Introduction
The rainforest of New Guinea is recognized as one of the last remaining wilderness areas but rapid development and human population growth are leading to high rates of deforestation and forest conversion. Besides disturbance from logging, large-scale oil palm plantations are the primary cause of the loss of lowland forest in Papua (Frazier, Reference Frazier, Marshall and Beehler2007). A number of conservation areas and protection forests have been established in this region (de Fretes, Reference de Fretes, Marshall and Beehler2007) but agricultural encroachment, illegal logging and hunting by immigrants and local communities are common. As in other parts of the tropics, local extinction of forest avifauna following forest fragmentation and extensive forest clearing is to be expected (Kattan et al., Reference Kattan, Alvarez-Lopez and Giraldo1994; Castelletta et al., Reference Castelletta, Sodhi and Subaraj2000; Waltert et al., Reference Waltert, Mardiastuti and Muehlenberg2004). Large forest birds such as cassowaries (Casuarius spp.) are particularly likely to disappear if their habitat is disturbed, as their persistence may depend on factors such as the presence of fruiting trees and water sources (Bentrupperbäumer, Reference Bentrupperbäumer1997). Cassowaries are flightless, obligate frugivores and as such they require abundant fruit on the forest floor, a resource that is in short supply or absent in logged or converted forest. Their long-term viability probably depends on the preservation of sufficiently large tracts of forest (Moore, Reference Moore2003). Roads associated with new logging concessions provide hunters with access to previously remote forest areas. Cassowaries are particularly sought by hunters because of their large body size, and in some areas they are under considerable pressure from hunting (Mack & West, Reference Mack and West2005; Pangau-Adam & Noske, Reference Pangau-Adam, Noske, Tidemann, Gosler and Gosford2010).
The northern cassowary Casuarius unappendiculatus is endemic to the lowlands of northern New Guinea. This species has two relatives: the dwarf cassowary Casuarius bennetti, which inhabits the montane areas of New Guinea and New Britain, and the southern cassowary Casuarius casuarius of southern New Guinea and north-east Australia. The northern cassowary is New Guinea's largest bird, with height up to 1.5 m and weight of 25–60 kg, and inhabits lowland and swamp forests up to 700 m altitude (Coates, Reference Coates1985; Beehler et al., Reference Beehler, Pratt and Zimmerman1986). Unlike the southern cassowary, the northern cassowary has not been studied in the field and its ecology is therefore poorly known (BirdLife International, 2013).
Cassowaries play a significant role as the major seed dispersers of numerous rainforest plants. All cassowary species are obligate frugivores (Crome, Reference Crome1976; Stocker & Irvine, Reference Stocker and Irvine1983), with fruit constituting 90–99% of their diet (Bentrupperbäumer, Reference Bentrupperbäumer1997; Wright, Reference Wright, Dew and Boubli2005). In Papua New Guinea the dwarf cassowary was found to be an important seed disperser for large-fruited plants and appeared to be the keystone frugivore for 67 species (Mack & Wright, Reference Mack, Wright, Dew and Boubli2005). In North Queensland the southern cassowary is the only major dispersal agent of c. 100 rainforest plants (Crome & Moore, Reference Crome and Moore1990). It is reasonable to assume, therefore, that the northern cassowary is the largest and principal seed disperser of many lowland forest plants in northern New Guinea (Beehler et al., Reference Beehler, Sengo, Filardi and Merg1995), making it an important target species for conservation, and a potential flagship species.
Despite its significant ecological role and ongoing rapid deforestation in Papua there have been no systematic assessments of the population status of the northern cassowary or of its response to disturbance. The aims of this study are to assess cassowary density in various habitat types along a forest disturbance gradient of northern Papua, to examine the relationship between cassowary abundance and habitat variables, especially those related to anthropogenic activities, and to discuss the implications for forest conservation strategies in Papua.
