Introduction
Macroecological patterns in body size are well documented for a wide variety of extant taxa, including endotherms and ectotherms in terrestrial, freshwater, and marine habitats worldwide (Ashton et al. Reference Ashton, Tracy and de Queiroz2000; Ashton Reference Ashton2002; Belk and Houston Reference Belk and Houston2002; Ashton and Feldman Reference Ashton and Feldman2003; Chown and Gaston Reference Chown and Gaston2010; Berke et al. Reference Berke, Jablonski, Krug, Roy and Tomasovych2013; Angielczyk et al. Reference Angielczyk, Burroughs and Feldman2015; Gohli and Voje Reference Gohli and Voje2016; Blackburn et al. Reference Blackburn, Redding and Dyer2019). Of these, the most commonly observed pattern conforms to Bergmann's rule, after Carl Bergmann, who described a relationship where larger body sizes are seen in species that live in colder climates compared with their smaller-bodied relatives that live in warmer climates (Reference Bergmann1847; see translation by James Reference James1970). Because the definition of the rule is itself a reformulated translation that has since been reinterpreted by various authors (e.g., Rensch Reference Rensch1938; Mayr Reference Mayr1963; James Reference James1970; Blackburn et al. Reference Blackburn, Gaston and Loder1999; Salewski and Watt Reference Salewski and Watt2017) and because no underlying mechanism is as widespread as the taxa that seem to follow it, the validity of Bergmann's rule has long been debated (Mayr Reference Mayr1956; McNab Reference McNab1971, Reference McNab2010; Blackburn et al. Reference Blackburn, Gaston and Loder1999; Ashton et al. Reference Ashton, Tracy and de Queiroz2000; Meiri and Dayan Reference Meiri and Dayan2003; Meiri et al. Reference Meiri, Yom-Tov and Geffen2007; Watt et al. Reference Watt, Mitchell and Salewski2010; Salewski and Watt Reference Salewski and Watt2017). The classical interpretation of Bergmann's rule is that larger endothermic animals withstand cold temperatures better by having a reduced surface to volume ratio (James Reference James1970). However, many studies have used definitions of Bergmann's rule that do not include a specific physiological mechanism or indeed any explicit underlying process: namely, that body size is positively correlated with latitude (Blackburn and Gaston Reference Blackburn and Gaston1996; Blackburn and Ruggiero Reference Blackburn and Ruggiero2001; Ashton Reference Ashton2002; Montgomery et al. Reference Montgomery, Mackessy and Moore2003; Meiri et al. Reference Meiri, Dayan and Simberloff2004).
Size–latitude relationships are a common test of Bergmann's rule, as climate is assumed to be colder at higher latitudes, although this correlation may be weak at small scales (Mayr Reference Mayr1963; Blackburn et al. Reference Blackburn, Gaston and Loder1999) or absent during times in Earth history with reduced temperature gradients from equator to poles. Importantly, latitude does not affect body size directly but is likely to correlate with body-size clines, because it encompasses more precise predictive variables that fluctuate over temporal and spatial scales to affect body size (reviewed in Yom-Tov and Geffen Reference Yom-Tov and Geffen2011). Indeed, body size has been shown to vary in response to precipitation (Nwaogu et al. Reference Nwaogu, Tieleman, Bitrus and Cresswell2018), elevation (Feder et al. Reference Feder, Papenfuss and Wake1982; Yu et al. Reference Yu, Wang, Busam and Deng2019), water depth (Timofeev Reference Timofeev2001), seasonality (James Reference James1970; Murphy Reference Murphy1985; Wells et al. Reference Wells, Saunders, Lea, Cortina-Borja and Shirley2019), geographic-range size (Ashton et al. Reference Ashton, Tracy and de Queiroz2000; Rodríguez et al. Reference Rodríguez, Olalla-Tárraga and Hawkins2008), resource availability (McNab Reference McNab2010; Correll et al. Reference Correll, Prowse and Prideaux2016; Brown et al. Reference Brown, Kotler and Porter2017; Kelly et al. Reference Kelly, Friedman and Santana2018), functional traits (Briscoe et al. Reference Briscoe, Krockenberger, Handasyde and Kearney2015), or predation risk and prey size (McNab Reference McNab1971), with only some correlations supporting a Bergmann-like pattern (e.g., Murphy Reference Murphy1985; Ashton et al. Reference Ashton, Tracy and de Queiroz2000; Timofeev Reference Timofeev2001; Rodríguez et al. Reference Rodríguez, Olalla-Tárraga and Hawkins2008; Briscoe et al. Reference Briscoe, Krockenberger, Handasyde and Kearney2015; Correll et al. Reference Correll, Prowse and Prideaux2016; Nwaogu et al. Reference Nwaogu, Tieleman, Bitrus and Cresswell2018; Yu et al. Reference Yu, Wang, Busam and Deng2019).
