Introduction
Habitat loss and fragmentation, hunting pressure and human–wildlife conflict threaten the survival of primates worldwide (Estrada et al., Reference Estrada, Garber, Rylands, Roos, Fernandez-Duque and Di Fiore2017). Deforestation is occurring rapidly within primate distributional ranges, with relatively intact habitats being converted to mosaics dominated by agriculture and agroforestry (Chapman & Peres, Reference Chapman and Peres2001; Laporte et al., Reference Laporte, Stabach, Grosch, Lin and Goetz2007; Plumptre et al., Reference Plumptre, Rose, Nangendo, Williamson, Didier and Hart2010). Deforestation can have a negative impact on some primate species (e.g. Cowlishaw & Dunbar, Reference Cowlishaw and Dunbar2000; Minhós et al., Reference Minhós, Chikhi, Sousa, Vicente, da Silva and Heller2016) but not all species respond in the same way to habitat loss and the expansion of anthropogenic land uses (Johns & Skorupa, Reference Johns and Skorupa1987). Considering the increasing isolation of protected areas throughout the tropics, there is a need to investigate the ability of primate communities to persist in human-dominated landscapes, to inform management practices outside protected areas.
In West Africa wooded savannahs, one of the predominant vegetation types across Africa (White, Reference White1983), are undergoing particularly rapid land-use changes (Brink & Eva, Reference Brink and Eva2009). The most dramatic changes in forest loss have occurred in the Guinean forest–savannah transition zone, where forest cover decreased by 20% during 1975–2000, mainly as a result of agricultural development (Brink & Eva, Reference Brink and Eva2009). Despite the importance of this vegetation formation and the potential effect that its deforestation may have on forest-dwelling species such as primates, the primate communities of this habitat in Western Africa have been little studied (Fimbel, Reference Fimbel1994; Galat Luong, Reference Galat Luong1995; Oates, Reference Oates2011; Gonedelé Bi et al., Reference Gonedelé Bi, Koné, Bitty, Béné Koffi, Akpatou and Zinner2012; Lambert, Reference Lambert2013; Ginn & Nekaris, Reference Ginn and Nekaris2014).
We assessed the populations of four arboreal primates: three threatened species (the Critically Endangered chimpanzee Pan troglodytes verus, the Endangered Temminck's red colobus Piliocolobus badius temminckii, and the Vulnerable king colobus Colobus polykomos) and one Near Threatened species (sooty mangabey Cercocebus atys), across a mixed wooded savannah–agricultural landscape, in the Boé region of Guinea-Bissau. Specifically, we investigated population densities of these species and their association with habitat and landscape features. Based on our results, we discuss the capability of the primate community to tolerate habitat conversion and persist in a human-dominated landscape.
To examine the extent to which arboreal primates can tolerate agricultural development, effects of land use must be separated from those of hunting (Blanco & Waltert, Reference Blanco and Waltert2013). In this sense, the Boé offers a unique context because, although sporadically reported, primate hunting is rare in this region as a result of religious taboos (van Laar, Reference van Laar2010). We can, therefore, assume that the composition and structure of the primate community are determined mainly by resource availability (i.e. food, habitat) and biotic interactions.
We provide comprehensive recommendations regarding conservation actions required to ensure the long-term persistence of these primate species in the region. These include a combination of adequate legislation, enforcement, habitat restoration, and community-based management.
Study area
The study was carried out in an area of 104 km2 around Béli, the largest and most populous village in Boé, in south-eastern Guinea-Bissau, West Africa (Fig. 1). The Boé region covers 3,289 km2 (Wit & Reintjes, Reference Wit and Reintjes1989). As part of the Fouta Djallon massif, the landscape consists of flat plateaux and steep-edged, narrow river valleys. Vegetation consists of a complex mosaic of savannah woodland, gallery forests on steep river slopes, and cultivated land. Cashew tree plantations and cultivated fields of rice, peanuts, maize, sorghum, and millet are expanding steadily. The climate is tropical, with an annual mean temperature of 28°C and annual rainfall of c. 1,500–1,750 mm (USGS, n.d.). The rainy season spans May–October. The area is sparsely populated, with c. 12,000 people living in 85 villages. However, the strong population growth in Guinea-Bissau (annual rate 2.44%) is reflected in the establishment of new villages in the central region of Boé (Chimbo Foundation, 2015), placing severe pressure on the already limited areas of gallery forests and intact tree populations, which remain almost exclusively on steep slopes of river valleys, with difficult access. Some parts of the gallery forest, mostly around headwaters, are protected by traditional beliefs (K. Kühnert et al., unpubl. data).
