INTRODUCTION
The Galápagos archipelago in the eastern Pacific Ocean is a biodiversity hotspot supporting many endemic species and a diverse assemblage of marine birds (Bensted-Smith, Reference Bensted-Smith2002; Palacios et al. Reference Palacios, Bograd, Foley and Schwing2006). In contrast to many islands settled by humans, there is no evidence of local extinction of seabird species in Galápagos (Steadman, Reference Steadman2006). Nevertheless, some species show clear evidence of recent reductions in population sizes (Vargas et al. Reference Vargas, Lougheed and Snell2005; Jiménez-Uzcátegui et al. Reference Jiménez-Uzcátegui, Milstead, Márquez, Zabala, Buitrón, Llerena and Fessl2006; Anderson et al. Reference Anderson, Huyvaert, Awkerman, Proaño, Milstead, Jiménez-Uzcátegui, Cruz and Grace2008), making discerning the causes of such declines a conservation priority. The blue-footed booby (Sula nebouxii) breeds in Galápagos and on islands and headlands on the west coast of South and Central America and Mexico. The Galápagos subspecies (S. nebouxii excisa) is both iconic and genetically distinct (Taylor et al. Reference Taylor, Maclagan, Anderson and Friesen2011) and was historically abundant, with probably >10 000 breeding pairs in the 1960s (Nelson, Reference Nelson1978). However, it has apparently declined by more than 50% in the last decade, probably due to persistent low breeding frequency and poor breeding success since around 1998, resulting in chronically low recruitment to the adult population (Anchundia et al. Reference Anchundia, Huyvaert and Anderson2014). It has been suggested that this recent poor breeding is due to low local abundance of the species’ principal prey (Pacific sardines Sardinops sagax). Other potential effects, such as impacts of introduced predators, were considered unlikely causes because breeding has been equally poor on islands with and without such factors (Anchundia et al. Reference Anchundia, Huyvaert and Anderson2014). The effects of parasites, however, have not previously been considered.
Tourism and human immigration to Galápagos have increased dramatically over the past 30 years (Taylor et al. Reference Taylor, Hardner and Stewart2009), posing a high risk of novel pathogen introduction that could negatively impact native species (Wikelski et al. Reference Wikelski, Foufopoulos, Vargas and Snell2004; Kilpatrick et al. Reference Kilpatrick, Daszak, Goodman, Rogg, Kramer, Cedeño and Cunningham2006; Bataille et al. Reference Bataille, Cunningham, Cedeño, Cruz, Eastwood, Fonseca, Causton, Azuero, Loayza, Martinez and Goodman2009). One such threat is infection with blood parasites, particularly those such as Plasmodium and Haemoproteus (Parker et al. Reference Parker, Whiteman and Miller2006; Levin et al. Reference Levin, Outlaw, Vargas and Parker2009) that can cause acute or chronic infections in the host. Infection with haemoparasites can cause anaemia, altering red blood cell (RBC) parameters and reducing oxygen transport (Booth and Elliott, Reference Booth and Elliott2002), affecting the overall physiological condition of infected individuals and their ability to perform energy-demanding activities such as raising chicks. Infected birds also mount an immune response which requires resources that are withdrawn from other activities, such as those involved in reproduction (Sheldon and Verhulst, Reference Sheldon and Verhulst1996). Accordingly, several studies have shown that avian haemoparasites can adversely affect reproduction through lowering hatching success and/or reducing provisioning and growth rates of chicks and survival to fledging (Merino et al. Reference Merino, Moreno, Sanz and Arriero2000; Marzal et al. Reference Marzal, De Lope, Navarro and Møller2005; Tomás et al. Reference Tomás, Merino, Moreno, Morales and Martínez-de la Puente2007). Plasmodium has been strongly implicated in driving declines in populations of birds elsewhere (Atkinson et al. Reference Atkinson, Woods, Dusek, Sileo and Iko1995). In contrast, even though Haemoproteus spp. have been known to cause disease and mortality in some avian hosts (Earle et al. Reference Earle, Bastianello, Bennett and Krecek1992; Davidar and Morton, Reference Davidar and Morton1993; Garvin et al. Reference Garvin, Homer and Greiner2003), they are generally regarded as benign, and multiple studies have not found any negative association between infection with Haemoproteus spp. and survival or reproduction of their host (e.g. Knutie et al. Reference Knutie, Waite and Clayton2013; Kulma et al. Reference Kulma, Low, Bensch and Qvarnström2014; Zylberberg et al. Reference Zylberberg, Derryberry, Breuner, Macdougall-Shackleton and Hahn2015).
Haemoparasites have recently been recorded in several seabird species within the Galápagos archipelago, including Galápagos penguin (Spheniscus mendiculus), swallowed-tailed gull (Creagrus furcatus), frigatebirds (Fregata spp.), Nazca booby (Sula grantii) and red-footed booby (Sula sula) (Padilla et al. Reference Padilla, Whiteman, Merkel, Huyvaert and Parker2006; Levin et al. Reference Levin, Outlaw, Vargas and Parker2009, Reference Levin, Valkiūnas, Santiago-Alarcon, Lee Cruz, Iezhova, O'Brien, Hailer, Dearborn, Schreiber, Fleischer, Ricklefs and Parker2011), but it is not known if infection has affected reproduction in any of these species. Blue-footed boobies are hosts to ticks (Argasidae and Ixodidae), louse flies (Hippoboscidae) and black flies (Simuliidae), all of which are potential vectors of haemoparasites (Valkiūnas, Reference Valkiūnas2005). Also the mosquito Culex quinquefasciatus, which is found around human settlements in the Galápagos, is a known vector of avian Plasmodium (Bataille et al. Reference Bataille, Cunningham, Cedeño, Cruz, Eastwood, Fonseca, Causton, Azuero, Loayza, Martinez and Goodman2009). Moreover, birds from one colony may disperse to other sites within the archipelago (Taylor et al. Reference Taylor, Maclagan, Anderson and Friesen2011), and so can potentially transport parasites between colonies. Here we assess if haemoparasites, specifically Haemoproteus sp. and Plasmodium sp., are present in adult blue-footed boobies at six breeding colonies in Galápagos. To evaluate if these haemoparasites could be implicated in the recent poor breeding of this species in Galápagos, we assess if, at two of these colonies, parasitic infection is associated with low body condition, altered haematological parameters of adults, or with low breeding success among those birds that initiated breeding. Low breeding success could also result from direct effects of parasites on chicks. Hence at two study sites, we also assess if parasites are present in chicks and, if so, whether they have detectable effects on growth, haematological parameters or survival to fledging.