Tests for Bergmann's rule at different taxonomic scales can also give different results, with interspecific analyses obscuring intraspecific patterns that may be better explained by the specific variables mentioned previously, rather than latitude alone (e.g., Ashton et al. Reference Ashton, Tracy and de Queiroz2000; Freckleton et al. Reference Freckleton, Harvey and Pagel2003; Gohli and Voje Reference Gohli and Voje2016; Pallarés et al. Reference Pallarés, Lai, Abellán, Ribera and Sánchez-Fernández2019; Romano et al. Reference Romano, Séchaud and Roulin2021). Nonetheless, modern birds, mammals, and salamanders generally show support for Bergmann's rule when average body sizes of numerous species are compared across a wide latitudinal range (mammals: Ashton et al. Reference Ashton, Tracy and de Queiroz2000; Meiri et al. Reference Meiri, Dayan and Simberloff2004; birds: Blackburn and Gaston Reference Blackburn and Gaston1996; salamanders: Ashton Reference Ashton2002). Modern turtles and frogs also follow a Bergmann-like pattern, although species sampling is comparatively scarce (Ashton Reference Ashton2002; Ashton and Feldman Reference Ashton and Feldman2003). Extant squamates tend to show the converse of Bergmann's rule, that body size decreases in colder climates (Ashton and Feldman Reference Ashton and Feldman2003); however, others argue that no spatial pattern in body size is seen (Pincheira-Donoso and Meiri Reference Pincheira-Donoso and Meiri2013; Slavenko et al. Reference Slavenko, Feldman, Allison, Bauer, Böhm, Chirio and Colli2019). Interestingly, many more taxa should follow Bergmann's rule, according to estimates using ancestral-state reconstructions among major tetrapod groups (de Queiroz and Ashton Reference Queiroz and Ashton2004). This provocative result may imply that the tendency to develop Bergmann-like clines is a shared pattern that is deeply nested in the evolutionary history of endothermic and ectothermic tetrapods (de Queiroz and Ashton Reference Queiroz and Ashton2004).
Bergmann's Rule in the Fossil Record
While Bergmann's rule is certainly widespread among extant taxa, comparatively few investigations have looked for similar results in the fossil record. Pterosaurs show the converse of Bergmann's rule during the Cretaceous and do not follow size–latitude clines during the Triassic–Jurassic (Villabolos et al. Reference Villabolos, Olalla-Tarraga, Vieira, Mazzei and Bini2017). Bivalve size distributions remain remarkably consistent across a global latitudinal distribution during the Miocene–Recent (Roy et al. Reference Roy, Jablonski and Martien2000). Similar investigations that focus on comparisons between extant and extinct members of the same clade within the same latitudinal region approximate tests for Bergmann's rule from a climatically driven standpoint but do not test for size–latitude patterns outright. For example, comparisons between Eocene–Recent Antarctic penguins, Pleistocene–Recent coyotes across North America, and equids, canids, and sciurids from the Oligo-Miocene of the northwest United States test for climate-driven patterns in body size (Jadwiszczak Reference Jadwiszczak2001; Orcutt and Hopkins Reference Orcutt and Hopkins2013; Meachen et al. Reference Meachen, Janowicz, Avery and Sadleir2014). These studies, while not a direct comparison of latitudinally driven changes in body size, indicate that penguin average body size tends to be larger in extinct taxa but that the underlying mechanism may be due to an adaptive radiation rather than warmer climates (Jadwiszczak Reference Jadwiszczak2001). Body-size trends are highly variable among North American mammals studied thus far, with coyotes showing a decrease from larger than Recent average size across the Pleistocene/Holocene boundary, perhaps due to species interactions and niche partitioning (Meachen et al. Reference Meachen, Janowicz, Avery and Sadleir2014). Equid, canid, and sciurid body-size variation during the Oligo-Miocene shows no consistent relationship with climate variables, nor were trends similar between orders (Orcutt and Hopkins Reference Orcutt and Hopkins2013). Taken together, investigations into the potential drivers of body-size patterns in the fossil record have yielded highly variable results. For the few taxa that have been studied during the Cenozoic, inferred climate variables do not have significant relationships to body-size variation, counter to what has been shown in modern ecosystems. Indeed, times in Earth history with a decreased temperature gradient from equator-to-pole may have removed key factors underlying Bergmann's rule. One extreme example of this is the earliest Triassic, where elevated CO2 levels, globally high sea level, and ice-free polar regions intensified global climate extremes across the supercontinent of Pangea (Kidder and Worsley Reference Kidder and Worsley2004; Winguth et al. Reference Winguth, Shields and Winguth2015; Mancuso et al. Reference Mancuso, Horn, Benavente, Schultz and Irmis2021).
A prime candidate for a test of Bergmann's rule in the fossil record is Lystrosaurus, a non-mammalian synapsid that survived the end-Permian mass extinction and rose to remarkable abundance across much of Pangea during the Early Triassic (King Reference King1990; Grine et al. Reference Grine, Forster, Cluver, Georgi, Carrano, Gaudin, Blob and Wible2006). Indeed, Lystrosaurus is exceptional among fossil tetrapods, because it is a globally distributed genus known from a relatively short time interval (~3 Myr). Fossils of Lystrosaurus have been recovered in Early Triassic–aged strata in South Africa, Antarctica, Mongolia, Russia, China, India, and possibly Australia and Mozambique (e.g., Tripathi and Puri Reference Tripathi and Puri1961; Colbert Reference Colbert1974; Sun Reference Sun1980; Thulborn Reference Thulborn1990; Gubin and Sinitza Reference Gubin, Sinitza, Lucas and Morales1993; Liu et al. Reference Liu, Li and Cheng2002; Ray Reference Ray2005; Botha and Smith Reference Botha and Smith2007; Araújo et al. Reference Araújo, Smith, Tolan, Angielczyk, Crowley, Milisse and Mugabe2020; Viglietti et al. Reference Viglietti, Benson, Smith, Botha, Kammerer, Skosan and Butler2021) (Fig. 1). Besides having a near-global distribution, Lystrosaurus is also remarkably abundant, especially in South Africa, where more than 2500 specimens are cataloged into museum collections (Smith et al. Reference Smith, Rubidge, van der Walt and Chinsamy-Turan2012).

Figure 1. Geographic distribution of Lystrosaurus fossils sampled. Paleogeographic map of the Early Triassic with estimated locations of geologic basins denoted by stars; sampled localities are labeled and filled in orange; unsampled localities are open. Paleomap modified from Scotese (Reference Scotese2016). Lystrosaurus silhouette from Phylopic.org.