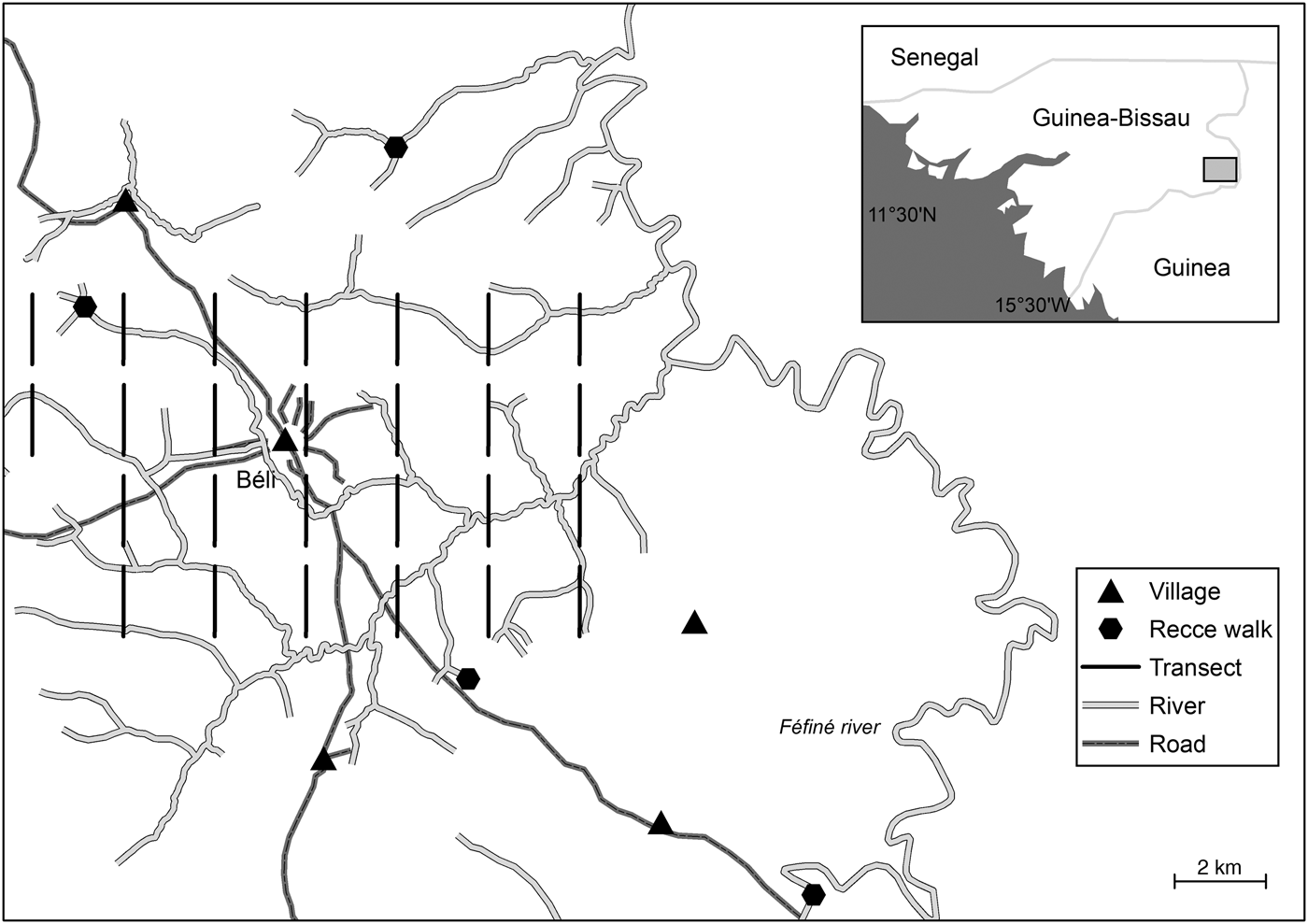
Fig. 1 Location of transect surveys and recce walks in the study area around the village of Béli, in the Boé region of Guinea-Bissau.
Methods
Data collection
Line transect sampling (Buckland et al., Reference Buckland, Plumptre, Thomas and Rexstad2010) was conducted in the dry season (March–April) in 2016. We systematically placed a rectangular 2 × 2 km sampling grid within the study area and placed one line transect of 1.5 km length within each of the 26 grid cells. Transect location was recorded using a global positioning system. All transects were surveyed four times, at intervals of 2 weeks, resulting in a total survey effort of 156 km.
