Introduction
Parasites of the genus Trypanosoma are unicellular flagellate microorganisms of the Trypanosomatidae. Haematophagous arthropods act as biological or mechanical vectors of different species of this family, infecting a wide range of vertebrate hosts (Hoare, Reference Hoare1972; Haag et al. Reference Haag, O'HUigin and Overath1998).
Although most Trypanosoma species are transmitted by blood-sucking insects, ticks are also likely to be vectors of some members of this genus (Morzaria et al. Reference Morzaria, Latif, Jongejan and Walker1986; Thekisoe et al. Reference Thekisoe, Honda, Fujita, Battsetseg, Hatta, Fujisaki, Sugimoto and Inoue2007). In Brazil, a trypanosome with morphological characteristics similar to Trypanosoma theileri, a species described as non-pathogenic to bovines and usually transmitted by horseflies, is often found parasitizing R hipicephalus microplus haemolymph (Ribeiro et al. Reference Ribeiro, Lima and Guimarães1988; Martins et al. Reference Martins, Leite and Doyle2008).
This study describes for the first time an isolate of the Trypanosoma genus naturally infecting R. microplus characterized through molecular, morphological and biological analysis.
Materials and methods
Origin of R. microplus ticks
Engorged R. microplus females were obtained from naturally infested cattle of the Federal Rural University of Rio de Janeiro (UFRRJ) dairy cattle sector in the municipality of Seropedica, state of Rio de Janeiro (22°45′42.0″S 43°42′09.6″W), Brazil. The bovine blood was evaluated by the Giemsa-stained smear method and was negative for Trypanosoma spp.
Isolation of trypanosomes
After 12 days of incubation at 27 °C with 80% relative humidity, haemolymph smears were made from a section of the distal portion of the first pair of legs of 24 engorged female ticks. The smears were fixed in methanol, stained with 10% Giemsa and examined under an optical microscope at a magnification of 100× for the presence of trypanosomes. Ticks that were positive for trypanosomes were subjected to isolation of the parasite as described below.
To obtain the haemolymph, the positive ticks were treated as follows. In a laminar flow cabinet, the live infected ticks were surface-sterilized by immersion in 70% ethanol for 1 min, 0·05% sodium hypochlorite solution for 30 s, 70% ethanol again for 1 min, detergent based on 2% chlorhexidine (Riohex® Rioquimica manufacturer, Brazil) for 30 s, a third wash in 70% ethanol for 1 min and finally sterile ultrapure water with penicillin (100 IU ml−1), streptomycin (100 µg ml−1) and amphotericin B (250 µg ml−1) for 1 min. The ticks were then dried on sterile gauze and transferred with sterile forceps to a sterile Petri dish. Subsequently, around 5 µl of haemolymph was obtained from each tick by severing the first pair of its legs with sterile forceps and ophthalmic scissors, pooled and added to a 25 cm2 flask containing a monolayer of cells of the Ixodes scapularis embryo-derived cell line IDE8 (Munderloh et al. Reference Munderloh, Liu, Wang, Chen and Kurtti1994) at the 110th passage in 5 ml of L15B medium, supplemented with 10% inactivated fetal calf serum (FCS), 10% phosphate tryptose broth, 0·1% bovine lipoprotein concentrate (MP Biomedicals, United Kingdom), 2 mm L-glutamine, 100 IU ml−1 penicillin and 100 µg ml−1 streptomycin. The pH of the medium was adjusted to 6·6–6·8. The inoculated culture was incubated at 30 °C and monitored by examination of Giemsa-stained smears (prepared by spreading a drop of culture supernatant on a slide and air-drying) every 3 days post-infection (DPI).
Maintenance and monitoring of trypanosome cultures
Following initial isolation in IDE8 cells, trypanosome cultures were maintained in two ways: after the fourth co-cultivation with IDE8 cells, axenic culturing was also performed in L15B medium, both incubated in 25 cm2 culture flasks in bacterial incubators at 30 °C. Renewal of the IDE8 cells’ culture medium was performed weekly by removal and replacement of approximately two-thirds of the medium. Subcultures were made after a confluent monolayer of IDE8 cells had formed on the surface of the culture flask.
