Introduction
Having been isolated from other land masses for at least 500,000 years (Verstappen, Reference Verstappen1973), the 7,800 km2 Mentawai Island Archipelago west of Sumatra, Indonesia, supports numerous endemic mammals. Endemism of non-volant mammals is exceptionally high (WWF, 1982), including five endemic primate species in four genera: the monotypic pig-tailed snub-nosed langur or simakobu Simias concolor, Kloss's gibbon Hylobates klossii, the Mentawai langur Presbytis potenziani, and two species of macaque (Macaca pagensis and Macaca siberu). H. klossii occurs throughout the archipelago (Whittaker, Reference Whittaker2005a). M. pagensis occurs on the three smaller islands of Sipora, North and South Pagai, and M. siberu is confined to the largest and most northerly island, Siberut (Kitchener & Groves, Reference Kitchener and Groves2002; Roos et al., Reference Roos, Ziegler, Hodges, Zischler and Abegg2003). Subspecies have been described for both langur species based on morphological characters only (Chasen & Kloss, Reference Chasen and Kloss1927).
This high level of primate endemism is a priority for conservation (Chivers, Reference Chivers and Benirschke1986; MacKinnon, Reference MacKinnon and Benirschke1986; Mittermeier et al., Reference Mittermeier, Ratsimbazafy, Rylands, Williamson, Oates and Mbora2007) but the Mentawai primates are under threat from habitat loss resulting from extensive deforestation, both legal and illegal, for agricultural use. This has reduced habitat for primates on the Mentawai islands by at least 50% in the last 25 years (from an estimated 4,200 km2 to only 2,400 km2; Chivers, Reference Chivers and Benirschke1986; Whittaker, Reference Whittaker2005b). Furthermore, hunting primates for food is an important part of Mentawaian culture. According to local hunters (pers. comm.) S. concolor is the easiest to hunt and its meat is the best tasting; it is therefore the main prey species (Mitchell & Tilson, Reference Mitchell, Tilson, Else and Lee1986). The species is listed globally as one of the 25 most endangered primates (Mittermeier et al., Reference Mittermeier, Ratsimbazafy, Rylands, Williamson, Oates and Mbora2007).
Although all Mentawai primate taxa are categorized as threatened on the IUCN Red List (Vulnerable: H. klossii, P. potenziani; Endangered: S. concolor; Critically Endangered: M. pagensis, M. siberu; IUCN, 2007), there is relatively little reliable and up-to-date information on their population sizes and distribution within the archipelago (Tenaza, Reference Tenaza1987; Paciulli, Reference Paciulli2004). Data for Siberut, accounting for over half (4,480 km2) of the total land mass (Whitten, Reference Whitten1982b; Schefold, Reference Schefold1988) are particularly limited and no transect surveys of primate densities have been carried out. Siberut still has considerable forest cover and, in contrast to the southern islands of the archipelago, a comparatively low human population density, especially in the north (Tenaza, Reference Tenaza1987; Fuentes & Ray, Reference Fuentes and Ray1995; Yanuar et al., Reference Yanuar, Fuentes and Studd1998).
Previous estimates of the density of Siberut's primates were made by Tilson (Reference Tilson1977), Watanabe (Reference Watanabe1981) and Whittaker (Reference Whittaker2005b). Tilson (Reference Tilson1977) estimated densities of S. concolor at one site in central Siberut, and Watanabe (Reference Watanabe1981) studied S. concolor and P. potenziani at two sites, in north and south Siberut. Whether these studies are representative is difficult to ascertain because density estimates were mainly inferred from home ranges within comparatively small areas of c. 2 km2. In addition, the widely differing results from the two locations of Watanabe's study (7 and 220 individuals per km2, for S. concolor) complicate interpretation of the data. Call monitoring was used by Whittaker (Reference Whittaker2005a,Reference Whittakerb) to calculate estimates of population size and density of H. klossii in northern Siberut.
Here we present data from the Peleonan forest, a 40 km2 conservation area within a belt of production forest in northern Siberut, close to ongoing logging activities in the east and to the Siberut National Park in the south and west. The Peleonan forest is one of the few remaining relatively undisturbed primary rainforests on the island and is currently assigned for the research and conservation activities of the Siberut Conservation Project under an agreement with the local clans. In addition to conducting primatological research, one of the main aims of the Project is the establishment of a database to support the development of conservation measures for the region and for other areas situated within production forests. As a part of this we surveyed the four primate taxa present, and here we present data on abundance, density and biomass using line transect data collected in 2005. These data provide a baseline for future island-wide population estimates.