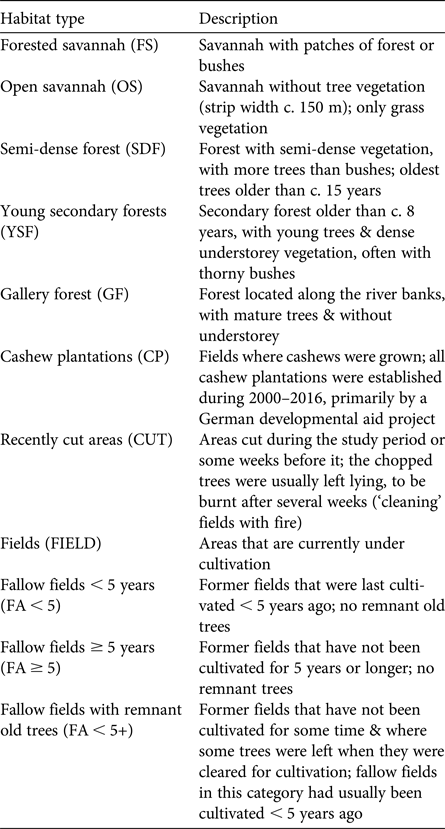
Along transects we recorded both visual observations and acoustic detection of all four primate species. For chimpanzees we also counted sleeping nests during the first survey of each transect (i.e. total survey effort of 39 km), following the standing crop nest count method for density estimation (Tutin & Fernandez, Reference Tutin and Fernandez1984). For every observation or detection we noted the species, time, surrounding habitat type (Table 1), the tree species in which sleeping nests were found, and perpendicular distance to the transect. The latter was measured horizontally from the transect line to a fixed reference object near the estimated centre of a primate group or nest cluster, usually a tree stem, using a laser range-finder. Additionally, we recorded landscape changes along transects, noting the start and end coordinates of the various habitats (Table 1) we crossed.
Table 1 Descriptions of habitat types used in this study of primates in the Boé region of Guinea-Bissau (Fig. 1).
As no groups of colobus species were observed during the line transect survey, we conducted additional reconnaissance (recce) walks at four sites in the study area (Fig. 1), based on recommendations of local guides. All four sites were located in or close to rivers and gallery forests. We also recorded opportunistic sightings of primates on our way to those sites. The recce walks were conducted in May 2016, and each site was surveyed for at least 2 hours. The total distance walked was 24 km (range 0.1–5 km).
Data analysis
Distance sampling analysis requires a minimum number of sightings to model detection probability and estimate density reliably (Buckland et al., Reference Buckland, Plumptre, Thomas and Rexstad2010). From our data we were able to calculate density estimates only from chimpanzee nest data. Using DISTANCE 6.1 (Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010) we estimated the density of chimpanzee nest clusters (D Nest), using the equation D Nest = n / (2·w·L·P a), where n = number of nest clusters observed along the transect, w = strip width (i.e. the maximum distance from the line at which clusters were included in the analyses), L = transect length, and P a = probability that clusters were detected within w. An estimate of P a was obtained from the detection function ![]() $\int_{\rm o}^w {\hat g(x)dx} $, where g(x) = probability of detecting a nest, provided that it is at a perpendicular distance x from the transect. We used the conventional distance sampling analysis engine to fit the detection function. A key assumption of this method is that objects close to the line are detected with certainty (e.g. Buckland et al., Reference Buckland, Plumptre, Thomas and Rexstad2010; Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010). Truncation and grouping of data can be useful if detections occur in irregular clusters, and Distance analysis is robust to these modifications (Ekblom, Reference Ekblom2010). Based on visual examination and a χ2 goodness-of-fit test, a half-normal function with one cosine adjustment term fitted to data truncated to w = 50 m and unequally grouped data with six intervals provided the best fit to our data. We used a nest production rate r of 1.09 nests per day (Plumptre & Reynolds, Reference Plumptre and Reynolds1997), a nest decay rate t of 194 days (Fleury-Brugiere & Brugiere, Reference Fleury-Brugiere and Brugiere2010) and our own empirical estimate of nest cluster size Ê (arithmetic mean) to obtain an estimate of chimpanzee individual density D Ind (D Ind = D nests·Ê/t·r).