To obtain a pure culture of trypanosomes free of tick cells for axenic culture, the isolated trypanosomes were resuspended, collected by rinsing and transferred to a sterile 15 ml tube for centrifugation at 700 × g for 10 min. The supernatant was transferred to a new sterile 15 ml tube and centrifuged at 200 × g for 10 min. Then the supernatant was discarded and the pellet was resuspended in 8 ml of phosphate-buffered saline and examined by inverted microscope to rule out the presence of tick cells. After a further centrifugation at 200 × g for 10 min, the resultant pellet was resuspended in 5 ml of complete L15B medium, transferred to a 25 cm2 culture flask and incubated at 30 °C. The culture medium was renewed weekly, with removal and replacement of approximately two-thirds of the medium. Cultures were monitored with an inverted phase contrast microscope and by examination of Giemsa-stained smears of culture supernatant as above.
Isolated trypanosomes were passaged by transfer of 1 ml of culture supernatant (1 : 5 dilution) in both IDE8 and axenic cultures. Aliquots of the isolated trypanosomes were cryopreserved with 10% DMSO in liquid nitrogen at −196 °C. For freezing, the isolate was resuspended and transferred to a 15 ml sterile centrifuge tube and centrifuged at 500 × g for 5 min. Thereafter, the supernatant was removed and the pellet resuspended in 2 ml of L15B culture medium. An equal volume of ice-cold culture medium with 20% filtered DMSO was added dropwise and slowly mixed. The cell suspension was divided between four labelled cryotubes, placed in a Nalgene™ Cryo 1 °C isopropanol freezing container, and held at −80 °C for at least 90 min. Subsequently the cryotubes were transferred to a liquid nitrogen storage container (−196 °C). The cryopreserved trypanosomes were resuscitated by thawing slowly in a water bath at 34 °C and maintaining in culture as above.
Propagation in different culture conditions
Trypanosomes were propagated in axenic culture in triplicate. After four passages, they were tested in the following culture media: MEM, DMEM (supplemented with 2 mm L-glutamine and 10% fetal bovine serum), M199 (supplemented with 10% fetal bovine serum), BHI, BHI supplemented with blood agar and Schneider's insect medium. Propagation was tested at the following incubation temperatures over 15 days: 26, 28, 30, 32, 34 and 37 °C.
Growth profile and developmental forms in axenic culture
The developmental profile of the isolated trypanosomes was evaluated in a young axenic culture, 4 days after subculture. Initially, the trypanosomes were counted in a Neubauer chamber to prepare the inoculum concentration of 1 × 104 parasites ml−1 and subsequent transfer to 25 cm2 culture flasks with L15B medium. Growth curves were performed in triplicate at 30 °C. Aliquots of 10 µl of each of the three replicates were collected after resuspension of the culture at 48 h intervals up to the 30th day and quantified as above with analysis of developmental forms in Giemsa-stained smears by examination of 50–100 trypanosomes per sample, based on published descriptions (Barros et al. Reference Barros, Fonseca, Macedo-Silva, Côrte-Real, Toma and Madeira2014). No medium change was performed during this 30-day period.
Morphometric analysis
The morphometry was performed on randomly selected stained trypanosomes, evaluated with a light microscope (OLYMPUS BX45®) coupled with a photo documentation system (D'Cell®software). The measurements were performed according to Hoare (Reference Hoare1972), from axenic trypanosome cultures, by evaluating the total length of the parasite (from the anterior end to the posterior end), the free flagellum length, nucleus diameter, kinetoplast diameter, distance from the posterior end to the centre of the nucleus, distance from the posterior end to the centre of the kinetoplast, distance from the centre of the nucleus to the centre of the kinetoplast, distance from the centre of the nucleus to the anterior end and the relationship between the posterior end–nucleus and nucleus–anterior end measurements (nuclear index).
DNA extraction and polymerase chain reaction
DNA extraction from trypanosome cultures was performed using a Qiagen® Quiamp kit, according to the manufacturer's recommendations.
Nested polymerase chain reaction (PCR) was performed for amplification of a partial region of the 18S rRNA gene specific to the family Trypanosomatidae using TRY927F (5′-GAAACAAGAAACACGGGAG-3′) and TRY927R (5′-CTACTGGGCAGCTTGGA-3′) external primers, which amplify a fragment of approximately 900 bp, and SSU561F (5′-TGGGATAACAAAGGAGCA-3′) and SSU561R (5′-CTGAGACTGTAACCTCAAAGC-3′) internal primers that amplify a fragment of approximately 700 bp, according to the protocol of Smith et al. (Reference Smith, Clark, Averis, Lymbery, Wayne, Morris and Thompson2008).