Here, we examine whether body-size proxies vary predictably with paleolatitude in Triassic species of Lystrosaurus. Our sample includes nearly 500 specimens recovered from four basins that span a range of paleolatitudes from approximately 73°S–55°S and ~45°N (van Hinsbergen et al. Reference Hinsbergen, de Groot, van Schaik, Spakman, Bijl, Sluijs, Langereis and Brinkhuis2015; Yang et al. Reference Yang, Wan, Crowley, Wang, Luo, Tabor and Angielczyk2021) (Fig. 1). This sample spans the entirety of all known Early Triassic–aged deposits, but unfortunately, Lystrosaurus-bearing strata from equatorial regions are lacking. If Lystrosaurus followed a size–latitude pattern consistent with Bergmann's rule, the largest specimens would be expected from the highest southern paleolatitudes (i.e., the Transantarctic Basin of Antarctica). If some other body-size cline is detected, this could indicate that Lystrosaurus is an exception to the rule, along with modern lizards and snakes. If, however, no size–latitude pattern is detected, sampling inconsistencies between Early Triassic basins could play a role in overprinting potential patterns. Finally, a failure to detect body-size clines in Lystrosaurus could also imply a previously unrecognized macroevolutionary consequence of climatic warming.
Data Collection and Analysis
Distribution of Lystrosaurus Species across Pangea
Lystrosaurus is found in Early Triassic–aged strata on every continent except North and South America (Tripathi and Puri Reference Tripathi and Puri1961; Colbert Reference Colbert1974; Sun Reference Sun1980; Thulborn Reference Thulborn1990; Gubin and Sinitza Reference Gubin, Sinitza, Lucas and Morales1993; Liu et al. Reference Liu, Li and Cheng2002; Ray Reference Ray2005; Botha and Smith Reference Botha and Smith2007; Araújo et al. Reference Araújo, Smith, Tolan, Angielczyk, Crowley, Milisse and Mugabe2020; Viglietti et al. Reference Viglietti, Benson, Smith, Botha, Kammerer, Skosan and Butler2021). Species-specific distributions are more clearly defined from southern Pangean deposits, where the most recent systematic appraisal of Lystrosaurus recognized four valid species in South Africa: Lystrosaurus maccaigi, Lystrosaurus curvatus, Lystrosaurus murrayi, and Lystrosaurus declivis (Grine et al. Reference Grine, Forster, Cluver, Georgi, Carrano, Gaudin, Blob and Wible2006). These four species have been used in numerous studies since (e.g., Botha and Smith Reference Botha and Smith2007; Botha-Brink et al. Reference Botha-Brink, Codron, Huttenlocker, Angielczyk and Ruta2016; Botha Reference Botha2020). All four species occur in varying abundances across southern Pangea, except for L. maccaigi, which is only found in South Africa and Antarctica (Colbert Reference Colbert1974; Peecook et al. Reference Peecook, Smith and Sidor2019), and L. declivis, which is currently only recognized from South Africa and India (Gupta and Das Reference Gupta and Das2011).
Within the Karoo Basin of South Africa, L. curvatus is the rarest species followed by L. maccaigi. These species are locally considered Permian taxa but likely survived during the extinction interval based on in situ specimens found in strata within the lithologic boundary of the vertebrate-defined Permo-Triassic boundary (Botha-Brink et al. Reference Botha-Brink, Huttenlocker, Modesto, Kammerer, Angielczyk and Fröbisch2014; Viglietti et al. Reference Viglietti, Benson, Smith, Botha, Kammerer, Skosan and Butler2021). Additionally, L. maccaigi and L. curvatus are found in the lower Fremouw Formation of Antarctica, indicating that these species persisted at higher latitudes in the Early Triassic (Colbert Reference Colbert1974; Collinson et al. Reference Collinson, Hammer, Askin and Elliot2006; Peecook et al. Reference Peecook, Smith and Sidor2019).
In contrast to the sparse occurrences of L. maccaigi and L. curvatus, thousands of specimens of L. murrayi and L. declivis have been recovered from the lowermost Triassic of the Karoo Basin, the more abundant L. declivis giving its name to the assemblage zone that typifies the faunal assemblage immediately after the end-Permian mass extinction (Botha and Smith Reference Botha and Smith2020). Lystrosaurus murrayi is also known in Antarctica and India (Colbert Reference Colbert1974; Ray Reference Ray2005; Peecook et al. Reference Peecook, Smith and Sidor2019). Two specimens referred to L. curvatus and one to L. declivis have also been recovered from the Panchet Formation in the Damodar Basin of India, further suggesting the cosmopolitan distribution of all four species across southern Pangea (Gupta and Das Reference Gupta and Das2011).
In the greater Turpan-Junggar Basin of Xinjiang, China, numerous species of Lystrosaurus have been described, including Lystrosaurus youngi, Lystrosaurus robustus, Lystrosaurus latifrons, and Lystrosaurus hedini, which has also been found in Mongolia (Yuan and Young Reference Yuan and Young1934; Young Reference Young1939; Sun Reference Sun1964, Reference Sun1973; Gubin and Sinitza Reference Gubin, Sinitza, Lucas and Morales1993; Liu et al. Reference Liu, Li and Cheng2002). Cosgriff et al. (Reference Cosgriff, Hammer and Ryan1982) suggested that some of the northern Pangean forms were likely synonymous with L. murrayi. More recently, work by Camp and Liu (Reference Camp and Liu2011) and Kulik et al. (Reference Kulik, Lungmus, Angielczyk and Sidor2021), has shown that Chinese specimens have significantly different cranial morphologies compared with South African specimens. Therefore, it is unlikely that northern Pangean species are synonymous with southern species. Furthermore, is it not currently possible to provide a reliable taxonomic assignment to Chinese specimens, as additional work is needed to clarify the number of valid taxa there, as well as in Russia (Surkov et al. Reference Surkov, Kalandadze and Benton2005). For our analysis, we refer to all species recovered from China as Lystrosaurus sp.