MATERIALS AND METHODS
Study sites and sampling
To examine the prevalence of haemoparasite infection and impacts on haematological parameters, we obtained blood samples from adults at six breeding sites in Galápagos (Fig. 1) during the main breeding periods (April–September) of 2007 and 2008. Adults were captured at the nest site during the daytime using a telescopic pole with a brass noose, following Hamer et al. (Reference Hamer, Phillips, Wanless, Harris and Wood2000); most adults had eggs or chicks at the time of sampling. Bill length was measured to the nearest 1 mm using metric tape, and body mass was measured using a spring balance (±10 g) when captured. Sex was determined by examination of the iris and by vocalization (Nelson, Reference Nelson1978), and most birds were fitted with a numbered metal leg-ring to allow individual identification. Birds were handled for no longer than 5 min (and typically much less) and, upon release, all birds immediately returned to the nest-site from which they were captured and resumed normal behaviour.
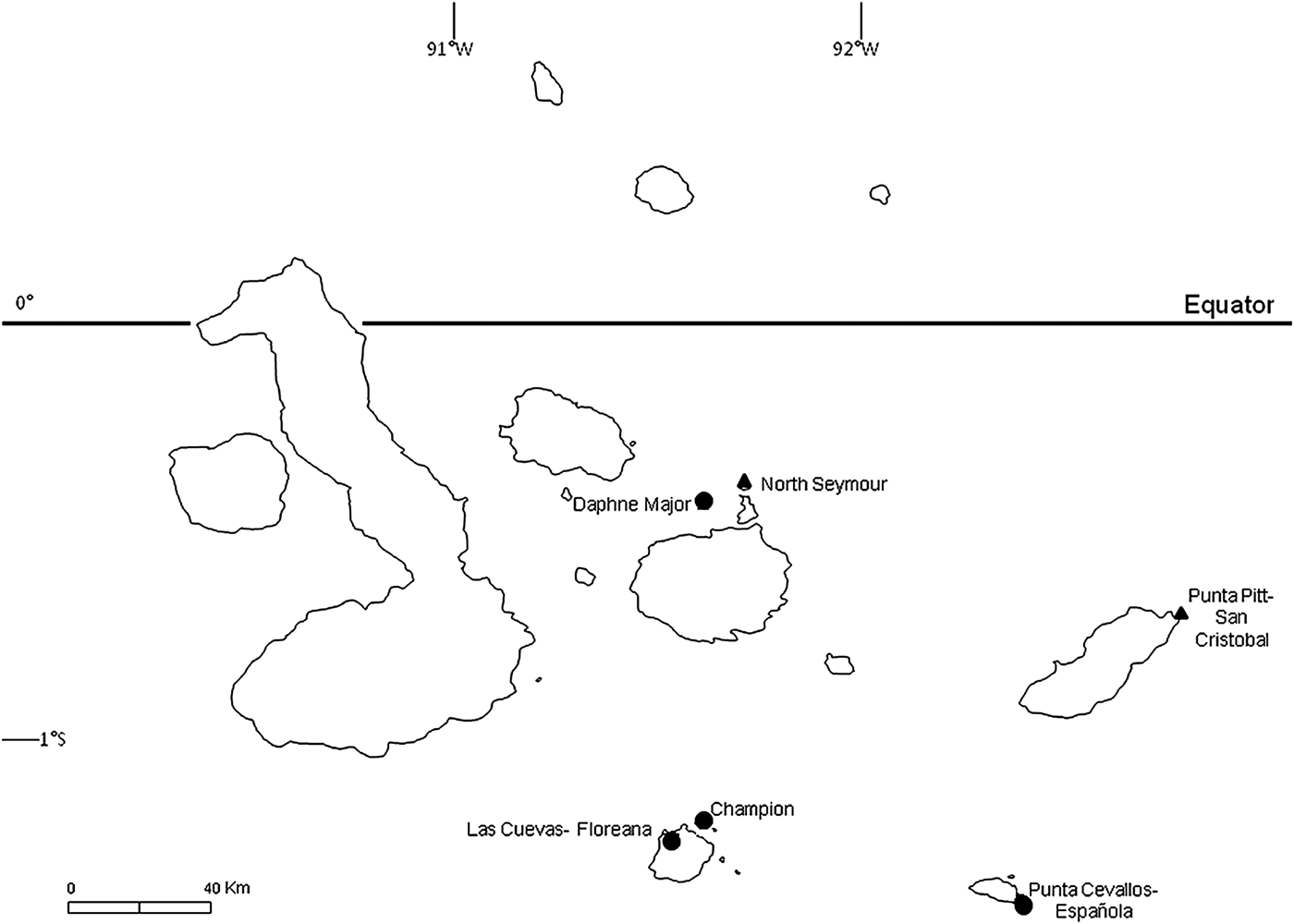
Fig. 1. Blue-footed booby breeding colonies sampled during 2007 and 2008. Focal colonies (see text) are marked with a ▲; other sampled colonies are marked with a ●.