Study area
The study was conducted in Nimbokrang, c. 110 km west of Jayapura, the capital of Papua Province, Indonesia (Fig. 1). The Nimbokrang lowlands contain a mosaic of forest habitats, including large areas of intact forest. As a result of its high diversity of endemic birds, Nimbokrang forest has become an internationally renowned bird-watching destination. Typical canopy tree genera are Intsia, Pometia, Ficus, Canarium, Alstonia and Terminalia, and understory trees include Myristica, Syzygium, Garcinia, Diospyros, Pandanus and palms. Significant areas of the forest are claimed as traditional or clan forest by local people, resulting in several different land-use systems, including shifting cultivation and forest garden.

Fig. 1 The various habitat types in the study area in northern Papua, Indonesia. The inset shows the distribution of the northern cassowary Casuarius unappendiculatus, with a rectangle marking the location of the main map.
The study area comprises 304 km2 of continuous lowland forest and was chosen based on the availability of different habitat types representing a gradient of disturbance from undisturbed primary forest to unlogged but hunted natural forest, > 30-year-old secondary forest, logged forest and forest garden. Primary forest is defined here as forest that has reached a steady state without significant disturbance. Natural forest is original forest with infrequent collecting of non-timber forest products (NTFP) and subsistence wildlife hunting by local people. Secondary-growth forest was logged selectively > 30 years prior to this study and is now entered frequently by local people for hunting, bird trapping and NTFP collection. Logged forest habitat is selectively logged forest < 3 years old. Forest garden is secondary forest and/or abandoned logged forest that contains a mixture of cultivated plant species and forest vegetation.
Methods
All fieldwork was carried out during May 2011–April 2012 by MP-A and four local field assistants.
Surveys were conducted using the line transect method (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). In each habitat 10–14 transects were established, at least 500 m apart, with a total of 58 transects. Transects were 0.8–3 km in length and each transect was walked in the morning (06.30–11.30) and afternoon (13.00–17.00) during 2011–2012. For each direct encounter of a cassowary we measured the perpendicular distance from the bird (or from its estimated position at detection) to the transect. Most sightings were of single individuals; only 5 of 95 encounters were with an adult bird with chicks. Distance data were therefore analysed using single individuals as the sampling unit. We used DISTANCE v. 6.2 (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001) to model a global detection probability, using habitat as a covariate to estimate cassowary density for each habitat. We calculated an overall density based on stratum estimates, weighted by stratum area, as well as effective strip width (μ), defined as the distance from the line at which as many animals are detected beyond μ as are missed within μ (Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). We investigated the relationship between cassowary abundance and habitat variables, with univariate and multivariate analyses, using numbers of bird encounters per transect after they were standardized for equal survey effort.
Habitat variables were measured and analysed at transect level, with habitat as the grouping variable. The circular plot method was used to collect vegetation data. At least three plots of 5-m radius were established 30 m from, and perpendicular to, either side of each transect. Within each plot the diameter at breast height (DBH) was measured and the height of all trees with DBH ⩾ 10 cm estimated. At the centre of each plot the percentage of vegetation cover was estimated visually at three strata: ground level (< 1 m), understorey level (1–5 m) and canopy level, and the mean was calculated for each transect.
Differences in vegetation parameters between habitats were tested using the Kruskal–Wallis test and the P-values were adjusted using the Bonferroni correction (Fahrmeir et al., Reference Fahrmeir, Künstler, Pigeot and Tutz2010). We also assessed bivariate relationships between cassowary abundance and vegetation variables, using Spearman rank correlations. As measures of forest disturbance we counted human trails and hunting traps encountered along each transect, and measured the distance from the starting point of each transect to the nearest village.