$\int_{\rm o}^w {\hat g(x)dx} $, where g(x) = probability of detecting a nest, provided that it is at a perpendicular distance x from the transect. We used the conventional distance sampling analysis engine to fit the detection function. A key assumption of this method is that objects close to the line are detected with certainty (e.g. Buckland et al., Reference Buckland, Plumptre, Thomas and Rexstad2010; Thomas et al., Reference Thomas, Buckland, Rexstad, Laake, Strindberg and Hedley2010). Truncation and grouping of data can be useful if detections occur in irregular clusters, and Distance analysis is robust to these modifications (Ekblom, Reference Ekblom2010). Based on visual examination and a χ2 goodness-of-fit test, a half-normal function with one cosine adjustment term fitted to data truncated to w = 50 m and unequally grouped data with six intervals provided the best fit to our data. We used a nest production rate r of 1.09 nests per day (Plumptre & Reynolds, Reference Plumptre and Reynolds1997), a nest decay rate t of 194 days (Fleury-Brugiere & Brugiere, Reference Fleury-Brugiere and Brugiere2010) and our own empirical estimate of nest cluster size Ê (arithmetic mean) to obtain an estimate of chimpanzee individual density D Ind (D Ind = D nests·Ê/t·r).
For the other primate species, and data collected during additional recce surveys, we report only encounter rates.
To quantify habitat selection by chimpanzees we used the selection index (i.e. forage ratio) proposed by Savage (Reference Savage1931) and by Williams & Marshall (Reference Williams and Marshall1938): w i = o i/p i, where w i = selection index for species i, o i = proportion of chimpanzee nest clusters observed in the habitat type, and p i = proportion of habitat type available in the study area. This selection ratio was estimated using the data on chimpanzee nest observations from all four line transect surveys. Therefore, the selection index refers only to preferences for nesting, rather than overall habitat preference. To estimate the coverage of each habitat type we assumed that the habitat composition of the line transects was representative of the whole study area. Thus, we calculated the length covered by each habitat type along each of the transects. We summed the lengths for all 26 transects and calculated the percentage of each habitat type along the entire distance surveyed (i.e. 39 km, considering 26 transects of 1.5 km each).
Additionally, for sooty mangabeys and chimpanzees we characterized the locations where these primates and their nests were observed during line transect surveys in relation to various anthropogenic and natural landscape features and contrasted them with those of random points, using ArcGIS 10.2.2 (ESRI, Redlands, USA). A number of random points equalling the total number of records for each species (Table 2) were generated along the transect lines, given that the exact location of observations was not recorded. We calculated distances between villages, roads and rivers and both random and observation points. We used analysis of variance (ANOVA) to analyse the differences in the mean distances between observation points and the landscape features, and those between random points and landscape features.
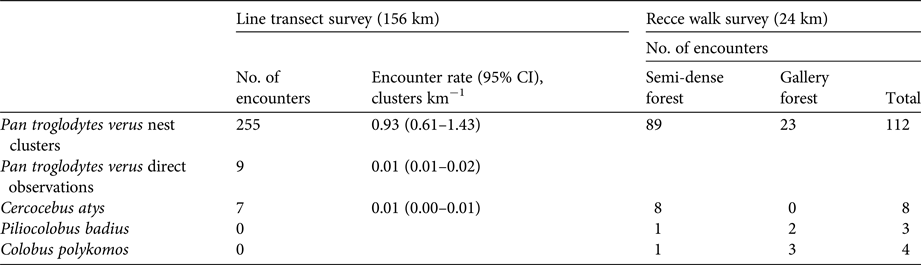
Table 2 Results from line transect surveys in the 104 km2 study area and recce walks in four additional sites to assess populations of four primate species (chimpanzee Pan troglodytes verus, sooty mangabey Cercocebus atys, Temminck's red colobus Piliocolobus badius, king colobus Colobus polykomos) in the Boé region of Guinea-Bissau (Fig. 1). For line transects, total numbers of detections (both visual and acoustic) and encounter rates of clusters are given, based on the cumulative survey effort from four visits to each transect. For recce walks, observations are separated according to the habitat type where they were made.
Results
Chimpanzees
During the first survey of the line transects (39 km) we recorded 319 nests distributed over 171 clusters. After truncating to a strip width of 50 m, 153 nest clusters remained for analysis, resulting in a nest cluster encounter rate of 3.92 clusters per km (95% CI 2.32–6.62 clusters per km). Detection probability for this strip was estimated to be P a = 0.44 (95% CI 0.39–0.49), and nest cluster size to be 1.82 nests per cluster (95% CI 1.63–2.04), resulting in a density estimate of 0.77 individuals per km2 (95% CI 0.45–1.34), and a total abundance estimate of 80 (95% CI 46–140) weaned chimpanzees for the 104 km2 study area.