Body-Size Proxies
We gathered cranial measurements from 482 skulls to compare body-size proxies from Early Triassic specimens of Lystrosaurus. Nine specimens were from the Transantarctic Basin of Antarctica, 411 were from the Karoo Basin of South Africa, 27 were from the Damodar Basin of India, and 35 were from the Turpan-Junggar Basin of China (see Supplementary Material for associated data). Specimens were selected to best represent the maximum distribution of Lystrosaurus skull size from all known Early Triassic localities. Paleolatitude was estimated from tectonic plate reconstructions made available through the online calculator developed by van Hinsbergen et al. (Reference Hinsbergen, de Groot, van Schaik, Spakman, Bijl, Sluijs, Langereis and Brinkhuis2015) and range from approximately 73°S–55°S and ~45°N (van Hinsbergen et al. Reference Hinsbergen, de Groot, van Schaik, Spakman, Bijl, Sluijs, Langereis and Brinkhuis2015; Yang et al. Reference Yang, Wan, Crowley, Wang, Luo, Tabor and Angielczyk2021). These ranges were reconstructed from the basins preserving Early Triassic Lystrosaurus, but importantly lack records from low paleolatitudes (Romano et al. Reference Romano, Bernardi, Petti, Rubidge, Hancox and Benton2020). Indeed, the Triassic terrestrial fossil record is exceedingly sparse from low paleolatitudes, which complicates our understanding of Pangean species’ distributions but does not preclude an assessment of body sizes at high-southern and mid-northern paleolatitudes.
We selected cranial measurements that were easily identifiable, reproducible, and most often free of encasing matrix (i.e., the interorbital region and snout are often prepared, because they provide taxonomically informative details for species-level identification). As shown in Figure 2, these include: (1) basal skull length, as measured from the anterior extremity of the snout to the occipital condyle; (2) minimum interorbital width; and (3) tusk diameter at its point of eruption from the maxilla (averaged when both tusks could be measured). When skulls were not sufficiently prepared or were incomplete, we measured the dorsal snout length, as a midline horizontal line from the anteriormost point of the parietal foramen to the anterior extent of the premaxilla, and/or dorsal skull length, as a midline horizontal line from the anterior extent of the premaxilla to the posterior end of the parietal. Linear regressions were computed based on complete and prepared specimens of the four southern Pangean species to estimate basal skull length in specimens that were incomplete or not completely prepared. Skulls that were crushed, distorted, or too fragmentary were excluded from our analysis.
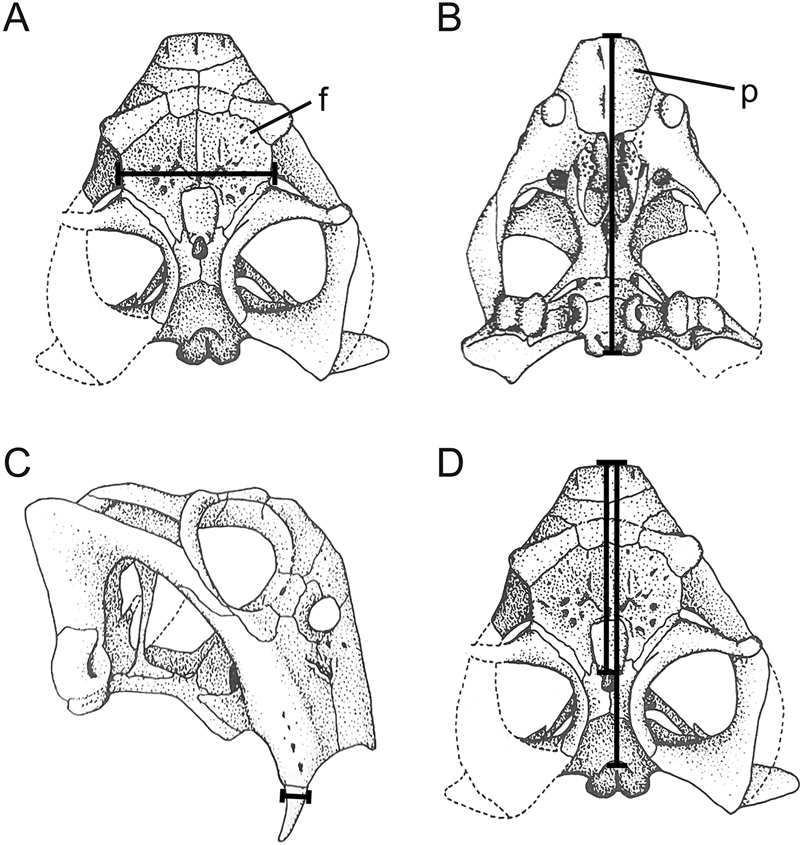
Figure 2. Cranial measurements used to estimate body size in Lystrosaurus. A, Minimum interorbital width, B, basal skull length, C, tusk diameter at eruption, and D, dorsal snout length and dorsal skull length were measured from incompletely prepared or broken specimens. Abbreviations: f, frontal; p, premaxilla. Skull drawings adapted from King (Reference King1990).
Measurements were taken using Mitutoyo digital calipers (±0.02 mm) at the following institutions: Burke Museum of Natural History and Culture, Washington, U.S.A.; American Museum of Natural History, New York, U.S.A.; Evolutionary Studies Institute (formerly Bernard Price Institute for Paleontological Research), University of the Witwatersrand, Johannesburg, South Africa; the Field Museum of Natural History, Chicago, Illinois, U.S.A.; Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China; National Museum, Bloemfontein, South Africa; and Iziko, the South African Museum, Cape Town, South Africa. Measurement data were also compiled from a survey of the literature (Ray Reference Ray2005; Gupta and Das Reference Gupta and Das2011; Botha Reference Botha2020; see Supplementary Material). All subsequent data manipulations and visualizations were carried out in R (v. 2022.02.0).