To determine the suitability of habitat for cassowaries we counted all fruiting trees with fruits > 1 cm diameter within 15 m of the transect. We also collected fresh droppings of cassowaries at each site and identified the seeds present in the samples, to facilitate the comparison of consumed plant species with tree species known to fruit during the survey periods. Because water sources are amongst the most important factors for cassowaries (Crome & Moore, Reference Crome and Moore1990; Bentrupperbäumer, Reference Bentrupperbäumer1997) we quantified the number of swamp pools, streams and other water sources during the survey. To analyse the influence of habitat requirements and distance from village on cassowary abundance a covariate analysis was performed using generalized linear modeling in R v. 2.12.1 (R Development Core Team, 2011). Given the low number of cassowary records in forest gardens, this habitat type was excluded from this analysis.
Results
Cassowary density
Cassowaries were encountered on all transects in primary and natural forest, 13 of 14 transects in secondary forest, and seven of 10 transects in logged forest, but on only two of 10 transects in forest gardens. A total of 95 cassowaries (56 adults, 28 subadults and 11 chicks) were located during the survey, with 292 km of survey effort. Density estimates varied among habitat types, the highest being 14.1 individuals km−2 (95% CI 9.2–21.4) in primary forest and the lowest 1.4 individuals km−2 (95% CI 0.4–5.6) in forest garden. Densities were intermediate in natural forest (7.7 km−2, 95% CI 4.5–13.0) and in > 30-year-old secondary forest (9.8 km−2, 95% CI 5.5–17.3) but were considerably lower in recently logged (< 3 years) forest (4.2 km−2, 95% CI 2.1–8.4; Table 2). Combining the data from all habitats the overall density estimate was 8.5 individuals km−2 (95% CI 6.5–11.1) or 3.9 adult individuals km−2 (95% CI 2.8–5.4).
Table 1 Species of feeding trees recorded from faecal samples of northern cassowary Casuarius unappendiculatus, collected in different habitats in northern Papua, Indonesia (Fig. 1).

Table 2 Mean encounter rate, effective strip width, detection probability and estimated mean density for northern cassowaries in five habitats in northern Papua, Indonesia, based on line-transect data. Detection probability was modelled using a hazard rate key with cosine adjustment.

* Coefficient of variation
Vegetation parameters and anthropogenic activities
Almost all habitat variables differed significantly between habitat types (Table 3). Primary forest had a significantly higher mean canopy cover (74.8%), tree height (H = 20.6 m) and tree diameter (D = 31.1 cm) and significantly lower understorey and ground cover (40.0 and 26.6%, respectively) than the other habitats (Table 3). Mean canopy cover of natural forest (71%) was not significantly different from that of primary forest. Secondary forest showed intermediate values for vertical levels of vegetation cover and tree height but had a mean tree diameter similar to logged forest (24.7 and 22.2 cm, respectively). Logged forest had a lower mean tree diameter, tree height and canopy cover, and forest garden had the smallest and shortest trees and sparsest canopy cover (Table 3).
Table 3 Mean values of % canopy cover, % understorey cover, % ground cover, tree height, diameter at breast height (DBH), numbers of water sources, human trails and hunting traps, and P-values for each of the five habitat types studied.
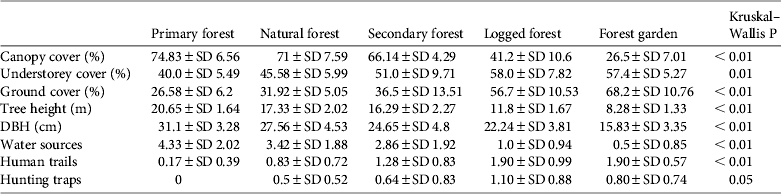
Mean numbers of fruiting trees were highest in primary forest (17.4 ± SD 7.6), followed by natural and secondary forest (14.5 ± SD 6.4 and 12.1 ± SD 7.2, respectively). Low numbers of fruiting trees were counted in logged forest, with the lowest number in forest garden (Fig. 2). Cassowaries were observed feeding on tree species of the families Palmae, Pandanaceae, Myrtaceae, Lauraceae and Combretaceae and, including the records from faecal analysis, 23 families and 61 species of fruiting trees were found in the cassowary's diet (Table 1). They were also observed drinking water in swamp pools, in small streams and rivers. These water sources were found more frequently in primary forest, followed by natural forest and secondary forest, and less frequently in logged forest and forest garden (Table 3). Few human trails and no wildlife traps were found in primary forest, intermediate numbers of each were found in natural forest and secondary forest, and the highest numbers were found in logged forest and forest garden (Table 3).