For the complete line transect survey (156 km) the cumulative number of nest clusters of all age classes amounted to 255, with 549 nests. Most nests (65.8%) were found in semi-dense forests, and a substantial proportion (17.3%) were found in forested savannah (Fig. 2). Only a small proportion of nests were found in other habitats (Fig. 2). Similarly, most nests detected during additional recce walks were located in semi-dense forest (Table 2). When contrasted to habitat availability, the selection index indicated a clear preference for semi-dense forest, followed by fallow fields with remnant old trees, gallery forests, and fallow fields older than 5 years (Fig. 2).
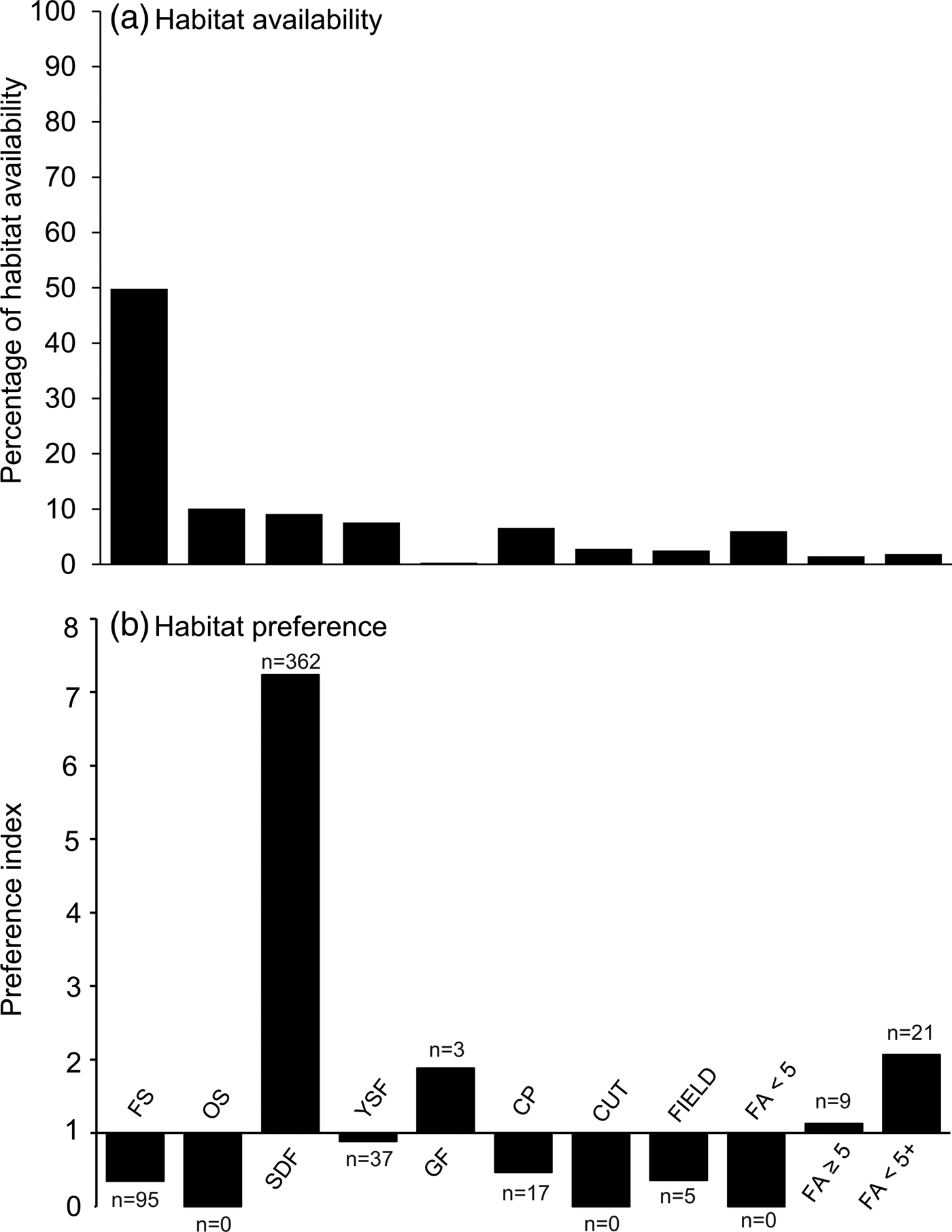
Fig. 2 (a) Habitat availability and (b) habitat preferences of chimpanzees Pan troglodytes verus for nesting in the Boé region of Guinea-Bissau (Fig. 1) for the 11 habitat types (see Table 1 for description), based on data from all four line transect surveys. Habitat availability is represented as the percentage of line transects covered by each habitat. Preference index values > 1.0 indicate preference, and < 1 avoidance. n = number of nest clusters.