Testing for Bergmann's Rule
We assessed whether Lystrosaurus body-size proxies varied with paleolatitude by comparing average basal skull length and average tusk diameter between interspecific samples binned into the four basins included in our study (Fig. 3). Interspecific averages could be influenced by species-specific body-size distributions in Lystrosaurus, particularly because each species overlaps in its body-size range with at least one other species. For example, L. murrayi has a maximum known size of 21.3 cm (BP/1/ 3236), whereas L. curvatus has a maximum known size of 20 cm (BP/1/ 3976). To account for species-specific differences in body size, we standardized basal skull length and tusk diameter as a proportion of the maximum known size per species. We treated the unidentified southern Pangean specimens as a unique species (Lystrosaurus sp. indet.), which corresponds to a grouping of small skulls that are most likely unidentifiable to the species level because they lack developed diagnostic features. Similarly, we treated Chinese Lystrosaurus sp. as a separate species and computed proportional skull and tusk size accordingly.

Figure 3. Plots comparing Lystrosaurus size and geographic position using: A, basal skull length; B, standardized basal skull length as a percent of maximum size; C, tusk diameter; and D, standardized tusk diameter. Lystrosaurus does not follow a pattern that is consistent with Bergmann's rule. Skull size is significantly larger in the midlatitude Turpan-Junggar Basin and greater in the Karoo Basin than in the Damodar Basin. Red diamonds and gray bars indicate the mean and median values, respectively.
Results
Considering both raw and standardized body size across geologic basins, Lystrosaurus body-size proxies do not vary with paleolatitude in a pattern that is consistent with Bergmann's rule (Fig. 3). The largest average skull and tusk sizes are from Lystrosaurus specimens from the Turpan-Junggar Basin, which have an estimated paleolatitude of ~45°N (Yang et al. Reference Yang, Wan, Crowley, Wang, Luo, Tabor and Angielczyk2021). Indeed, average basal skull length and tusk diameter are between 16% and 17% larger in the Turpan-Junggar Basin compared with southern congeners. However, when only the basins in the Southern Hemisphere are considered, average skull and tusk size increase toward the pole (Fig. 3). These results, however, are not statistically significant at high paleolatitudes between the Karoo (~55°S–65°S) and Transantarctic Basins (~65°S–70°S) (t = 0.87124, df = 8.0759, p = 0.4088). Interestingly, mean tusk diameter and basal skull length are significantly smaller in the Damodar Basin when compared with the Karoo dataset (tusk: t = −8.4281, df = 37.01, p < 0.05; skull: t = −7.0031, df = 37.384, p < 0.05).
Based on these results, it is possible that intraspecific differences in maximum size could be driving the pattern of large specimens (i.e., large species) in high southern paleolatitudes. To address this, we compared species-specific average body size across southern Pangean basins and show that Bergmann's rule is not detected (Fig. 4). Median body size remains constant across southern Pangean species, except for large Lystrosaurus maccaigi from the Transantarctic Basin, which are much larger than the median size from the Karoo Basin. However, maximum size is remarkably similar across both basins. Indeed, interspecific maximum size is comparable across the Transantarctic, Karoo, and Turpan-Junggar Basins (between 22 and 28 cm), indicating that Lystrosaurus species in both the Northern and Southern Hemispheres could reach similar large sizes (Figs. 3, 5). However, the frequency of large Lystrosaurus specimens is highest in the Northern Hemisphere, as most sampled individuals are larger than ~50% maximum known size (Fig. 5). This is in contrast to the Karoo Basin, where the highest frequency of skulls is less than 50% maximum known size. Importantly, the Karoo Basin dataset is an order of magnitude larger than those of the other basins, which prompts us to consider the effects that different sample sizes have on average body size in Lystrosaurus.

Figure 4. Plots comparing skull length in four species of Lystrosaurus against geographic position. At the species level, median body size remains constant between Triassic basins, except for Lystrosaurus maccaigi; note that the outlier from the Karoo Basin is approximately the same size as the individuals from the Transantarctic Basin.

Figure 5. Distributions of skull size of Lystrosaurus collected from four geographic areas. A, interspecific Lystrosaurus basal skull length (BSL) and proportional skull size (%BSL max) in the Transantarctic Basin, B, normally distributed skull size in the Karoo Basin, C, right-skewed distribution in the Damodar Basin, and D, left-skewed distribution in the Turpan-Junggar Basin when skull length is standardized as a proportion of the maximum known size per species.
Assessing Sampling Bias through Resampling
Despite Lystrosaurus being one of the most abundant terrestrial fossils found in Early Triassic–aged strata, substantial differences exist in the number of specimens recovered outside the Karoo Basin. To assess how different sample sizes could be affecting our results, we subsampled the Karoo Basin dataset to the size of the other basins, respectively. We then tested for statistically significant differences between the downsampled Karoo dataset and the remaining datasets from the other basins. In addition, we also calculated the probability of recovering the largest known skull when the Karoo dataset was downsampled to further demonstrate the rarity of large sizes known from the Karoo Basin. We found that there is a 7% probability of recovering the maximum known skull size when the Karoo Basin dataset is reduced to the sample size of the Damodar or Turpan-Junggar Basin and only a 2% probability of recovering the largest individual when the Karoo is downsampled to the size of the Transantarctic Basin. We then tested whether average body-size proxies (i.e., basal skull length and tusk diameter) were significantly different between the downsampled Karoo dataset and the datasets from other basins using Welch's t-test of unequal variance. Average skull and tusk size were similarly not significantly larger between the Transantarctic Basin and downsampled Karoo Basin (t = −0.89094, df = 8.0035, p = 0.399), consistent with the raw and standardized results reported earlier. In addition, body-size proxies from the Damodar Basin remained significantly smaller when compared with the downsampled Karoo dataset (t = 7.5418, df = 26.176, p > 0.05). These results indicate a significant difference in body size between basins at relatively high southern paleolatitudes (~50°S–65°S) but not between these basins and the most poleward sample in our dataset, which is not consistent with the predictions of Bergmann's rule. However, comparisons between downsampled results remove much of the available data. Therefore, we employed rarefaction methods to extrapolate body-size abundance at larger sample sizes.