To amplify the partial conserved sequence of the ribosomal gene largest subunit (24Sα rDNA) of members of the family Trypanosomatidae, PCR was performed using D75 (5′-GCAGATCTTGGTTGGCGTAG-3′) and D76 (5′-GGTTCTCTGTTGCCCCTTTT-3′) primers, which amplify a fragment of approximately 270 bp, according to the protocol of Souto et al. (Reference Souto, Vargas and Zingales1999).
PCR products were subjected to 2% agarose gel electrophoresis run at 90 W for 30 min. The gels were stained with ethidium bromide and visualized with a UV light transilluminator.
Sequencing and phylogenetic analysis
The amplification products were purified using the QIAquick® PCR Purification Kit (Qiagen), according to the manufacturer's recommendations. After purification, the DNA was sequenced using a capillary-type Sanger platform in an ABI 3730 DNA Analyser (Applied Biosystems, Life Technologies®). The obtained sequences were compared with published sequences using the NCBI Nucleotide BLAST programme.
Phylogenetic trees were built from the partial sequences of 18S rRNA and 24Sα rDNA genes using the Mega 6 programme, the Maximum Likelihood test and the Tajima–Nei model.
Results
Trypanosome isolation and culture
Among the 24 engorged females evaluated, epimastigote trypanosome forms were observed in the haemolymph of three specimens (Fig. 1). After inoculation of pooled haemolymph from these three ticks into an IDE8 cell culture, typical forms of Trypanosoma were seen from the seventh DPI onwards.

Fig. 1. Photomicrographs of haemolymph smears. Visualization of epimastigote forms of trypanosomatid and Rhipicephalus microplus haemocytes stained with Giemsa, 100× objective.
The isolated trypanosomes, designated strain P1RJ, grew well axenically in L15B medium at temperatures of 30, 3 and 34 °C. However, in all other culture media tested (MEM, DMEM, M199, BHI, BHI supplemented with blood agar, Schneider's Insect Medium) and at all other temperatures examined, there was no parasite growth and no viable trypanosomes were seen after the seventh DPI (data not shown).
The trypanosome culture remained viable in L15B axenic culture medium over 14 passages at 30 °C. Trypanosomes cryopreserved at the third passage in axenic culture were viable when resuscitated after 60 days of storage in liquid nitrogen and kept in culture. Figure 2A shows the growth curve obtained over a 30-day period in L15B axenic culture at 30 °C.

Fig. 2. Axenic growth of Trypanosoma rhipicephalis sp. nov. in L15B medium. (A) Growth curve determined by counting total numbers of trypanosomes at 2-day intervals over 30 days; mean of three replicate cultures. (B) Proportions of different developmental forms determined by examination of Giemsa-stained smears prepared at 2-day intervals over 26 days.
Morphometric analysis
Morphometric variations between different developmental forms were observed. The morphometric measurements of trypanosome strain P1RJ epimastigote and spheromastigote developmental forms are presented in Table 1. Epimastigote forms had greater body length on average (32·44 µm) and the spheromastigote forms had the shortest average total body length (16·13 µm). The average flagellum length in spheromastigote forms was greater (11·66 µm) than those of the trypomastigote (5·72 µm) and epimastigote (6·90 µm) forms.
Table 1. Morphometric data (μm) obtained from epimastigote and spheromastigote developmental forms of Trypanosoma rhipicephalis sp. nov

PK, posterior end to kinetoplast; KN, kinetoplast to middle of the nucleus; PN, posterior end to middle of the nucleus; NA, middle of the nucleus to anterior end; FF, free flagellum; TL, total length, nuclear index (PN/NA); NL, nucleus length. Average ≠ standard deviation (minimum−maximum value).
The trypomastigote flagellum length of strain P1RJ trypanosomes showed a lower average value (5·72 µm) than reference values of different species of Trypanosoma (Table 2).