To examine impacts of haemoparasites on breeding birds, we studied two focal breeding sites on the islands of San Cristobal and North Seymour (Fig. 1) in 2007 and 2008, respectively. In each case, all known nest sites occupied by adults were surveyed daily to record eggs or chicks, except for 12 nests in 2008 which were surveyed every 6 days. The extended laying periods of birds at each site precluded obtaining complete data on breeding success, but we were able to determine the fates of eggs laid and, if hatched, the growth and survival of chicks for most study pairs. For chicks, body mass was measured every 6 days using a spring balance (±5 g for chicks weighing up to 1 kg; ±10 g for heavier chicks), and bill and ulna length were measured on each occasion to the nearest 1 mm using metric tape. Chicks were also blood-sampled to examine the prevalence of haemoparasites and potential impacts on haematological parameters and growth rates. Sexes of chicks were determined from blood samples using the PCR protocol of Fridolfsson and Ellegren (Reference Fridolfsson and Ellegren1999). Average ages of chicks when blood-sampled were 72 ± 17 days post-hatching at San Cristobal and 65 ± 14 days at North Seymour.
Blood sampling
Roughly 1–3 mL of blood were taken from the ulnar vein of each bird using a 23-gauge needle and syringe, collected in ethylenedimine tetra-acetic acid (EDTA) tubes and stored on ice until taken to the laboratory, where they were stored at 4 °C for a maximum of 48 h before haematological parameters were measured. Fresh blood smears were made immediately after blood sampling using one drop of blood smeared on a slide. Birds were blood-sampled only once except for 30 adults at North Seymour that were sampled during both incubation and chick-rearing, to examine the temporal persistence of blood parasite infections.
Screening for parasites
Parasite screening was performed by microscopic examination of blood smears, viewing 100 fields per smear using a 100× objective. We also screened for Haemoproteus spp. and Plasmodium spp. using a nested polymerase chain reaction (PCR) specific for detecting these genera (Waldenström et al. Reference Waldenström, Bensch, Hasselquist and Östman2004). DNA was extracted from 30 µL of EDTA-preserved blood according to Bermúdez-Humarán et al. (Reference Bermúdez-Humarán, García-García, Leal-Garza, Riojas-Valdes, Jaramillo-Rangel and Montes-de-Oca-Luna2002). We analysed 95 samples using the nested PCR method. For 154 samples we used the same nested PCR method with slightly modified primers as follows: nHaemNF (5′-CAACATATATTAAGAGAATTATGGA-3′) and nHaemNR2 (5′-AACAATATGTAGAGGAGTAACATAT-3′) for the first PCR, and HaemF from Waldenström et al. (Reference Waldenström, Bensch, Hasselquist and Östman2004) and the new primer nHaemR2 (5′CATTATCAGGATGWGCMTTAATGGTA-3′) for the second PCR. PCR reactions were performed on 25 µL volumes using Flexi Go Taq (Promega), 0.2 mm of each dNTP (i.e. nucleotides), 3 mm MgCl2, 0.02 mm of each primer and 2 µL of template DNA. The first PCR was carried out with an initial denaturation step of 94 °C for 3 min, then 20 cycles with 94 °C for 30 s, 50 °C for 30 s and 72 °C for 45 s, and a final extension at 72 °C for 10 min. The second PCR followed the same thermal profile as the first but was run for 35 cycles with an annealing temperature of 52 °C. PCR products were separated on a 2% agarose gel where the presence of a band of the expected size (520 bp) indicated the presence of blood parasites. All PCR reactions were run with a negative control (ultrapure water; Sigma, UK) and a positive control (Plasmodium falciparum DNA). Positive PCR products from five different birds were sequenced using an ABI 3730 Automated Sequencer (PE Applied Biosystems Ltd., USA) at the NERC Molecular Genetics Facility, University of Sheffield. DNA sequences were compared with publicly available sequences in the GenBank database using BLASTN 2.2.4 (Altschul et al. Reference Altschul, Madden, Schaffer, Zhang, Zhang, Miller and Lipman1997). No PCR products using the modified primers were sequenced, but two positives and one negative were used in each run.
Haematology
RBC counts were performed on a Neubauer haemocytometer using 20 µL of blood diluted in 1000 µL of Reese–Eckers dying solution (Campbell, Reference Campbell1995). Haemoglobin (Hb) concentration was measured using the Hemocue system (Hemocue® Limited) based on absorbance of light. Mean corpuscular volume (MCV) was calculated using a standard formula (Campbell, Reference Campbell1995).
Total white blood cell (WBC) counts were performed on a Neubauer haemocytometer using 20 µL of whole blood diluted in 380 µL of Reese–Eckers dying solution (Campbell, Reference Campbell1995). Leucocyte differentials were determined by examination of whole-blood smears made from fresh blood or from blood preserved in EDTA-tubes. All smears were air dried, fixed in methanol and stained with May–Grünwald–Giemsa stain within the first 24–48 h after preparation. If the quality of fresh-blood smears was poor, EDTA-preserved blood smears were used instead. EDTA does not affect leucocyte counts within the first 24–72 h for blood stored at 4 °C (Buttarello, Reference Buttarello2004). Leucocyte differentials for each bird were determined by the percentage of heterophils, eosinophils, lymphocytes, monocytes and basophils in a total count of 100 leucocytes. Leucocyte counts were performed using a 100× objective lens, and were always performed by the same person (LLC).