Assessing Sampling Bias Using Rarefaction
To test the effects that different sample sizes had on body-size distributions without reducing sample sizes to the smallest dataset in our results, we employed rarefaction methods originally developed to assess species diversity from unequal sample sizes. This provides a new approach to estimate whether body sizes are likely to remain large if more specimens were recovered from the Turpan-Junggar Basin, or conversely, specimens are likely to remain small if more were recovered from the Damodar Basin. To investigate whether body-size distributions are likely to remain consistent when sample sizes are increased, diversity metrics (i.e., richness, Shannon diversity, and Simpson diversity) were used to calculate rarefaction curves using the R package iNEXT (Hsieh et al. Reference Hsieh, Ma and Chao2016). This method is preferred over traditional rarefaction methods, because it does not reduce sample sizes. Importantly, diversity metric calculations are iterated over numerous sample sizes and are typically reliable to twice that of the reference sample (Chao and Jost Reference Chao and Jost2012). Unfortunately, this does not allow comparisons between equal sample sizes in our dataset, as the doubled Transantarctic Basin sample size is less than the reference sample size of the remaining datasets.
In our application of these rarefaction metrics, rather than thinking of richness as a measure of the number of species within a population, we treat it as the number of individuals at a given size class within a population. With this in mind, we made the following adjustments to our data. First, we binned Lystrosaurus skull sizes into 25 size classes using 10 mm increments, from 21 to 270 mm. Next, we calculated the interspecific abundances of each size class within each basin. This allowed us to compare body-size abundance between basins, analogous to traditional diversity metrics that compare species abundances between sites. Our goal was to be able to determine whether each basin had reached its asymptote in the number of body-size classes filled.
Our results indicate that skull sizes do not readily span the full range of sizes known for Lystrosaurus in any of the four basins studied here (Fig. 6). Of the 25 size classes generated by Lystrosaurus skull size, none of the richness estimates reach the expected asymptote (Fig. 6). The Karoo Basin comes closest to the asymptote, with 21 size classes filled in the reference sample. In the remaining basins, only a small portion of size classes are filled in the reference and extrapolated sample, indicating that sampling is sparse overall. The wide confidence intervals in the rarefaction curves for the Damodar and Transantarctic Basins further indicate that small sample size is skewing the distribution of body sizes in those basins (Fig. 6). The current sample also makes it impossible to extrapolate what sample size is needed for the asymptotes to be reached.

Figure 6. Plots comparing rarefaction curves estimating the number of body-size categories that are filled by fossil specimens in each basin studied here. A, summary of rarefaction curves for each basin, scaled to extrapolate twice that of the reference sample. B–E, rarefaction curves for each basin. Sample size–based rarefaction curves indicate that all Lystrosaurus specimens collected from Early Triassic basins fail to capture the total expected diversity of Lystrosaurus body sizes. In A–E, the solid lines represent the total number of size classes that are filled by at least one individual in the reference sample, and the extrapolated dotted line shows how many additional size classes would be filled at larger sample sizes with 95% confidence intervals. Note that extrapolated sample sizes are scaled based on the size of the reference sample.
With so many size classes left empty in our data, we cannot reliably estimate body-size distributions at larger sample sizes. Instead, we can compare the frequency and skew of body sizes from the four basins (Fig. 5) and rarefaction curves (Fig. 6) to see that specimens recovered from the Transantarctic Basin are either large or quite small, whereas specimens from the Damodar Basin tend to be small, and specimens from the Turpan-Junggar Basin are quite large. Taken together, our results indicate that additional sampling efforts outside the Karoo Basin will improve the accuracy of average body-size estimates within each basin as well as the accuracy of what sample size is needed to reach rarefied asymptotes of body-size abundances. Furthermore, our resampling and rarefaction results indicate that average body-size estimates are likely to change with the addition of new specimens outside the Karoo Basin, but that maximum body-size estimates are less likely to change, as maximum known skull size is comparable across northern and southern basins, regardless of paleolatitude. These results imply that Bergmann's rule did not apply for Lystrosaurus during the Early Triassic.
Discussion
The myriad drivers suggested as underlying Bergmann's rule in modern ecosystems make it difficult to narrow down mechanisms that could have affected body-size distributions and body-size clines in Lystrosaurus in the fossil record. In addition to sample size differences, local environmental differences within each basin could explain why body-size distributions are left or right skewed, which can influence body-size clines more broadly. Furthermore, physiological interpretations and thermal tolerances inferred for Lystrosaurus could also help to explain the potential trend of large body size in southern polar regions. As outlined in this section, we consider these potential drivers of Lystrosaurus body-size clines and discuss some of the unique considerations of size–latitude assessments in the fossil record.