Chimpanzee nests were considerably closer to rivers than randomly generated points (ANOVA, P < 0.001, F = 66.35), with the former being at a mean minimum distance of 283 ± SD 251 m to the closest water course, and random locations being at a mean distance of 432 ± SD 292 m. Conversely, chimpanzee nest clusters were significantly further from roads than random points (P < 0.001, F = 12.39), and significantly further from villages (P = 0.016, F = 5.78). Nest clusters were, on average, at least 2,160 ± SD 1,072 m from roads and 3,603 ± SD 1,025 m from villages, compared to random points, which were at a mean distance of 1,810 ± SD 1,349 m from roads and 3,380 ± SD 1,239 m from villages.
Sooty mangabeys
During the transect survey 11 sooty mangabeys were observed (a single individual and a group of 10), and nine additional records were made based on vocalizations (Table 2). Overall, two records could be assigned to young secondary forests, two to semi-dense forests, and three to gallery forests. During the recce surveys the species was found at three of four additional sites surveyed (Table 2). One group, composed of c. 20 adults and five infants, was sighted in the gallery forest of the Féfiné River on the border with Guinea. In total, seven records of vocalizations were made in three gallery forests.
Sooty mangabeys were recorded significantly further from villages than random points (ANOVA, P = 0.01, F = 8.74). Locations where sooty mangabeys were observed were at a mean distance of 4,196 ± SD 1,071 m from the nearest village, whereas random locations were at a mean minimum distance of 3,684 ± SD 1,193 m. The species was not observed significantly closer to roads than random points (2,144 ± SD 983 and 2,544 ± SD 1,431 m, respectively; P = 0.14, F = 2.63). All observations were made on or close to rivers.
King colobus and Temminck's red colobus
No king colobus or Temminck's red colobus were observed during the line transect survey (Table 2). During the additional recce surveys nine king colobus individuals (in groups of two, three and four) were recorded at the Féfiné River, and one group of five adults was observed in a small patch of gallery forest at Bantenjam. In association with king colobus, 27 Temminck's red colobus, in three groups of 2–20 individuals, were recorded at the Féfiné River (Table 2). Two groups were observed at the river edge, and one foraging in fruit trees close to the river.
Discussion
Chimpanzees
We estimated the chimpanzee population in a comparatively large section of the populated part of the Boé region, a typical mosaic landscape composed of savannah, agricultural land and remnant forest. Although our density estimate of 0.77 individuals per km2 is close to that reported by studies conducted in a non-protected area in southern coastal Guinea-Bissau (0.89 individuals per km2, Sousa, Reference Sousa2009) and the Haut Niger National Park in neighbouring Guinea (0.87 individuals per km2, Fleury-Brugiere & Brugiere, Reference Fleury-Brugiere and Brugiere2010), it is significantly higher than densities reported by most similar surveys in other West African countries (e.g. 0.13 individuals per km2 in Niokolo Koba National Park in Senegal, Pruetz et al., Reference Pruetz, Marchant, Arno and McGrew2002; < 0.4 individuals per km2 in Mali and the Ivory Coast, Granier & Martinez, Reference Granier and Martinez2004; Campbell et al., Reference Campbell, Kuehl, Kouamé and Boesch2008) and by another recent assessment in coastal Guinea-Bissau (0.22 individuals per km2, Carvalho et al., Reference Carvalho, Marques and Vicente2013).
Two previous estimates of chimpanzee density in Boé are available: one from a small, forested locality c. 20 km south of our study area (1.80 individuals per km2, Wenceslau, Reference Wenceslau2014), and one estimate (based on interview data) of c. 710 chimpanzees in the whole of the Boé savannah (Schwarz et al., Reference Schwarz, Serra and Lopes2007). Our findings suggest there may be 1,465–4,415 chimpanzees in Boé (i.e. more than double the estimates of Schwarz et al., Reference Schwarz, Serra and Lopes2007). The use of nest decay rates obtained from both wet and dry season data (Fleury-Brugiere & Brugiere, Reference Fleury-Brugiere and Brugiere2010) may have caused a slightly high bias in our estimates, given that nest decay is usually slower in the dry season (when our study was conducted). Thus, the estimates may have to be reassessed as decay rates for the dry season become available. However, there are reasons to believe that the chimpanzee population in the Boé is even larger than our estimate, given that unsurveyed areas in the region appear to be less affected by habitat conversion than those included in this study (authors, pers. obs.).