Study area
The c. 40 km2 Peleonan forest is in the north-east of the island and connected to the large forest block of Siberut National Park by a wide corridor of mixed primary and secondary forest. It borders the coast to the north, extends from sea level to 180 m, and consists of primary dipterocarp rainforest dominating high ridges and hills. On slopes and lower elevations primary mixed forest, characterized mainly by Myristicaceae, Euphorbiaceae, Dilleniaceae and Dipterocarpaceae, dominates. Near the northern coast the Peleonan forest turns into waterlogged peat swamp forest, characterized by tree species of Myristicaceae and Sapotaceae, and fringed by permanently wet freshwater swamp forest dominated by Terminalia phellocarpa associated with feather palms, rattans, pandans and aroids (WWF, 1982). These swampy areas are separated from the sea by a narrow belt of coastal vegetation. The Siberut Conservation Project field station is located in the south of the Peleonan forest (Fig. 1) and its main study site is a circular area of 10.7 km2 around the station that has been made accessible by 13 systematically placed radially oriented transects with a mean length of 1.5 km (range 1–3 km, 19.85 km in total).
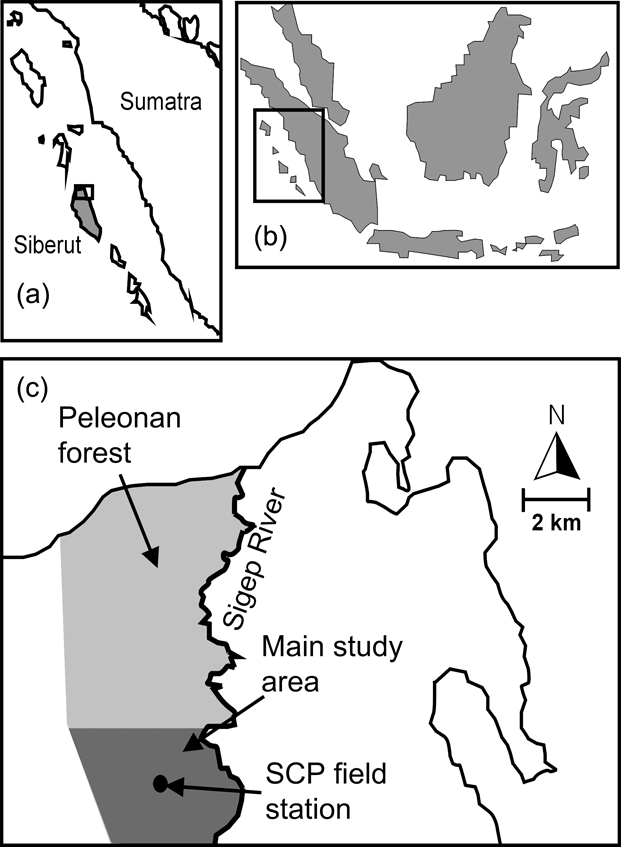
Fig. 1 Location of Siberut (a) off West Sumatra (b), and the Siberut Conservation Project's (SCP) field station and the main study site within the Peleonan forest (c).
Methods
Surveys for primates were carried out between 9 July and 29 December 2005 in a total survey effort of 104.1 km. Surveys were conducted over 6.30–11.30 and 15.30–18.00. Protocols, including searching behaviour and data analysis (see below), followed those of earlier primate surveys (Waltert et al., Reference Waltert, Lien, Faber and Mühlenberg2002) and general recommendations for line transect surveys (Peres, Reference Peres1999; Buckland et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001; Plumptre & Cox, Reference Plumptre and Cox2006). For each observation the species, group size and perpendicular distance from the group or subgroup's centre to the transect (measured using a laser range finder), were noted. We assessed detection probabilities by fitting half-normal detection functions with hermite adjustments to perpendicular distance data, using the software Distance v. 4.1 (Thomas et al., Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001), and calculated density and abundance for the survey site.
As radially oriented transects could potentially lead to biased density estimates by over-covering the area surrounding the station we tested whether survey parameters differed between distances from the station. For each species we calculated encounter rates, detection probability and cluster size for three transect sections, i.e. 0–500, 500–1,000 and 1,000–1,500 m from the starting points. Only S. concolor had a significant drop in encounter rate within the first transect section compared to the second and third (Kruskall-Wallis test P < 0.001). Detection probability and cluster size did not differ significantly (z-test, P > 0.1). To avoid a biased density estimate for this species we stratified the data by dividing the 10.7 km2 study area into the inner belt surrounding the station (a circular area with 600 m radius), and the remaining (c. 1,200 m wide) outer belt.