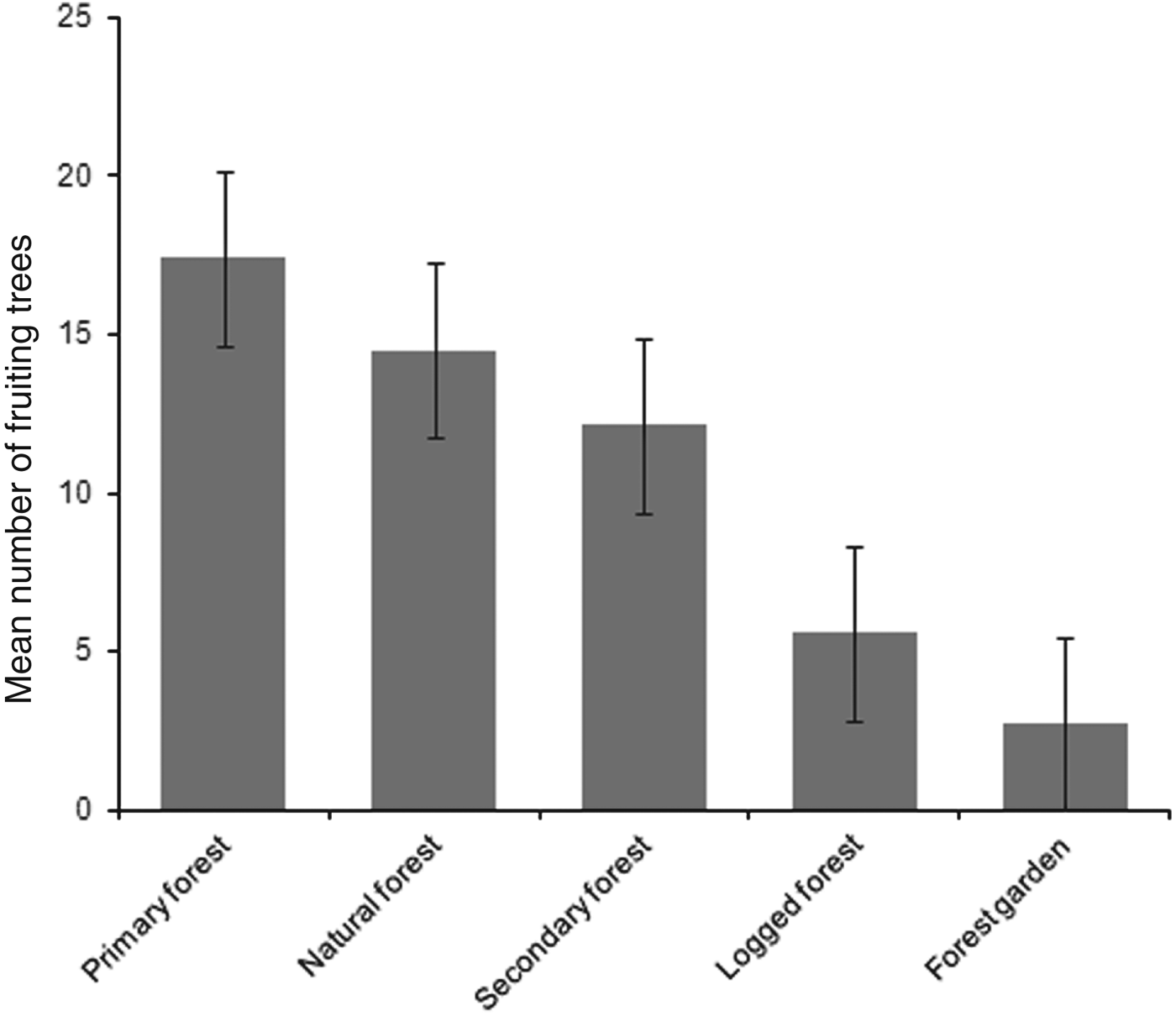
Fig. 2 Mean number of fruiting trees per 2.5 km transect in each habitat. Error bars are standard errors.