Chimpanzees usually occur at high density only in areas without logging and human disturbance (Tutin & Fernandez, Reference Tutin and Fernandez1984; Hashimoto, Reference Hashimoto1995). However, our density estimates and the presence of nests in remnant trees within cultivations indicate that the species can tolerate human presence to a considerable degree when unhunted. Hunting of chimpanzees is rare in Boé because of religious taboos (van Laar, Reference van Laar2010), and this is supported by the lack of fear exhibited by the individuals encountered in this study.
In our study area chimpanzees nested mainly in semi-dense forest, to an even greater extent than reported by Fleury-Brugiere & Brugiere (Reference Fleury-Brugiere and Brugiere2010). This preference for old-growth and structurally diverse forest has been described elsewhere (Fleury-Brugiere & Brugiere, Reference Fleury-Brugiere and Brugiere2010; Torres et al., Reference Torres, Brito, Vasconcelos, Catarino, Gonçalves and Honrado2010; Carvalho et al., Reference Carvalho, Marques and Vicente2013). Unfortunately, given the limited remnants of mature gallery forest in the study site it was not possible to assess whether chimpanzees positively select this habitat. However, given the importance of gallery forests in terms of availability of food tree species (McGrew et al., Reference McGrew, Baldwin and Tutin1988; Bryson-Morrison et al., Reference Bryson-Morrison, Matsuzawa and Humle2016) it is likely that gallery forests are also a preferred habitat for nesting. We found that chimpanzee nests were closer to rivers than random points; however, the mean distance from nests to rivers was 283 m, indicating that sites further from rivers may also be suitable. Chimpanzees did not avoid young fallow fields, provided that some trees remained. Chimpanzee nests were found c. 300 m further from villages and roads than random points, which could be attributed to active resource selection rather than human avoidance; for example, the apparent avoidance of villages could be a result of the lower suitability of areas close to villages for nesting (e.g. cultivated fields with no trees). Similarly, although avoidance of roads has been reported elsewhere (e.g. Vanthomme et al., Reference Vanthomme, Kolowski, Korte and Alonso2013), avoidance of roads in the study area may be a consequence of these usually being constructed in areas of open savannah with lower tree density.
Sooty mangabeys
For some time after the first record of sooty mangabeys in Guinea-Bissau by Frade et al. (Reference Frade, Bacelar and Gonçalves1946), the species was considered to be extinct (Limoges, Reference Limoges1989). Karibuhoye (Reference Karibuhoye2004) mentioned sightings of sooty mangabeys reported by local people in coastal Guinea-Bissau, although these were rare. In 2009, sooty mangabeys were recorded in the Boé, using camera traps, for the first time in decades (Chimbo Foundation, 2011). These records along with our own findings confirm the presence of the species in Guinea-Bissau, which also occurs in neighbouring Guinea.
King colobus and Temminck's red colobus
In 1989 an alarming decrease in colobine numbers at the national level was reported (Limoges, Reference Limoges1989), the major threat being habitat fragmentation (Casanova & Sousa, Reference Casanova and Sousa2007). Temminck's red colobus and king colobus have been reported to occur at very low densities after suffering a demographic collapse (Minhós et al., Reference Minhós, Chikhi, Sousa, Vicente, da Silva and Heller2016). Temminck's red colobus appears to be particularly vulnerable to forest destruction because of its group size, dispersal behaviour and limited ability to adapt to changing environments (Minhós et al., Reference Minhós, Chikhi, Sousa, Vicente, da Silva and Heller2016).
The low number of observations of both species in our study underlines their critical status in Guinea-Bissau, as elsewhere in West Africa (Casanova & Sousa, Reference Casanova and Sousa2007; Craigie et al., Reference Craigie, Baillie, Balmford, Carbone, Collen, Green and Hutton2010; Minhós et al., Reference Minhós, Chikhi, Sousa, Vicente, da Silva and Heller2016). In the study area it seems colobus monkeys occur in a few sacred forests (local guides, pers. comm.). During our surveys we recorded both species in the largest gallery forest in the study area, and a small group of king colobus in an isolated fragment of gallery forest. The composition of this group appears to have remained unchanged for the last year (local guides, pers. comm.) and we observed no infants during this study, which suggests the group is not reproducing. Given the poor connectivity of such forests in the region, such isolated groups are likely to be lost in the near future, even in the absence of hunting.
Recommendations for conservation
Our results confirm that Boé is still home to threatened primate species. However, some of them occur at very low densities and could disappear from the area unless immediate action is taken.
Although hunting may not be a major threat in the area currently, it has recently increased near the country's border with Guinea and may increase in the future as a result of immigration from neighbouring countries (Schwarz et al., Reference Schwarz, Serra and Lopes2007). As primates may be able to tolerate a mosaic of agriculture and natural forests provided that poaching pressure is low (Torres et al., Reference Torres, Brito, Vasconcelos, Catarino, Gonçalves and Honrado2010), we strongly recommend enforcing laws regarding primate hunting, and extending legal protection to all the species studied.
