No 112), with unit-cell parameters a = 5.7487(3) Å, c = 11.5106(6) Å, V = 380.40(3) Å3, led to Rp = 9.0%, Rwp = 9.9%, Rexp = 7.2%, S = 1.4 for 134 independent reflections. This model has the following Wyckoff site atomic distribution: Cu in 2e (0,0,0); In in 2b (½,0,¼), 2d (0,½,¼), and 2f (½,½,0);□ in 2f (½,½,0); Se in 8n (x,y,z).
$\overline 4 $ 2c (No 112), with unit-cell parameters a = 5.7487(3) Å, c = 11.5106(6) Å, V = 380.40(3) Å3, led to Rp = 9.0%, Rwp = 9.9%, Rexp = 7.2%, S = 1.4 for 134 independent reflections. This model has the following Wyckoff site atomic distribution: Cu in 2e (0,0,0); In in 2b (½,0,¼), 2d (0,½,¼), and 2f (½,½,0);□ in 2f (½,½,0); Se in 8n (x,y,z).
Keywords
- Type
- New Diffraction Data
- Information
- Copyright
- Copyright © International Centre for Diffraction Data 2018
References
- 5
- Cited by




