No CrossRef data available.
Article contents
Citellinema (Nematoda: Heligmosomidae) from North America with descriptions of 2 new species from the red squirrel Tamiasciurus hudsonicus and 1 from the Canadian woodchuck, Marmota monax
Published online by Cambridge University Press: 27 May 2022
Abstract
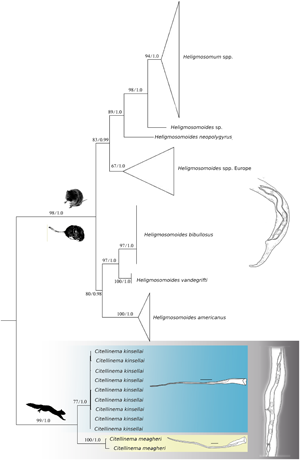
Citellinema Hall, 1918 includes 6 valid species of gastrointestinal nematodes of sciurids. Two species occur in the Palearctic and 4 in the Nearctic, 3 of which occur minimally across Colorado, Idaho and Oregon and 1, Citellinema bifurcatum, has a wide distribution across North America. Members of the genus are didelphic, possess a cephalic vesicle, a terminal spine-like process in females and feature robust spicules, consisting of a proximal end fused and semicylindrical shaft connected to a lamina supported by 2 terminal filiform processes. Typically, the size of the spicules is used to differentiate species. As part of the Beringian Coevolution Project, specimens provisionally identified as C. bifurcatum were collected through intensive field sampling of mammals and associated parasites from across localities spanning the Holarctic. These specimens revealed considerable genetic variability at both mitochondrial and nuclear loci, supporting the identification of deeply divergent clades. Examination of these new specimens, along with the holotypes of C. bifurcatum and Citellinema quadrivittati indicates that Citellinema monacis (previously synonymized with C. bifurcatum) should be resurrected and 3 additional species described. We suggest that the apparent bifurcated nature of the spicule should be considered a generic diagnostic trait, while the proportional length of the lamina relative to that of the spicule is used as a specific character. We demonstrate the critical need for continued inventory of often poorly known assemblages of hosts and parasites, contributing to a growing baseline of archival specimens, collections and information that make explorations of faunal structure and diversity possible.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2022. Published by Cambridge University Press