Furthermore, we advise the implementation and enforcement of clear land-use policies and regulations to avoid further forest loss. Gallery forests and mature forests with old trees in particular must be strictly protected as crucial habitats and ecological corridors for forest vertebrates (McGrew et al., Reference McGrew, Baldwin and Tutin1988; Bryson-Morrison et al., Reference Bryson-Morrison, Matsuzawa and Humle2016; K. Kühnert et al., unpubl. data). It is likely that gallery forests are also a preferred habitat for nesting. Protecting intact gallery forests is also beneficial to people, as the forests prevent soil erosion, mineral loss and a drop in water levels, and provide food (e.g. faroba, wild yams), medicines and building materials. Some farmers already blame others for cutting down trees too close to rivers. This shows that there is potential for garnering local support for establishing and enforcing rules to improve protection of the remaining gallery forests, and reforesting fallows at such sites.
At a regional level, habitat assessments to ascertain priority sites for restoration are advisable. Isolated habitats of major conservation importance, such as the study site Bantenjam, which holds one of the few colobus groups in the area, should be connected with intact forest sites to prevent local primate extinctions. Additionally, the possibility for primates to move through the agricultural matrix needs to be facilitated by maintaining trees in cultivated fields (Dunham, Reference Dunham2011). This will also be beneficial for chimpanzees, which have been shown to nest in cultivated fields provided that some trees remain. In this regard, it is necessary to implement initiatives to train farmers in primate-friendly practices and to educate them about the importance of trees within croplands. Improved knowledge and environmental awareness have often been linked to improved attitudes towards conservation (Lepp & Holland, Reference Lepp and Holland2006; Waylen et al., Reference Waylen, Fischer, McGowan, Thirgood and Milner-Gulland2010). However, further research on how to incentivize such practices will be required.
Although overall there is a positive attitude towards primates among the local communities in Boé, there are indications that human–wildlife conflict linked to crop raiding is likely to increase in the near future, and early action is advisable (Hockings & Humle, Reference Hockings and Humle2009). There are already complaints about chimpanzees (local guide, pers. comm.) and these are likely to intensify if natural feeding resources continue to decrease. We recommend an ethnoprimatological study to investigate people's perceptions of chimpanzees and record existing and potential conflicts.
Community-based conservation has the potential for success when people have a sense of ownership, and perceive direct and indirect benefits from conservation (e.g. financial incentives, employment, infrastructure, education; Mishra et al., Reference Mishra, Allen, McCarthy, Madhusudan, Bayarjargal and Prins2003; Webber et al., Reference Webber, Hill and Reynolds2007). In Boé initiatives to raise awareness among communities regarding the potential effects of further landscape conversion on their livelihoods are already being implemented (Chimbo Foundation, pers. comm.), and there are efforts to increase ownership among the communities through, for example, the active involvement of local people in wildlife monitoring (Chimbo Foundation, pers. comm.). We recommend maintaining these efforts in the region to continue fostering positive attitudes within the communities of the Boé region.
Acknowledgements
We thank the Chimbo Foundation for making this research in Guinea-Bissau possible, and Gerco Niezing, research coordinator of Chimbo, for his helpful advice and support. We are grateful to all field assistants and members of the Chimbo Foundation who collaborated during the field work, and to an anonymous reviewer who commented on this work. We acknowledge the stipend programme Kreativität im Studium of Georg-August-Universität Göttingen and the AKB-Stiftung for financial support.
Author contributions
AB collected, analysed and interpreted data, and drafted the article. PRB participated in habitat selection analyses, presented the results in graphs and tables, and finalized the article. MW and EWH designed the study, guided data analysis and edited the article.
Biographical sketches
Anna Binczik is interested in the effects of human population growth on the conservation of natural resources, and in strategies that facilitate human–wildlife coexistence. Paula Roig-Boixeda is interested in the mitigation of illegal activities, and in understanding and predicting human behaviour and its influence on the effectiveness of conservation interventions. Eckhard Heymann leads research projects on New World primates and is interested in the behavioural ecology and ecology of primates. He is also the director of a field research site in Peruvian Amazonia and a member of the IUCN-SSC Primate Specialist Group. Matthias Waltert works on wildlife conservation research and teaching, and has experience in the Old World tropics and the Middle East. He has worked on improving wildlife population assessments in West and East Africa and has been particularly interested in the impacts of deforestation and land use on tropical biodiversity.