Table 2. Comparison of morphometric data (μm) for trypomastigote forms of Trypanosoma rhipicephalis sp. nov. with published data from other Trypanosome species
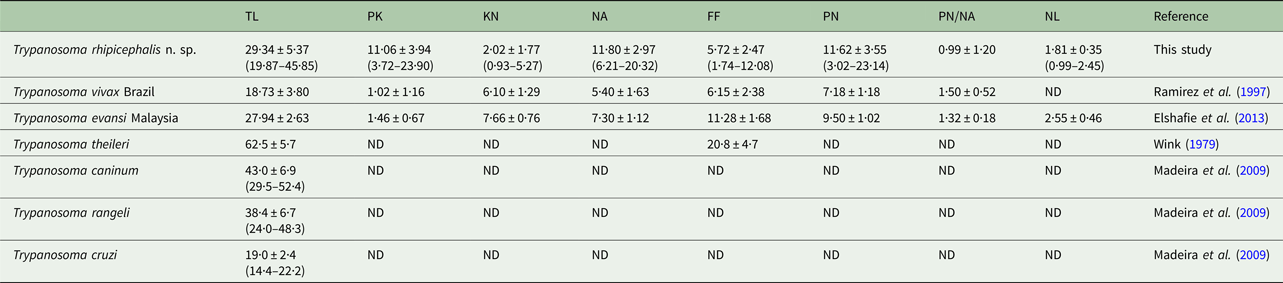
PK, posterior end to kinetoplast; KN, kinetoplast to middle of the nucleus; PN, posterior end to middle of the nucleus; NA, middle of the nucleus to anterior end; FF, free flagellum; TL, total length, nuclear index (PN/NA); NL, nucleus length. Average ≠ standard deviation (minimum−maximum).
The proportions of the different developmental forms over 26 days in axenic culture at 30 °C (Fig. 3) are shown in Fig. 2B. On day 0, 75% of the (1 × 104 ml−1) parasites were typical epimastigote forms with elongated bodies and sharp posterior ends (Fig. 3E), 13% were trypomastigote forms (Fig. 3G,H), 7% were epimastigote forms without flagella (Fig. 3A) and with shorter body length compared with classical epimastigote forms (Fig. 3E), and 5% of the forms were spheromastigotes (Fig. 3I). Trypanosome growth peaked at day 20 (Fig. 1A) with a predominance of classical epimastigotes (Fig. 2B). The proportion of trypomastigote forms remained low throughout. An increase in spheromastigote forms was seen from the 22nd to the 26th DPI.
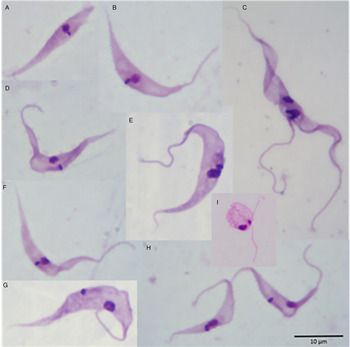
Fig. 3. Photomicrographs showing the morphological diversity of Trypanosoma rhipicephalis sp. nov. in axenic culture in the L15B medium at 30 °C. (A) Atypical epimastigote form with no apparent flagellum; (B, D, F) Forms in transition to trypomastigote; (C) dividing form; (E) classical epimastigote; (G, H) trypomastigote, (I) spheromastigote. Giemsa-stained smears; scale bar = 10 µm.
Molecular analysis
From the PCRs of the 18S rRNA and 24Sα rDNA genes, fragments of approximately 658 and 262 bp were obtained, respectively. The fragments were sequenced and analysed to determine their similarity with sequences from other trypanosome species deposited in GenBank. The partial sequence of the 18S rRNA gene showed 92% similarity (query coverage 100%) with Trypanosoma sp. KG1 (accession number AB281091). It also showed 99% similarity (query coverage 48%) with sequences from Trypanosoma ralphi (accession number KP768296), Trypanosoma sp. strain 1100 (accession number EU596260) and Trypanosoma grayi (accession number AJ005278), and showed 89% similarity (query coverage 61%) with the sequence of Trypanosoma caninum (accession number KF805470).
For the 24Sα rDNA gene, there were similarities of 96% (query coverage 62%) with Trypanosoma rangeli strain BL711 (accession number JN016743), 96% (query coverage 65%) with Trypanosoma grosi AKHA (accession number AB175624), 94% (query coverage 65%) with Trypanosoma pestanai (accession number KR527480) and 92% similarity (query coverage 64%) with the sequence of T. caninum (accession number KF805494).