Statistical analyses
To examine the potential factors associated with variation in infection at a site, we analysed prevalence of haemoparasitism at our two focal study sites using a generalized linear model (GLM) with binomial distribution of errors, with sex, breeding phase (incubation or chick-rearing) and their interaction as factors. We then applied a similar model to chicks with sex as a factor and age at sampling as a covariate. We also compared haemoparasite prevalence between adults and chicks at each site with a similar model using age-class (adult or chick) as a factor.
Impact of parasitism on adults
To examine effects of haemoparasitism on adult body mass, we used a linear model with infection status, sex, breeding phase and all two and three way interactions as factors and bill length as covariate. For North Seymour, where 30 adults had been sampled during both incubation and chick-rearing, we included bird identity as a random factor to account for birds sampled during both breeding phases.
We assessed the effect of blood parasites on haematological parameters of adults within each focal study colony separately. We fitted a linear model (LM), GLM, or generalized mixed-effects models (GLMM) with each haematological parameter in turn as the response variable. We used breeding phase, presence or absence of parasites (binary variable), sex and all two- and three-way interactions as factors for this analysis. For North Seymour, bird identity was again included as a random factor. Overdispersion in GLMs was accounted for using a quasipoisson distribution of errors; in GLMMs we included an additional term based on the total number of observations to account for overdispersion, and therefore we removed bird identity if its standard deviation was less than that of the additional term. We added 1 to all lymphocyte count values to avoid log-transformation of any zero values. Non-significant interactions were removed sequentially.
Timing of breeding
We seldom observed egg-laying directly and so we used the median hatching date of chicks at each nest-site to measure timing of breeding. We estimated hatching dates of chicks by direct observation or, where hatching was not observed, from bill and ulna lengths calibrated against growth in first-hatched chicks of known age. Chick age was estimated from a logistic model fitted separately for bill and ulna lengths at each colony (r > 0.95 in all cases). The two estimates for age of chick (i.e. age estimated from bill and ulna length models) were then averaged.
Adults often changed their parasitaemia status between incubation and chick-rearing (see ‘Results’ section) and so we used only adults blood-sampled during incubation at North Seymour to determine whether or not haemoparasites affected timing of breeding (few adults were sampled during incubation at San Cristobal). We used a Mann–Whitney test to compare median hatching dates of clutches incubated by adults with and without haemoparasites, analysing males and females separately. We also used a Kruskal–Wallis test to assess if median hatching date differed among nests with neither, one or both parents infected.
Impact of parasitism on chicks
Blue-footed booby chicks typically attain maximum mass at 65–70 days post-hatching (Drummond et al. Reference Drummond, Osorno, Torres, García-Chavelas and Merchant Larios1991). To avoid including data during the period of pre-fledging mass recession, we therefore assessed the effect of haemoparasitism on body mass at the greatest age measured prior to 60 days post-hatching (Mean ± SE: 59.4 ± 0.56 and 58.7 ± 0.55 days old at San Cristobal and North Seymour, respectively) using a linear model with haemoparasitism status, sex and their interaction as factors and chick age as a covariate.
To assess the effect of haemoparasitism on haematological parameters of chicks, we used LMs or GLMs with each haematological parameter in turn as the response variable, with blood infection status, sex and their interaction as factors, and with chick age at sampling as a covariate. Overdispersion in GLMs was accounted for using a quasipoisson distribution of errors and non-significant interactions were removed sequentially.
Chick survival
At San Cristobal all but two chicks alive at the end of the study period had fledged. At North Seymour, survival to fledging was not recorded, but chicks alive at the end of the study were on average 60 ± 10 (s.d.) days old, and chicks that died did so on average at 26 ± 15 (s.d.) days post-hatching. However, at least one chick died at 70 days of age, thus it is possible that survival to fledging was slightly overestimated for North Seymour. We compared the number of chicks that survived until the end of the study period (i.e. 0–2), between parasitaemic and non-parasitaemic adults. We analysed males and females separately at each colony. For birds at San Cristobal we used a Mann–Whitney test. For North Seymour, 15 adult pairs were blood-sampled during both incubation and chick-rearing, and so we used a mixed effects generalized linear model with Poisson distribution of errors; parasites, breeding phase and their interaction were used as factors, and bird ID was included as a random factor. For females alone, however, as the standard deviation of bird ID was close to zero, we used a generalized linear model without any random factor. The interaction between haemoparasitism and breeding phase was non-significant in all cases, thus we removed it from the models. We also compared if chick survival differed among nests where none, one or both adults were parasitaemic using a Kruskal–Wallis test. For birds at North Seymour we used PCR data of chick-rearing birds for this analysis.
All analyses were performed using R version 2.13.1 (R Development Core Team, 2008).