For all species the size-bias regression method (natural log of cluster size against estimated detection probability) was used to correct average cluster size (sub-group size noted in the field) for distance effects. Because of the dense habitat it was often not possible to measure perpendicular distances accurately beyond 30 m, and only estimation of distances was possible. We therefore compared results from data truncated to < 30 m with only slightly truncated data and found that density estimates derived from slightly truncated data (75–99 m truncation distance) were not significantly different but coefficients of variation were much lower (12–28% instead of 15–40%) in all species.
The daily activity patterns of gibbons are usually reflected in encounter rates, which differ between morning and afternoon surveys. Because of the pooling robustness of the detection function such differences do not pose a problem for detection probability estimation, and hence should not lead to biased density estimates if stratified analyses are not made. We nevertheless also modelled detection probability using survey time (morning vs afternoon) as a covariate. Visual examinations of distance distributions showed there were differences in the shape of the detection functions between morning and afternoon surveys, suggesting that more cryptic behaviour in the afternoon could have led to reduced detection probability near the transect centre and that our density estimates of H. klossii may be conservative.
Biomass density was calculated according to the average weights of Mentawai primates (Rowe, Reference Rowe1996): S. concolor, 7.9 kg; P. potenziani, 6.5 kg; M. siberu, 9.5 kg; H. klossii, 5.8 kg.
Results
Average group sizes were 2.1–3.2 individuals (Table 1) but many observed M. siberu groups were sub-groups of larger social units. A total of 391 primate groups were encountered: S. concolor, 187; M. siberu, 71; P. potenziani, 67; H. klossii, 66. Detection functions fitted to data truncated to widths < 100 m resulted in 139, 41, 36 and 34 observations for each species, respectively (Table 1). S. concolor was the most abundant primate species, with an average density of 53.1 individuals km-2 (and a biomass density of 419 kg km-2) and an estimated population of 570 (range 420-780; Table 2). Stratifying data by distance from the station showed that average density of S. concolor decreases from c. 70 km-2 within 600 m of the station to c. 50 km-2 elsewhere; other than this decrease there was no evidence for further falls in encounter rate (and hence density) at distances > 600 m from the field station. All other primates were considerably less abundant than S. concolor (Table 2). Mean primate biomass was 679 kg km-2 and, based on 95% confidence limits of density, 460–1,011 kg km-2.
Table 1 Mean group encounter rate, size and density, and detection probability, with 95% confidence intervals (CI), for groups within truncation distance w, and number of groups encountered (n), of the four primate species in the Peleonan forest, the site of the Siberut Conservation Project in northern Siberut (Fig. 1).

Table 2 Mean density estimate, with 95% confidence intervals (CI) and coefficient of variation of density (CV), biomass density, and population estimates (based on 95% confidence intervals of mean density), of the four primate species in the Peleonan forest, the site of the Siberut Conservation Project in northern Siberut (Fig. 1).
* Estimate corrected for density decrease with increasing distance from field station.
Discussion
This study provides the first systematic line transect survey of primates on Siberut and the first to encompass all of its four endemic primate taxa. Given that the Peleonan forest has so far been relatively undisturbed by logging and hunting, the study provides an insight into the presumed natural structure of the endemic primate community. The most abundant primate of Siberut's forests appears to be S. concolor, and this species is probably capable of reaching high densities in areas with low disturbance and little hunting pressure (Watanabe, Reference Watanabe1981). Our calculated densities for this species are approximately double those obtained by Tenaza & Fuentes (Reference Tenaza and Fuentes1995) on North and South Pagai, underlining the importance of the Peleonan forest for this species. Thus, conservation efforts in undisturbed areas, even in small areas such as the Peleonan forest, could be successful in securing viable populations of this species.
In contrast, the density of the other Siberut colobine, P. potenziani, appears to be naturally much lower, probably < 50% of the density of S. concolor, confirming estimates made by Watanabe (Reference Watanabe1981). The reason for this large difference between the two species is unclear but could be due to their different ecologies (Tilson, Reference Tilson1977; Watanabe, Reference Watanabe1981).
Whittaker (Reference Whittaker2005b) found H. klossii at densities > 25 km-2 in the Peleonan forest but our estimates are > 50% lower. The reason for this discrepancy is difficult to determine because of the different sampling techniques used. While undercounting of gibbons can occur with line transects, even on the transect line (Brockelman & Ali, Reference Brockelman, Ali, Marsh and Mittermeier1987), and our estimate may therefore be conservative, our interpretation is that Whittaker may have overestimated density due to a combination of low sample size, lack of systematic sampling, and underestimation of gibbon call distance. Our estimate of 8–9 H. klossii km-2 is similar to that of Whitten (Reference Whitten1982a) who estimated 10.5 individuals km-2 based on home ranges, and to data for other Hylobates spp. (density ranges of 4–12 individuals km-2; McConkey & Chivers, Reference McConkey and Chivers2004; Nijman, Reference Nijman2004), although Brockelman et al. (Reference Brockelman, Reichard, Treesucon and Raemaekers1998) reported much higher densities for Hylobates lar (5 groups km-2). Whittaker's (Reference Whittaker2005b) estimate of average group size for H. klossii in the Peleonan forest (3.7) is also unusually high compared to other estimates for H. klossii (Whitten, Reference Whitten1982a; Paciulli, Reference Paciulli2004) and to the other four locations surveyed in her own study.