Relationships between cassowary abundance and habitat variables
Cassowary abundance, measured as the number of encounters per transect, was strongly correlated with canopy cover (r s = 0.76, P < 0.05), and moderately correlated with tree height and diameter (r s = 0.52, P < 0.05 and r s = 0.57, P < 0.05, respectively). In contrast, cassowary abundance was moderately to strongly negatively correlated with wildlife traps (r s = −0.67, P < 0.05), human trails (r s = −0.78, P < 0.05), understorey vegetation cover (r s = −0.60, P < 0.05) and ground vegetation cover (r s = −0.67, P < 0.05). The mean distance from transects to the nearest village was significantly different between habitat types (H = 36.94, df = 4, n = 58, P < 0.01), ranging from a mean of 3.94 km in forest gardens to 11.78 km in primary forest. This variable was moderately correlated with cassowary abundance (r s = 0.62, P < 0.05).
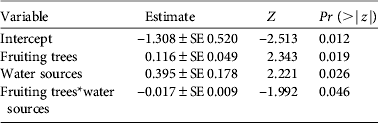
As with density estimates, cassowary abundance also differed significantly between habitats (H = 21.49, df = 4, n = 58, P < 0.01). However, natural forest and secondary forest had similar mean encounter rates, as had logged forest and forest garden (Table 2). In the multivariate analysis cassowary abundance was significantly influenced by the number of fruiting trees and potential water sources, and the interaction between these variables had a marginally significant effect (Table 4). The variable ‘distance to village’ was excluded from the model because of non-significant results.
Table 4 Covariates of a generalized linear model for the number of cassowaries encountered per transect. The model is Poisson distributed and was fitted using the Akaike information criterion.
Discussion
Cassowary density
Our density estimate for the northern cassowary (3.9 adults km−2) is higher than that inferred from the BirdLife International (2013) data for this species (c. 0.01–0.05 adult birds km−2 across its global range). It is also higher than that of Moore (Reference Moore2007), who estimated 0.48 adults km−2, but similar to that of Bentrupperbäumer (Reference Bentrupperbäumer1997), who estimated 3.8 adults km−2 (both for the southern cassowary). In calculating cassowary density, Bentrupperbäumer (Reference Bentrupperbäumer1997) sampled small areas (< 4 km2) in the Mission Beach region of North Queensland, whereas Moore (Reference Moore2007) surveyed a large area (102 km2) in the same region, suggesting that small sampling areas may have led to overestimation of cassowary density. We covered a study area of 304 km2, accumulating 56 observations of adult cassowaries. Comparing our results with those of Moore (Reference Moore2007) we cautiously conclude that our density estimate of northern cassowaries is higher than that of its southern counterpart in Australia. One possible reason for this difference is that our study area is embedded in large, continuous tracts of undisturbed primary rainforest, which provide abundant food and water resources. In contrast, much of the rainforest habitat of the southern cassowary in North Queensland is fragmented and this has resulted in population decline and local extirpation (Crome & Moore, Reference Crome and Moore1990; Latch, Reference Latch2007). Moreover, many southern cassowaries in Australia are killed by dogs or, accidentally, by motorists (Latch, Reference Latch2007) but these causes of mortality are absent in the northern lowlands of Papua.