There is considerable debate on the environmental conditions inferred during the Permian–Triassic (Thomas et al. Reference Thomas, Tabor, Yang, Myers, Yang and Wang2011; Li et al. Reference Li, Gastaldo, Neveling and Geissman2017; Tabor et al. Reference Tabor, Sidor, Smith, Nesbitt and Angielczyk2017; Yang et al. Reference Yang, Wan, Crowley, Wang, Luo, Tabor and Angielczyk2021). This complicates tests for Bergmann's rule using specific environmental indicators. Seasonally dry, subhumid to semiarid conditions have been inferred for the mid-Induan lower Olenekian Jiucaiyuan Formation in the Turpan-Junngar Basin (Yang et al. Reference Yang, Wan, Crowley, Wang, Luo, Tabor and Angielczyk2021). Seasonally dry conditions are inferred during the extinction interval in the Karoo Basin; however, aridity estimates are highly variable (e.g., Smith and Botha-Brink Reference Smith and Botha-Brink2014; Li et al. Reference Li, Gastaldo, Neveling and Geissman2017; Tabor et al. Reference Tabor, Sidor, Smith, Nesbitt and Angielczyk2017). Despite differing reports for environmental conditions in the Karoo Basin, ecosystem instability is widespread across Pangea and began in the latest Permian and continued into the Early Triassic. For example, macrofloral fossils indicate increased environmental stress in the Glossopteris forests of Antarctica (Gulbranson et al. Reference Gulbranson, Mellum, Corti, Dahlseid, Atkinson, Ryberg and Cornamusini2021) and fossilized charcoal shows evidence of wildfires in northwestern China (Wan et al. Reference Wan, Yang, Wan and Wang2021).
Considering the disturbed and highly turbulent ecosystems of the earliest Triassic, local environmental conditions within each basin could have provided different resources for Lystrosaurus to exploit. Resource availability could explain why similar maximum sizes are observed across assemblages in the Northern and Southern Hemispheres, and why the highest frequency of large individuals are recovered in the Turpan-Junggar Basin, where the inferred subhumid to semiarid environment likely supported more vegetation compared with the Karoo Basin (Yang et al. Reference Yang, Wan, Crowley, Wang, Luo, Tabor and Angielczyk2021). This interpretation is consistent with McNab's (2010) resource rule, which predicts that the quality and availability of resources will have a stronger effect on body-size clines than latitude or temperature. It follows that temperature and climate dictate resource availability and primary productivity (McNab Reference McNab2010). Because paleoclimates are inferred to be more favorable and therefore could have provided increased food availability for Lystrosaurus in the Turpan-Junggar Basin, we might expect to find additional large individuals with renewed sampling in this area. The presence of large individuals in Antarctica during the Early Triassic might also be explained by more favorable environments outside the Karoo and Damodar Basins, but specific climate estimates are limited.
An important consideration in climate and resource-related influences on body size is the physiology inferred for Lystrosaurus. A heterothermic endothermic physiology has been suggested based on histological analyses of hard tissues (Botha Reference Botha2020; Whitney and Sidor Reference Whitney and Sidor2020; Han et al. Reference Han, Zhao and Liu2021; Kulik et al. Reference Kulik, Lungmus, Angielczyk and Sidor2021). This flexible physiology, combined with its broad habitat and thermal tolerances (Retallack et al. Reference Retallack, Smith and Ward2003; Liu et al. Reference Liu, Abdala, Angielczyk and Sidor2021), burrowing lifestyle (Botha-Brink Reference Botha-Brink2017), and inferred generalist diet of tough plant material (e.g., Jasinoski et al. Reference Jasinoski, Rayfield and Chinsamy2009, Reference Jasinoski, Cluver, Chinsamy, Reddy, Kammerer, Angielczyk and Fröbisch2014) could have allowed Lystrosaurus to weather extreme ecosystem instability. Histological evidence from the long bones of specimens from South Africa, India, and China indicate that none of the sampled individuals had reached skeletal maturity at death (Ray et al. Reference Ray, Chinsamy and Bandyopadhyay2005; Botha Reference Botha2020; Kulik et al. Reference Kulik, Lungmus, Angielczyk and Sidor2021). Further, the intrinsically high rate of growth inferred from the bone tissue composition indicates that Lystrosaurus had the ability to reach large size and did so in the Turpan-Junggar Basin (Kulik et al. Reference Kulik, Lungmus, Angielczyk and Sidor2021), but that Southern Hemisphere assemblages suffered increased mortality when young or at small sizes in the Karoo and Damodar Basins (Ray et al. Reference Ray, Chinsamy and Bandyopadhyay2005; Botha Reference Botha2020).
As demonstrated in our results, differences in sample size also affect estimates of average body size between basins. The relative abundance of large-bodied Lystrosaurus from China could reflect more favorable environmental conditions in the Turpan-Junggar Basin during the Early Triassic. However, we might expect the right-skewed body-size distribution to change to a normal distribution when more specimens are recovered (Fig. 5). This is also true for the Transantarctic Basin, which is so sparsely sampled that no pattern is evident in the body-size distribution (Fig. 5). Overall, it is rare to record extremes of extant taxa, so it is likely even rarer to find fossils that are extremely small or large (Blackburn and Gaston Reference Blackburn and Gaston1994). Indeed, from our results, it is rare to find a wide distribution of body sizes at all, as the number of missing size classes from our rarefaction analyses indicates that the true shape of basin-specific body-size distributions is largely unknown outside the Karoo Basin.