RESULTS
Prevalence of haemoparasitism
We obtained 249 blood samples from 159 adult blue-footed boobies at six breeding colonies in Galápagos and from 60 chicks at our two focal colonies. Haemoparasite prevalence assessed using PCR of blood was high (33–83%) at all sampled sites and in both adults and chicks (Table 1). The primers used are specific for Haemoproteus sp. and Plasmodium sp., with a high detection rate of Plasmodium compared with other assays (Waldenström et al. Reference Waldenström, Bensch, Hasselquist and Östman2004). We sequenced five PCR products (GenBank accession numbers JF833060–JF833064), all of which were at least 99% similar to each other and at least 95% similar to published Haemoproteus sequences in GenBank (closest match to Haemoproteus sp. LIN27, accession number EF380192.1). Also there was no suggestion of mixed infection (i.e. no double peaks in the chromatogram). Thus we assume that all parasites detected by PCR were Haemoproteus sp. In contrast, of the 249 blood smears screened, only one contained one parasite, probably belonging to the genus Leucocytozoon (G. Valkiūnas, personal communication 2014). No evidence of Plasmodium sp. infection was found. Prevalence of circulating Haemoproteus sp. infection did not differ significantly between males and females in adults or chicks, or between incubation and chick-rearing for adults at either of our two focal colonies (Table 1; LRT1, P ≥ 0.1 in all cases). Chicks were marginally more likely to be parasitaemic than adults at San Cristobal (LRT1 = 3.73, P = 0.05) but not at North Seymour (P = 0.52). Adults sampled twice at North Seymour commonly changed the parasitaemia status; of 20 birds parasitaemic during incubation, 15 (75%) were PCR-negative for haemoparasites during chick-rearing, while of 10 birds that were apparently free of circulating parasites during incubation, 7 (70%) were PCR-positive during chick-rearing.
Table 1. Number of blue-footed boobies sampled for parasite screening and percentage of birds with Haemoproteus sp. parasitaemia at six breeding sites in the Galápagos Islands
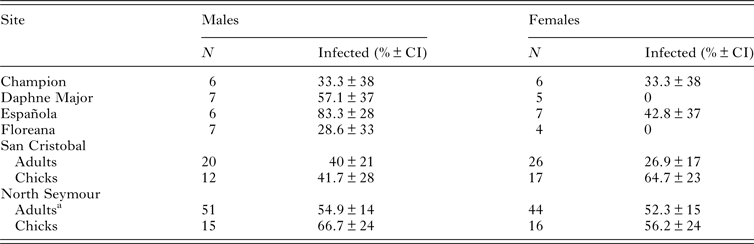
a 30 adults were sampled twice (i.e. during incubation and chick-rearing), thus N refers to number of samples.
Impact of parasitism on adults
The presence of blood parasites had no detectable effect on body mass or on any haematological variables in the birds sampled at San Cristobal (Table 2). At North Seymour, parasitaemia had no detectable effect on MCV or Hb but had marginally non-significant effects on body mass (P = 0.08) and heterophil count (P = 0.06); PCR-positive birds tended to be heavier and with a lower heterophil count than PCR-negative birds. Lymphocyte count and H/L ratio were affected by the presence of parasitaemia, although there was an opposite effect in males and females (Table 2).
Table 2. LM, GLM or GLMM of the presence of Haemoproteus sp. parasitaemia on physiological parameters of blue-footed booby adults at two breeding colonies in the Galápagos Islands
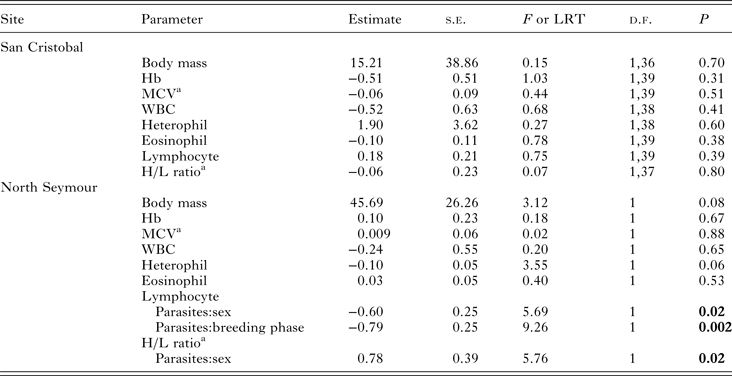
Breeding phase, sex, presence of parasites and all interactions were used as factors. Non-significant interactions were removed sequentially. Hb, haemoglobin; MCV, mean corpuscular volume; WBC, total white blood cell count.
a Variable was ln-transformed.
Timing of breeding
Hatching dates did not differ significantly between clutches incubated by parasitaemic and non-parasitaemic females or males at North Seymour (P ≥ 0.2 in both cases), and did not differ among nests where neither parent, one or other parent, nor both parents were parasitaemic (χ2 = 0.85, d.f. = 2, P = 0.65).
Impact of parasitism on chicks
Chick body mass at 60 days old did not differ in relation to the PCR result obtained from either their female or male parent at North Seymour (female, t 11 = 1.43, P = 0.18; male, t 8 = −0.12, P = 0.91), was marginally lower for chicks at San Cristobal for which the male parent was PCR-positive at the time of blood-sampling, (t 11 = −2.0, P = 0.07), but did not differ in relation to the blood PCR-status of their female parent (t 11 = 1.04, P = 0.32). Also, chick body mass at 60 days old did not differ between chicks that were parasitaemic and those that were not at the time of blood-sampling (all P > 0.05). Of the haematological parameters measured, only H/L ratio was apparently affected by nestling parasitaemia (San Cristobal: parasites–sex interaction, F 1,21 = 6.23, P = 0.02; North Seymour: parasites F 1,27 = 4.06, P = 0.05). This effect was, however, mainly attributable to the haematological results of two chicks at San Cristobal and one chick at North Seymour that had particularly high H/L ratios.
Chick survival
No effect of adult blood PCR-status was found on chick survival at San Cristobal (females, W = 20.5, P = 0.33; males, W = 27.5, P = 0.59) or North Seymour (females, LRT1 = 0.57, P = 0.45; males, LRT1 = 0.04, P = 0.83). Also, chick survival did not differ among nests where neither, one or other, nor both parents were PCR-positive (San Cristobal: χ 2 = 1.34, d.f. = 2, P = 0.51; North Seymour: χ 2 = 3.69, d.f. = 2, P = 0.16).