GenBank sequences were aligned with Mega software and phylogenetic trees constructed. Molecular analysis showed that our trypanosome isolate, strain P1RJ, was clearly separated from other species of the genus Trypanosoma, regardless of the molecular target used, with bootstrap values of 98 and 97 for trees built from sequences of the targets 18S rRNA and 24Sα rDNA, respectively (Fig. 4). The nucleotide sequences described were deposited in Genbank under accession numbers KX711901 and KY292287.
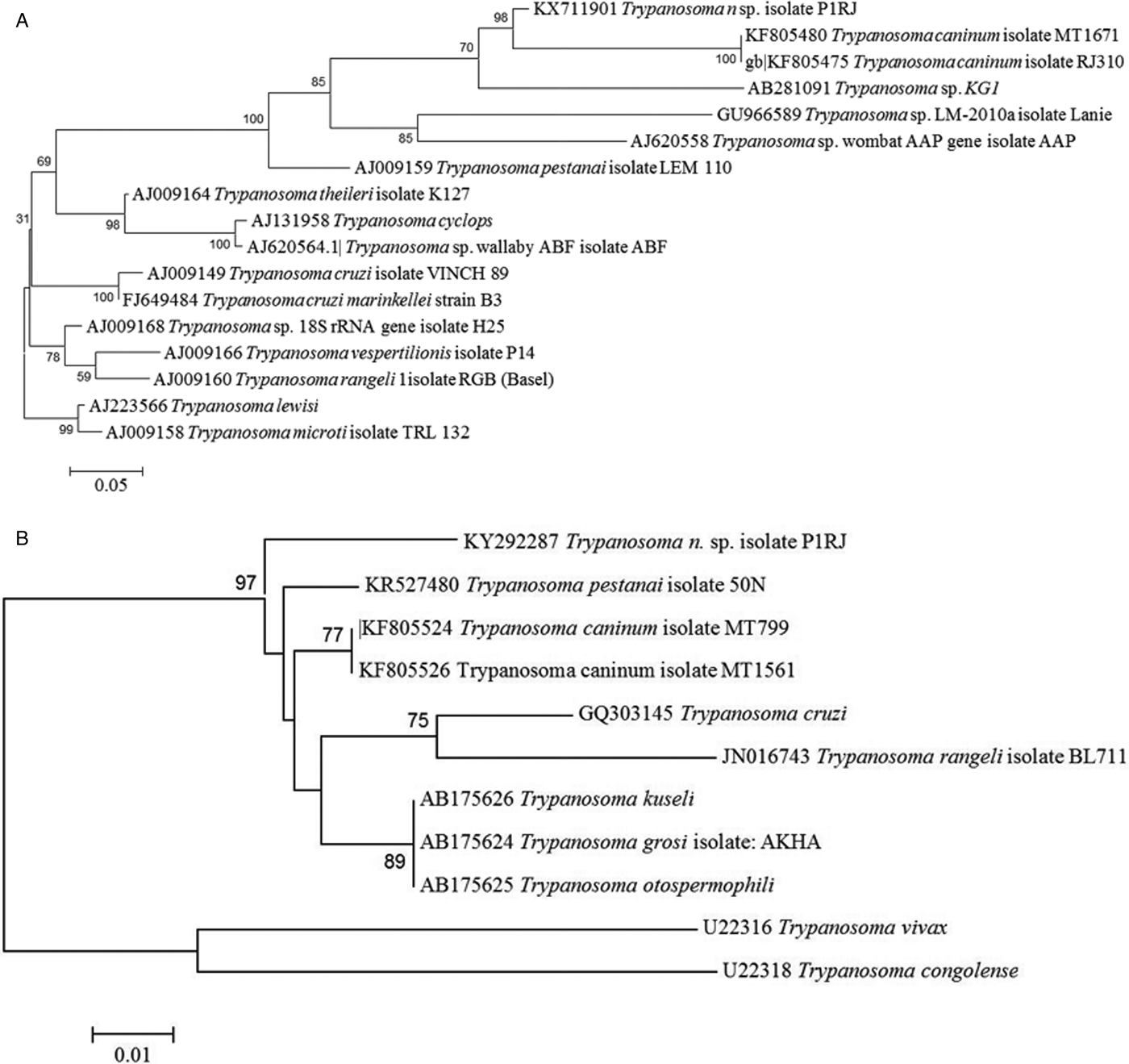
Fig. 4. Phylogenetic analysis of Trypanosoma rhipicephalis sp. nov. and other trypanosome species. (A) Phylogenetic tree based on 18S rDNA sequences analysis. Statistical method Maximum Likelihood-Kimura two-parameter model. Bootstrap: 1000. (B) Phylogenetic tree based on 24S rDNA sequences analysis. Neighbour-joining and Tamura-N method.