From the classical interpretation of Bergmann's rule with respect to cold climates, it is possible that the heterothermic endothermic physiology reconstructed for Lystrosaurus (based on the interpretation of hard tissues), could have allowed natural selection to act on individuals to impact growth rate, life history, and life span at different paleolatitudes, leading to variation in body size (Botha Reference Botha2020; Whitney and Sidor Reference Whitney and Sidor2020; Grigg et al. Reference Grigg, Nowack, Bicudo, Bal, Woodward and Seymour2021; Han et al. Reference Han, Zhao and Liu2021; Kulik et al. Reference Kulik, Lungmus, Angielczyk and Sidor2021). However, cold climates, akin to current Antarctic conditions, were not present at the poles during the Early Triassic (Boucot et al. Reference Boucot, Xu, Scotese and Morley2013), so any potential body-size trend in southern Pangea is not directly comparable to the energetic benefit of large body size at cold, high-latitude climates as classically interpreted for Bergmann's rule. Indeed, global warming caused severe dampening of the latitudinal temperature gradient and is thought to have permitted the migration of terrestrial tetrapods into southern polar regions during the Early Triassic (Collinson and Hammer Reference Collinson, Hammer, Cooper and Raymond2007; Fröbisch et al. Reference Fröbisch, Angielczyk and Sidor2010). In the absence of polar ice caps, it is possible that the comparatively cooler climate conditions in the highest southern regions could have allowed individuals to reach larger body sizes, as large archosauriforms are also known from the Early Triassic of Antarctica (Smith et al. Reference Smith, Crandall, Hellert, Hammer and Makovicky2011). The characteristic of large-bodied Triassic taxa in present-day Antarctica could have resulted from a release of constraints operating in mid-southern paleolatitudes to reduce body size (i.e., high mortality at small size), possibly due to increased resource quality and availability in temperate polar regions (Fröbisch et al. Reference Fröbisch, Angielczyk and Sidor2010; Romano et al. Reference Romano, Bernardi, Petti, Rubidge, Hancox and Benton2020).
A similar migration in the Northern Hemisphere may not have been necessary, as paleoclimate estimates indicate warm, subhumid to semiarid environments for the mid-paleolatitudes (Boucot et al. Reference Boucot, Xu, Scotese and Morley2013; Yang et al. Reference Yang, Wan, Crowley, Wang, Luo, Tabor and Angielczyk2021). The possibility that Bergmann's rule could operate across different latitudinal regions within each hemisphere cannot be ruled out, nor can it be tested, as the scarcity of terrestrial fossils from paleoequatorial regions makes it impossible to assess Bergmann's rule in the Northern Hemisphere alone (Romano et al. Reference Romano, Bernardi, Petti, Rubidge, Hancox and Benton2020).
A unique consideration when testing Bergmann's rule in the fossil record is the temporal scale that the sampled assemblages encompass. Age estimates for the terrestrial Permian/Triassic boundary are highly debated, especially in the Karoo Basin (e.g., Botha et al. Reference Botha, Huttenlocker, Smith, Prevec, Viglietti and Modesto2020; Gastaldo et al. Reference Gastaldo, Kamo, Neveling, Geissman, Looy and Martini2020), which raises the possibility that samples of Lystrosaurus within each basin represent different—and potentially non-overlapping—intervals of geologic time. Geochemical age estimates generated by independent working groups reveal that the terrestrial extinction event is decoupled from the end-Permian marine extinction event in the Karoo (Botha et al. Reference Botha, Huttenlocker, Smith, Prevec, Viglietti and Modesto2020; Gastaldo et al. Reference Gastaldo, Kamo, Neveling, Geissman, Looy and Martini2020; Viglietti et al. Reference Viglietti, Benson, Smith, Botha, Kammerer, Skosan and Butler2021). Further, U-Pb age estimates from the same localities in the Karoo Basin have recovered different dates, either placing the base of the Lystrosaurus declivis Assemblage Zone in the latest Permian (Gastaldo et al. Reference Gastaldo, Kamo, Neveling, Geissman, Looy and Martini2020) or in the Early Triassic after the lithological change taken to signify the Permian/Triassic boundary (Botha et al. Reference Botha, Huttenlocker, Smith, Prevec, Viglietti and Modesto2020). Age assessments for the Permian/Triassic boundary in China place specimens recovered from the Jiucaiyuan Formation as upper Induan–lower Olenekian (Yang et al. Reference Yang, Wan, Crowley, Wang, Luo, Tabor and Angielczyk2021). However, the remaining historical collection from the wider Turpan-Junggar Basin has not been correlated to this datum. In Antarctica and India, vertebrate biochronology suggests that both the lower Fremouw and Panchet Formations are Early Triassic in age, but age dates are not available (Collinson et al. Reference Collinson, Hammer, Askin and Elliot2006; Elliot et al. Reference Elliot, Fanning, Isbell and Hulett2017). Taken together, the possibility that our results include body-size data from Permian specimens cannot be entirely ruled out.
Although our results do not recognize a Bergmann's rule body-size distribution in Lystrosaurus, other geographically widespread tetrapods, such as Diictodon and Dicynodon during the Permian, might be more amenable to this type of analysis (Kammerer et al. Reference Kammerer, Angielczyk and Fröbisch2011). If body size varies predictably with paleolatitude for other Permian or Triassic taxa, this might suggest that our results are hampered by latitudinal range or small sample sizes in some geographic areas. Alternatively, repeated failures to detect Bergmann-like patterns during times in Earth history with drastically different global temperature regimes could indicate that Bergmann's rule is only valid for the icehouse conditions of the late Cenozoic. This has been suggested by neontologists who noticed a phenotypic change in body size of modern vertebrates due to anthropogenic climate change (Yom-Tov and Geffen Reference Yom-Tov and Geffen2011; McCoy Reference McCoy2012; Goldenberg et al. Reference Goldenberg, Bisschop, D'Alba and Shawkey2022). In sum, additional investigations of Bergmann's rule in the fossil record are critical for understanding how well patterns in the past can inform our understanding of the present.
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.j3tx95xhk.
Acknowledgments
We thank the following curators, collections managers, and colleagues for their help in providing museum collection access and support: Z. Skosan, C. Browning, C. Kammerer, R. Smith, J. Botha, E. Butler, B. Zipfel, B. Rubidge, S. Jirah, V. Radermacher, C. Mehling, M. Norell, K. Angielczyk, J. Liu, and A. Bailleul. We thank Neil Brocklehurst and one anonymous reviewer for their helpful suggestions on this manuscript. We acknowledge research funding from National Science Foundation EAR 1713787 and ANT 1341304 (to C.A.S.) and University of Washington Department of Biology Iuvo, Walker, and Snyder awards (to Z.T.K.).