DISCUSSION
Blood parasites were detected in blue-footed boobies from all of the six sites sampled. At the two focal breeding colonies, the prevalence of Haemoproteus sp. parasitaemia was high in both adults and chicks. This parasite was, however, detected only using PCR and was not found on microscopical examination of blood smears, which is consistent with low levels of parasitaemia (Bentz et al. Reference Bentz, Rigaud, Barroca, Martin-Laurent, Bru, Moreau and Faivre2006). Also, it is possible that in some cases our PCR assay detected free DNA rather than viable parasites. Only one parasite, probably Leucocytozoon sp., was found on examination of the blood smears. Importantly, no evidence of Plasmodium sp. infection was found from either microscopy or PCR of blood samples. We do not know of any case of Plasmodium sp. infection in boobies. However, given that mosquitoes can feed on seabirds in the Galápagos (Anderson and Fortner, Reference Anderson and Fortner1988) and that Plasmodium has been found in several bird species in the archipelago (Levin et al. Reference Levin, Zwiers, Deem, Geest, Higashiguchi, Iezhova, Jiménez-Uzcátegui, Kim, Morton, Perlut, Renfrew, Sari, Valkiūnas and Parker2013), there is potential of transmission of this parasite to Galápagos blue-footed boobies.
No differences in heterophil counts, eosinophil counts or in total WBC counts were detected between birds with and without parasitaemia. Increases in these haematological parameters can occur in response to high levels of Haemoproteus infection (Ots and Hõrak, Reference Ots and Hõrak1998; Garvin et al. Reference Garvin, Homer and Greiner2003; Dunn et al. Reference Dunn, Goodman, Benton and Hamer2013). Our haematology and PCR results, therefore, concur, suggesting that levels of parasitaemia were low in blue-footed boobies with negligible effects on circulating leucocytes. It is possible, however, that in our study birds negative for parasitaemia at the time of blood-sampling were not uninfected, but harboured only non-circulating, intra-endothelial tissue stages of Haemoproteus (Valkiūnas et al. Reference Valkiūnas, Bairlein, Iezhova and Dolnik2004). For instance, infections in which the life cycle of the parasite is not completed within the host can occur, resulting in the absence of gametocytes in the host's circulating blood (Valkiūnas et al. Reference Valkiūnas, Palinauskas, Ilgūnas, Bukauskaité, Dimitrov, Bernotiené, Zehtindjiev, Ilieva and Iezhova2014). This might also explain why the initial PCR-status of sampled adults at North Seymour during incubation was not a reliable indicator of parasitaemia at the time of the second blood-sampling period during chick-rearing. We did not analyse any other tissue apart from blood. Histological examination of naturally dead birds and more frequent, repeated blood sampling of individual birds would be required to identify infection by Haemoproteus reliably in Galápagos blue-footed boobies.
Based on our data, parasitaemia in adults affected only lymphocyte counts and H/L ratio, and only at North Seymour. Also, at this colony adults had similar prevalence of parasitaemia as chicks, whereas on San Cristobal the prevalence was significantly higher in chicks than in adults. Chicks are permanently exposed to biting vectors and are naïve to Haemoproteus infection; thus, it is common for them to reach higher infection loads, prevalences and durations of parasitaemia than adults (Merino and Potti, Reference Merino and Potti1995; Merino, Reference Merino2010). This apparent association between parasitaemia and haematological parameters and higher parasite prevalence only in adults at North Seymour may be related to overall poor condition of these birds compared with those at San Cristobal (adults at North Seymour were on average 14% lighter than those on San Cristobal). However, there could be other factors not detected by our study that affected adults’ physiological fitness on North Seymour.
We found no evidence of any association between Haemoproteus sp. parasitaemia and reproductive output at either focal breeding colony. Also, in contrast to other studies in which high levels of lymphocytes, heterophils, eosinophils and H/L ratio have been associated with haemoparasites in nestlings (Garvin et al. Reference Garvin, Homer and Greiner2003; Soler et al. Reference Soler, Neve, Pérez Contreras, Soler and Sorci2003; Norte et al. Reference Norte, Araújo, Sampaio, Sousa and Ramos2009), we did not find strong effects of parasitaemia on blood parameters on chicks at either colony. Only the H/L ratio appeared to be affected by parasitaemia, but this was due to two and one chick at San Cristobal and North Seymour, respectively, with particularly high H/L ratios. These three chicks had very low lymphocyte counts, two had a very high heterophil count and one had a particularly low body mass compared with other chicks its age. Even though it is possible that these leucocyte counts resulted from the Haemoproteus sp. infection detected in these chicks, other causes such as other pathogens, poor provisioning or sibling interactions cannot be ruled out (Maxwell, Reference Maxwell1993; Parejo et al. Reference Parejo, Silva and Avilés2007).