Discussion
This study describes the first isolation and molecular, morphological and biological analysis of a trypanosome of the genus Trypanosoma from naturally infected female R. microplus ticks collected from cattle in the municipality of Seropedica, Rio de Janeiro. However, the occurrence of epimastigote forms of a trypanosome in haemolymph of this tick species has also been reported by Ribeiro et al. (Reference Ribeiro, Lima and Guimarães1988) and Martins et al. (Reference Martins, Leite and Doyle2008) in Southern and central Brazil, respectively, and by Gaido et al. (Reference Gaido, Mangold, Aguirre and Guglielmone1989) in Argentina. Morphological evidence led the authors to suspect T. theileri, as this species was described as non-pathogenic in cattle and usually transmitted by horseflies. It is well known that arthropods not belonging to the genera Glossina, Rhodnius or Triatoma may act as vectors of trypanosomes, as seen with Trypanosoma lewisi, transmitted by fleas of the species Nosopsyllus fasciatus and Xenopylla cheopis (Rayat and Vasishta, Reference Rayat and Vasishta2014). Another example is the species Trypanosoma melophagium, which infects sheep and goats, and is transmitted by Melophagus ovinus (Nalbantoğlu and Karaer, Reference Nalbantoğlu and Karaer2008). The results of the analysis performed in this study suggest that strain P1RJ is a new species of trypanosome that we tentatively name Trypanosoma rhipicephalis sp. nov.
In axenic and cellular culture, microscopic examination of T. rhipicephalis strain P1RJ revealed classic morphological characteristics of the genus Trypanosoma, such as the nucleus, kinetoplast, undulating membrane and free flagellum, in addition to different epimastigote, trypomastigote and spheromastigote developmental forms.
The evaluation of morphological and morphometric aspects of trypanosomes provides valuable data for the identification and description of new species (Hoare, Reference Hoare1972). Trypanosoma rhipicephalis strain P1RJ is a large trypanosome, as evidenced by the average 29.34 µm total length of the trypomastigote form, which is larger than the strains of Trypanosoma vivax (Ramírez et al. Reference Ramírez, Dávila, Victório, Silva, Trajano and Jansen1997), Trypanosoma cruzi (Madeira et al. Reference Madeira, Sousa, Barros, Figueiredo, Fagundes, Schubach, De Paula, Faissal, Fonseca, Thoma and Marzochi2009) and Trypanosoma evansi (Elshafie et al. Reference Elshafie, Sani, Hassan, Sharma, Bashir and Abubakar2013), though smaller than T. theileri (Wink, Reference Wink1979), T. caninum (Madeira et al. Reference Madeira, Sousa, Barros, Figueiredo, Fagundes, Schubach, De Paula, Faissal, Fonseca, Thoma and Marzochi2009) and T. rangeli (Madeira et al. Reference Madeira, Sousa, Barros, Figueiredo, Fagundes, Schubach, De Paula, Faissal, Fonseca, Thoma and Marzochi2009; Madeira et al. Reference Madeira, Almeida, Barros, Oliveira, Sousa, Alves, Miranda, Schubach and Marzochi2014).
Here, we observed considerable variation in the total length of trypomastigote forms of T. rhipicephalis, ranging from 19.87 to 45.85 µm. A wide variation was also observed in other dimensions, such as the distance between the posterior end and the kinetoplast, and between the posterior end and the nucleus. Similar observations were made by Chagas (Reference Chagas1909) and Hoare (Reference Hoare1972) when they described the trypomastigote forms of T. cruzi, which also presented morphometric variation in total length [16.3–21.8 µm according to Hoare (Reference Hoare1972)]. Pleomorphism in T. rangeli, Trypanosoma minasense and Trypanosoma saimirii was also observed by Ziccardi and Lourenço-de-Oliveira (Reference Ziccardi and Lourenço-de-Oliveira1998) in primates of the Brazilian Amazon. Factors such as host species and stage of infection, as well as the Trypanosoma strain involved, may influence the occurrence of this variation (Brener and Chiari, Reference Brener and Chiari1965; Andrade, Reference Andrade1974; Urdaneta-Morales, Reference Urdaneta-Morales1983). Accordingly, characterization studies of Trypanosoma species should use more than just morphometry as a tool for specific identification; isozymes, molecular and phylogenetic analysis are currently widely used (Madeira et al. Reference Madeira, Sousa, Barros, Figueiredo, Fagundes, Schubach, De Paula, Faissal, Fonseca, Thoma and Marzochi2009).