Effects of habitat on density estimates
The northern cassowary was detected in each habitat type, showing that the species can use a complex array of habitats, including logged forest and forest garden. Nevertheless the variation in population density estimates between these habitats suggests it is sensitive to habitat alteration. The highest density was found in undisturbed habitat and the lowest in logged forest and forest garden, where the level of disturbance was highest. Moderately disturbed habitats supported intermediate densities. These results suggest that the northern cassowary may be tolerant of moderate disturbance but intolerant of severe disturbance. Damage to habitat, including the loss of canopy cover and other forest vegetation, was also found to be detrimental to the southern cassowary (Crome & Moore, Reference Crome and Moore1990).
Logging may be the biggest threat to the studied population of northern cassowary. Unsustainable logging practices can destroy the forest canopy and reduce the quality of the habitat for cassowaries, especially if food plants are removed. Most of the differences in cassowary density between habitats may be attributed to the variation in food availability and vegetation structure. Fruit availability is considered to be a major factor influencing population dynamics in frugivorous animals (e.g. Innis, Reference Innis1989; Bentrupperbäumer, Reference Bentrupperbäumer1997). The highest density of cassowaries was found in primary forest, where fruiting trees were most abundant. Because access to the primary forest is relatively difficult, this forest area has not been exploited for timber, and therefore the natural vegetation there still provides abundant food sources, particularly large trees that produce large fruits (Takeuchi, Reference Takeuchi, Marshall and Beehler2007; Waromi, 2011, pers. comm.). The areas of natural forest have low levels of disturbance and are visited by hunters only rarely, so the vegetation structure and abundance of fruiting trees are similar to primary forest. Most of the secondary forest surveyed is still recovering from past disturbance but this habitat already provides sufficient food sources for cassowaries. The abundance of fruiting trees in moderately disturbed habitats may be attributable to improved light conditions after the opening of forest canopy (e.g. for Neotropical forests: Gomes et al., Reference Gomes, Oostra, Nijman, Cleef and Kappelle2008; Lefevre, Reference Lefevre2008). However, the tree species composition of forest disturbed by logging is different from that of primary forest, being characterized by more light-demanding plants and probably resulting in different spatio-temporal availability of fruit and the complete loss of certain fruiting trees. Many Papuan forest trees that produce large fruits (e.g. Terminalia spp. and Canarium spp.) also have high-quality timber and are therefore the first to be extracted (Forestry Services of Papua Province, 2001).
Another important factor influencing the density of cassowaries is water availability. Water sources were found to a lesser extent in heavily disturbed habitats compared to less disturbed habitats. The sparse canopy cover in heavily disturbed forest probably increases the amount of heat (infrared) radiation reaching the forest floor, causing the water in swamp pools and at the base of large trees to evaporate. Although the study was conducted in mixed swamp lowland forest, pools were found at only two logged forest sites during the survey. Pools had probably disappeared from the other sites as a result of heat radiation and anthropogenic activities. As cassowaries require regular access to water throughout the year (Crome & Moore, Reference Crome and Moore1990) it is possible that individuals utilizing logged forest could suffer from a lack of water resources during the dry season. Although our findings indicate that northern cassowaries make use of disturbed habitat such as recently logged forest and forest gardens as they provide food resources, only primary and natural forest may provide sufficient food and water throughout the year.
The effect of forest disturbance on the northern cassowary may be exacerbated by hunting, facilitated by increased access to forest from logging roads. We found that hunting signs were more frequent in highly disturbed habitat, followed by secondary forest, but were absent in primary forest, seemingly because of the natural barrier formed by a river, which impeded access. Because of its large body size the northern cassowary is the main target bird species for meat consumption among the Genyem tribe in Jayapura region, and its meat is traded in local markets (Pangau-Adam & Noske, Reference Pangau-Adam, Noske, Tidemann, Gosler and Gosford2010).