Several studies have reported the presence of blood parasites in wild birds in Galápagos (Padilla et al. Reference Padilla, Whiteman, Merkel, Huyvaert and Parker2006; Santiago-Alarcon et al. Reference Santiago-Alarcon, Outlaw, Ricklefs and Parker2010; Levin et al. Reference Levin, Valkiūnas, Santiago-Alarcon, Lee Cruz, Iezhova, O'Brien, Hailer, Dearborn, Schreiber, Fleischer, Ricklefs and Parker2011), including one report of the malarial parasite Plasmodium sp. in the Galápagos penguin (Levin et al. Reference Levin, Outlaw, Vargas and Parker2009), and there is great interest in the threat that such pathogens might present to the endemic Galápagos avifauna (Miller et al. Reference Miller, Hofkin, Snell, Hahn and Miller2001; Wikelski et al. Reference Wikelski, Foufopoulos, Vargas and Snell2004). Here we found that Haemoproteus sp. is prevalent in blue-footed boobies by PCR, but not by microscopy. This has also been the case for Plasmodium parasites in the Galápagos, with migratory birds passing through the archipelago being a possible reservoir of this pathogen (Levin et al. Reference Levin, Zwiers, Deem, Geest, Higashiguchi, Iezhova, Jiménez-Uzcátegui, Kim, Morton, Perlut, Renfrew, Sari, Valkiūnas and Parker2013). We obtained DNA sequences of parasites isolated from five different adult birds. The closest match to all five sequences was to a Haemoproteus sp. LIN 27 belonging to a chestnut bunting (Emberizidae) (Ishtiaq et al. Reference Ishtiaq, Gering, Rappolel, Rahmani, Jhala, Dove, Milensky, Olson, Peirce and Fleischer2007). Our sequences were also included in a phylogenetic analysis of Haemoproteus spp., and the results showed that the five sequences were closely related to Haemoproteus balmorali (see sequences for S. nebouxii in phylogenetic tree in Levin et al. Reference Levin, Valkiūnas, Santiago-Alarcon, Lee Cruz, Iezhova, O'Brien, Hailer, Dearborn, Schreiber, Fleischer, Ricklefs and Parker2011), found in members of the Turdidae and Muscicapidae families (Hellgren et al. Reference Hellgren, Križanauskiene, Valkiūnas and Bensch2007). These parasites belong to the Parahaemoproteus subgenus which is found in passerines. Thus, it is possible that Parahaemoproteus parasites are transmitted to blue-footed boobies in Galápagos from passerine hosts common around blue-footed boobies’ nests, such as finches or mockingbirds. Haemoproteus and Parahaemoproteus are two subgenera that form distinct clades within the genus Haemoproteus (Valkiūnas et al. Reference Valkiūnas, Santiago-Alarcon, Levin, Iezhova and Parker2010). Haemoproteus and Parahaemoproteus are transmitted by hippoboscid flies (Hippoboscidae) and biting midges (Culicoides, Ceratopogonidae), respectively. Hippoboscid flies are ectoparasites of passerines and marine birds in Galápagos (Levin et al. Reference Levin, Valkiūnas, Santiago-Alarcon, Lee Cruz, Iezhova, O'Brien, Hailer, Dearborn, Schreiber, Fleischer, Ricklefs and Parker2011; Štefka et al. Reference Štefka, Hoeck, Keller and Smith2011), but the role of biting midges as vectors of Haemoproteus spp. has not be confirmed in Galápagos.
Anchundia et al. (Reference Anchundia, Huyvaert and Anderson2014) suggested chronic breeding failure of blue-footed boobies in the Galápagos may be due to lack of their preferred prey, sardines. They pointed out that there is little evidence to suggest infectious disease as a possible cause of the observed decline. We found parasitaemic birds in all six colonies sampled, but results from our two focal colonies showed negligible impact on reproductive output in spite of high prevalence of Haemoproteus spp. parasitaemia in adults and chicks. No impact of Haemoproteus on survival or reproduction has been reported for several bird species (e.g. Knutie et al. Reference Knutie, Waite and Clayton2013, de Jong et al. Reference de Jong, Fokkema, Ubels, van der Velde and Tinbergen2014). Moreover, higher survival and reproduction has been related to Haemoproteus infection (Zylberberg et al. Reference Zylberberg, Derryberry, Breuner, Macdougall-Shackleton and Hahn2015). The authors of this study suggest that infected individuals may not invest in immune defence in the case of less virulent pathogens, if such investment would result in reduced lifetime reproductive success. This could also be the case on long-lived birds such as many marine birds. Additionally, long embryonic development in seabirds may allow for a strong immune system better able to fight infection (Ricklefs, Reference Ricklefs1992).
Chick survival at our two focal colonies was not related to parasitaemia. In contrast, it was closely associated with variation in growth: chicks that survived were much heavier from the third week post-hatching compared with age-specific body masses of chicks that died before fledging (GLMM, chick age-survival interaction, San Cristobal, LRT1 = 18.25, P < 0.0001; North Seymour, LRT = 13.23, P = 0.0003, Fig. 2). Chick growth at our study sites was also poor compared with that reported for chicks elsewhere. For instance, chicks 45 days old at San Cristobal and North Seymour had body masses roughly 30% lower compared with chicks the same age on Isla de Lobos de Tierra, Perú (Velando, Reference Velando2002). This difference was especially marked for chicks at North Seymour, which were lighter than those in San Cristobal, and also compared with chicks in Mexico (Drummond et al. Reference Drummond, Osorno, Torres, García-Chavelas and Merchant Larios1991) and to chicks measured at 13 sites in the Galápagos in 1981 (Ricklefs et al. Reference Ricklefs, Duffy and Coulter1984). These data support the hypothesis that the low breeding success of this species in Galápagos is related at least partly to scarce prey availability.