Trypanosoma rhipicephalis strain P1RJ presents some biological characteristics uncommon in protozoa of the genus Trypanosoma. For example, the isolation performed into a tick cell line was unusual, as the isolation of trypanosomes is usually carried out in a biphasic NNN blood agar culture medium (Madeira et al. Reference Madeira, Sousa, Barros, Figueiredo, Fagundes, Schubach, De Paula, Faissal, Fonseca, Thoma and Marzochi2009). Moreover, developmental forms without a visible free flagellum were observed in axenic cultures. Recently, Barros et al. (Reference Barros, Fonseca, Macedo-Silva, Côrte-Real, Toma and Madeira2014) described epimastigote forms without flagella in T. caninum, confirmed by electron microscopy. The flagellum is considered a sophisticated structure in trypanosomes and problems regarding the expression of proteins responsible for the development of this organelle are related to the appearance of mutant forms without flagella. Bastin et al. (Reference Bastin, MacRae, Francis, Matthews and Gull1999) and Li et al. (Reference Li, Gerdes, Haycraft, Fan, Teslovich, May-Simera, Li, Blacque, Li, Leitch, Lewis, Green, Parfrey, Leroux, Davidson, Beales, Guay-Woodford, Yoder, Stormo, Katsanis and Dutcher2004) studied axoneme proteins; comparing flagellated and non-flagellated parasites, they noticed that these proteins were present in flagellated forms and absent in non-flagellated forms. The reasons for this phenomenon are still unknown, but these facts point to the possibility that the appearance of these atypical forms may be the result of disruption during cell division, which leads to the inability to form and expend the flagellum. As T. rhipicephalis is a new species, this information may be useful to clarify aspects of the life cycle of this parasite.
Another atypical feature is the failure of T. rhipicephalis to grow when incubated at 26, 28 and 37 °C either in conventional culture media or in L15B. In vitro culture approaches are fundamental to understand important parasite characteristics, such as morphological patterns and multiplication profiles. The great diversity of culture media may, for example, provide nutritional requirement information for these parasites (Schuster and Sullivan, Reference Schuster and Sullivan2002). Knowledge of the replication stage of trypanosomes is important when studying new species. For example, in some species of the subgenus Megatrypanum (T. theileri, T. melophagium and Trypanosoma conorhini), replication occurs in the epimastigote stage in mammals (Hoare, Reference Hoare1972).
Several genetic markers have been used for the molecular analysis of parasites of the genus Trypanosoma. For conserved regions of the genome, genes of the large and small subunits of rDNA (18S rRNA and 24Sα rDNA) are often used for diagnosis, particularly for the identification of new species (Lima et al. Reference Lima, Da Silva, Neves, Attias, Takata, Campaner, De Souza, Hamilton and Teixeira2012; Simo et al. Reference Simo, Sobgwi, Njitchouang, Njiokou, Kuiate, Cuny and Asonganyi2013; Villareal et al. Reference Villareal, Mingala and Rivera2013) and phylogenetic studies of related species (García et al. Reference García, Ortiz, Osorio, Torrico, Torrico and Solari2012).
Phylogenetic analysis of the 18S rRNA gene (Fig. 3A) showed that T. rhipicephalis strain P1RJ clusters within a clade with T. caninum and Trypanosoma KG1. In the 24Sα rDNA phylogenetic analysis (Fig. 3B), T. rhipicephalis is on a separate branch within a single clade, along with T. caninum. 18S rRNA and 24Sα rDNA sequencing confirmed the authenticity of T. rhipicephalis as a new species.