Conservation implications
We estimate the population of adult cassowaries in our study area to be 851–1,652 (95% CI), and therefore the Nimbokrang lowlands are a potentially important region for cassowary conservation. Both northern and southern cassowaries are categorized as Vulnerable and the dwarf cassowary as Near Threatened on the IUCN Red List (IUCN, 2013). The Australian population of the southern cassowary is threatened by habitat fragmentation and housing development (Latch, Reference Latch2007). In Papua New Guinea all three species are already extirpated from several regions as a result of habitat destruction and overhunting (Beehler et al., Reference Beehler, Sengo, Filardi and Merg1995; Johnson et al., Reference Johnson, Bino and Igag2004; Wright, Reference Wright, Dew and Boubli2005). The decline and loss of cassowaries may affect the abundance and risk of extinction of forest flora that depend on these frugivores to disperse their seeds. In turn, because cassowaries depend on the fruit of rainforest trees for their survival (Stocker & Irvine, Reference Stocker and Irvine1983; Mack & Dumbacher, Reference Mack, Dumbacher, Marshall and Beehler2007), loss of their food plant species may lead to their extirpation.
The forests of northern Papua, including protection forests, have increasingly been exploited for timber, and cleared for plantations and settlements, accelerated by the special regional autonomy laws enacted in 2001, which allow local authorities to determine the exploitation of natural resources (de Fretes, Reference de Fretes, Marshall and Beehler2007). Moreover, the only conservation area with law enforcement staff in this region, the Mt Cyclops Nature Reserve, is only c. 225 km2 (Table 5) and even there the majority of the foothills have already been converted to small-scale agriculture and settlements. Such imminent forest conversion underlines the importance of law enforcement to prohibit illegal logging in northern Papua and to maintain healthy populations of the northern cassowary in the long term. We propose the establishment of strictly controlled and guarded conservation areas. By law, forests can be upgraded to conservation areas (nature reserves and wildlife reserves) if they are home to unique biodiversity and have significant ecosystem functions (Government Regulation, PP RI No. 68/1998). Enforcing the protection of conservation areas in Papua generates conflicts between government and traditional communities (de Fretes, Reference de Fretes, Marshall and Beehler2007), and therefore it is important that local communities are engaged in the establishment of such areas. Most lowland forests in the Jayapura region are designated as production and conversion forest but parts of Nimbokrang, Unurumguay and Nimboran forests should be upgraded to protected areas (e.g. wildlife reserves) because the region still supports large populations of the northern cassowary and is home to many other range-restricted birds and marsupials, and there is already a functioning cooperation between researchers from Cenderawasih University and the local tribes. This cooperation will be instrumental in securing the support of indigenous tribes for the preservation of traditional land rights and development of protected areas. University staff and Nature Conservation Agency Papua have already conducted an interactive workshop with local communities to examine the value of forest and the possibility of establishing a nature reserve, and local people have expressed an interest in maintaining forest ecosystem services and supporting the protection of Nimbokrang forest.
Table 5 Habitat and forest-type classification for lowland forest in the Jayapura region of Papua, Indonesia, including designated conservation areas (data based on Landsat images from 2009 reported by the Agency for Forestry Area Consolidation (BPKH) Papua, 2010).

* Mt Cyclops Nature Reserve
Acknowledgements
This study was funded by the Dorothea Schlözer Fellowship Programme, University of Göttingen. MW is supported by a grant from the Volkswagen Foundation, Hanover. We thank Richard Noske for his useful suggestions on the manuscript.
Biographical sketches
Margaretha Pangau-Adam is running a research project on the northern cassowary in Papua, Indonesia. She is interested in frugivores and seed dispersal, ecosystem functioning and biodiversity conservation. Michael Mühlenberg's research interests lie in the management of protected areas, tropical ecology and conservation biology. Matthias Waltert studies the effects of forest land use on tropical biodiversity and teaches international nature conservation and wildlife assessment to university students.