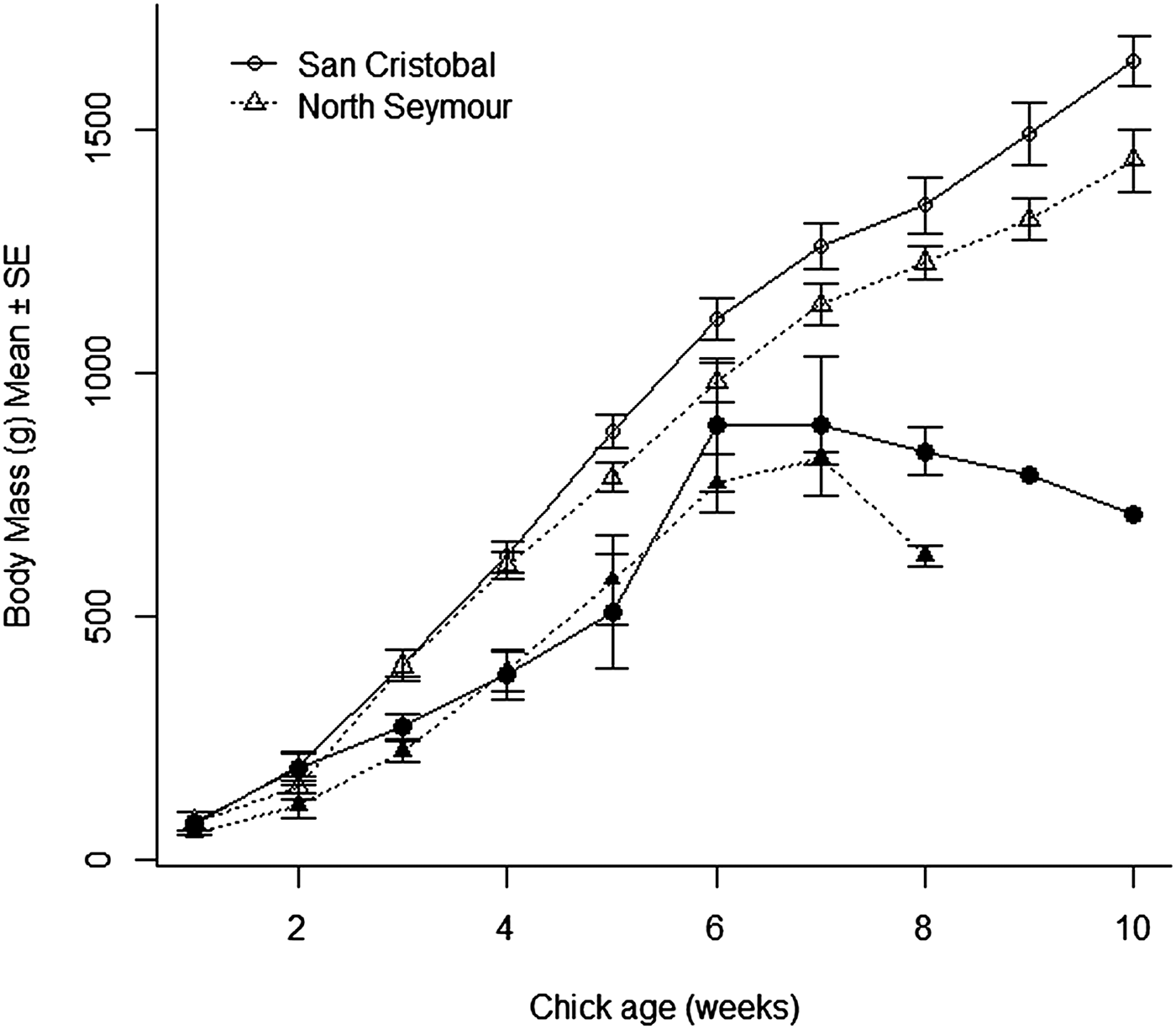
Fig. 2. Body mass of blue-footed booby chicks in relation to age of chicks that survived (open symbols) and chicks that died (filled symbols) at two breeding colonies in the Galápagos Islands.
Overall, our results suggest that the decline in blue-footed boobies in Galápagos is not strongly associated with Haemoproteus infection and we did not detect any infection with Plasmodium spp. malarial parasites. However we found a parasite, probably Leukocytozoon, on one of the smears, and future studies should use molecular methods to elucidate the prevalence of infection with this parasite in blue-footed boobies. This would be relevant as concurrent infections can be more harmful than single ones (Marzal et al. Reference Marzal, Bensch, Reviriego, Balbontin and De Lope2008). We did find a difference in body condition between birds in San Cristobal and North Seymour, with the latter generally being in poorer condition. Birds in poor condition can be more vulnerable to infectious diseases (Lochmiller and Deerenberg, Reference Lochmiller and Deerenberg2000, Alonso-Alvarez and Tella, Reference Alonso-Alvarez and Tella2001) and we also found higher Haemoproteus prevalence in both adults and chicks at North Seymour. There was, however, no significant difference between the two islands in breeding success of birds. Parasitic infection and poor body condition can have synergistic effects, which can impact on population dynamics (Beldomenico and Begon, Reference Beldomenico and Begon2010). Regular monitoring of the blue-footed booby population in the Galápagos archipelago is needed to assess if it continues to decline and if so, to help identify the causes.
ACKNOWLEDGEMENTS
We thank Sarah-L. Smith, Alberto Vélez, and Pablo Mejía for their help in the field, and David Anderson, Virna Cedeño, Washington Tapia, David Vizuete, Miton Mora, Efraín García and Nelson García for logistical support and advice. PCR products were sequenced at the NERC Molecular Genetics Facility, University of Sheffield. We also thank two anonymous reviewers for their comments on a previous version of this manuscript.
FINANCIAL SUPPORT
This study was funded by Consejo Nacional de Ciencia y Tecnología, México, postgraduate studies scholarship granted to L.L.C., and carried out in collaboration with the Galápagos National Park Service, and the Galápagos Genetics, Epidemiology and Pathology Laboratory, with support from the UK Government (DEFRA Darwin Initiative Grants 162-12-17 and EIDPO15 to S.J.G. and A.A.C.
CONFLICT OF INTEREST
None.
ETHICAL STANDARDS
The authors assert that all procedures including blood-sampling protocols contributing to this work comply with the ethical standards of the Galápagos National Park Service.