Trypanosoma sp. KG1 was originally described after isolation from naturally infected Hemaphysalis hystricis ticks in Japan (Thekisoe et al. Reference Thekisoe, Honda, Fujita, Battsetseg, Hatta, Fujisaki, Sugimoto and Inoue2007). The phylogenetic proximity of T. rhipicephalis to Trypanosoma sp. KG1 may be related to both Trypanosoma being isolated from a naturally infected tick. However, Latif et al. (Reference Latif, Bakheit, Mohamed and Zweygarth2004) showed that Hyalomma anatolicum could be infected with a trypanosome described as T. theileri when feeding as nymphs on an infected calf and that T. theileri survived the moult and multiplied in the resulting unfed adult ticks. Another species with phylogenetic proximity to T. rhipicephalis is T. caninum isolated in axenic culture from the skin of domestic dogs captured in different states of Brazil, including Rio de Janeiro (Madeira et al. Reference Madeira, Sousa, Barros, Figueiredo, Fagundes, Schubach, De Paula, Faissal, Fonseca, Thoma and Marzochi2009; Barros et al. Reference Barros, Toma and Madeira2015). Although the vector of T. caninum is still unknown, it is possible that it is transmitted by ticks, which might validate the phylogenetic proximity of T. rhipicephalis to T. caninum. Based on the detection of developmental forms of Trypanosoma in naturally infected tick haemolymph, we suggest a possible involvement not only of the R. microplus tick in the biological transmission of T. rhipicephalis, but also the involvement of other species of ticks as biological vectors of other species of the genus Trypanosoma. The isolation of this new species of the genus Trypanosoma in a line of embryonic tick cells suggests that these cells served as a substrate for the development of trypanosomes. Further studies are needed to investigate whether Trypanosoma internalization occurs in tick cells, which may indicate some intracellular phase of its life cycle and the vector potential of ticks.
The study of new species should consider both the molecular profile and aspects related to biology, such as the developmental forms that the parasite can display during its life cycle. For T. rhipicephalis, pathogenicity, involvement with vertebrate hosts, epidemiology, the developmental cycle and transmission mechanisms are still unknown and more studies are necessary.
Description
Name: Trypanosoma rhipicephalis sp. nov.
Mammalian host: Unknown.
Location: City of Seropedica, State of Rio de Janeiro, Brazil.
Vector: Possibly R. microplus tick.
Biology and morphology
This species was isolated in co-culture with the tick cell line IDE8 and grows well in L15B medium supplemented with FCS. The developmental stages found in axenic cultures were epimastigotes (predominantly), trypomastigotes and spheromastigotes. The total average body length of the epimastigote form was 32·44 µm, free flagellum 6·90 µm and the longitudinal axis of the kinetoplast was 1·21 µm. The total body length of trypomastigotes averaged 29·34 µm, free flagellum 5·72 µm and the longitudinal axis of the kinetoplast was 1·02 µm. The total average body length of the spheromastigote form was 16.13 µm, free flagellum 11·66 µm and the longitudinal axis of the kinetoplast was 1·06 µm.
Molecular characteristics
The trypanosome presents amplified products for the 24Sα rDNA gene of about 250 bp using D75/D76 primers. In the first nested-PCR reaction for the 18S rDNA gene with primers TRY927F and TRY927R, the amplified fragment was 900 bp. In the second reaction, using the SSU561F and SSU561R primers, the amplified fragment was 700 bp. In the phylogenetic analysis of ribosomal genes, this trypanosome is completely different from other described trypanosome species and closely related to Trypanosoma sp. KG1 and T. caninum.
Storage
Axenic cultures of these trypanosomes are cryopreserved in 10% DMSO, stored in liquid nitrogen at –196 °C and deposited in the Parasitic Diseases Laboratory (LDP), located in Annex I of the Veterinary Institute, Department of Epidemiology and Public Health, Federal Rural University of Rio de Janeiro (UFRRJ), municipality of Seropedica, state of Rio de Janeiro, Brazil.
Acknowledgements
The authors thank Prof Ulrike Munderloh, University of Minnesota, and the Tick Cell Biobank for providing the tick cell line IDE8.
Financial support
This study was supported by grants from the Fundação de Amparo à Pesquisa do Rio de Janeiro (A.H.F., grant number E 26/201.144/2014) – (Research Support Foundation of the State of Rio de Janeiro – FAPERJ); Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development – CNPq) (A.H.F. 305480/2013–8); and the Biotechnology and Biological Sciences Research Council (L.B.S., grant numbers BBS/E/1/00001741 and BB/P024270/1).
Conflict of interest
None.