Our estimate of the density of M. siberu is similar to that of Paciulli (Reference Paciulli2004) for M. pagensis in 10–20 year-old logged forests on North and South Pagai but is somewhat higher than those for undisturbed habitats. Nevertheless, we consider our estimate to be conservative, given the difficulties in obtaining reliable counts of group sizes in this species, in which groups can be relatively large and variable. It is therefore possible that we underestimated group size and that actual densities for the species may be > 16 km-2. Other data for Siberut itself are not available and comparisons with other macaque species in the Sundaland region are not useful because of the extreme degree of variation (< 5 to > 120 km-2) reported (MacKinnon, Reference MacKinnon and Benirschke1986).
Density estimates of S. concolor, P. potenziani and H. klossii are all higher in the Peleonan forest than in any of the locations surveyed on North and South Pagai by Paciulli (Reference Paciulli2004). The high primate densities we found in the Peleonan forest may reflect its relatively undisturbed structure and low hunting pressure by humans. Assuming that data from the main study site are representative of the 40 km2 Peleonan forest as a whole (despite the inclusion of additional swamp forest habitat), approximate population sizes in the region could be 2,100 S. concolor, 600 M. siberu, 360 H. klossii, and 330 P. potenziani. However, these estimates need to be verified by further surveys. Our data nevertheless show that the Peleonan forest supports a substantial proportion of the global populations of all four Siberut primates and hence is of major importance for the conservation of Mentawai's endemic primates.
The threats posed to Mentawai's primates by logging activities have been documented for > 20 years (Watanabe, Reference Watanabe1981; Fuentes & Ray, Reference Fuentes and Ray1995; Paciulli, Reference Paciulli2004; Whittaker, Reference Whittaker2005b). Whilst effects of forest destruction through logging are likely to be species-specific and recovery after logging can only be detected in time-lagged responses, all Mentawaian primate species, and especially the two species of langur, appear to be negatively affected by logging even at low cutting intensities (Whitten, Reference Whitten1982b; Marsh et al., Reference Marsh, Johns, Ayres, Marsh and Mittermeier1987; Paciulli, Reference Paciulli2004). In north Siberut logging and deforestation have so far not been as destructive as on North and South Pagai, and there is still hope that ecologically sound land-use strategies, and proper management of Siberut National Park, will play a much larger role on this island than on North or South Pagai. However, given the uniqueness of the so far largely unexplored fauna and flora of the island, more primate surveys and other biodiversity research are required. In particular, we advocate that wildlife studies on Siberut be carried out in both principal forest types, i.e. freshwater and peat swamp forest and dipterocarp forest. Most of the swamp forests are situated within production forests and will be subject to logging, whereas most of Siberut National Park is dipterocarp forest. The consequences of logging, particularly of swamp forests, for Siberut's endemic wildlife, are still largely unknown. Because the Siberut Conservation Project already operates in production forest, encompassing both forest types, Project activities offer future possibilities for research and for the development of conservation strategies on Siberut and other Mentawai islands.
Acknowledgements
We thank Conservation International, the Critical Ecosystem Partnership Fund, Margot Marsh Biodiversity Foundation, and the German Primate Centre for partly funding this survey. We particularly thank our field guides Pak Nauli and Pak Tarzan, as well as Muhamad Agil, Christos Astaras, Simone Riedelbauch, Andrea Herrmann and Juliane Geyer for very valuable assistance.
Biographical sketches
Matthias Waltert studies the effects of forest land use on tropical biodiversity and teaches wildlife population and biodiversity assessment within the integrated bi-national MSc International Nature Conservation at Georg-August-Universität Göttingen, Germany, and Lincoln University, New Zealand. Christophe Abegg is a specialist in macaque evolution and sociobiology. His work in Indonesia led to the instigation of the Siberut Conservation Project in 2002. Thomas Ziegler's research interests include mating strategies and ecological adaptations of primates, primarily langurs. Dodi Priata is the field manager of the Siberut Conservation Project. Keith Hodges has research interests in evolutionary endocrinology and comparative reproduction in primates.







