Introduction
Milk is a complex physiological liquid that represents an important source of many potentially bioactive components such as hormones, immunoglobulins, growth factors, carbohydrates and high-quality proteins(Reference Shah1). In the last two decades, there has been an interest in the beneficial effects of milk proteins on human health as a potential ingredient of functional foods aimed at controlling elevated blood pressure (BP) and improving vascular reactivity. Previous reviews have mainly focused on the effects of the casein-derived lactotripeptides (LTP; valine-proline-proline (VPP) and isoleucine-proline-proline (IPP)) on BP (see Boelsma & Kloek(Reference Boelsma and Kloek2) and Geleijnse & Engberink(Reference Geleijnse and Engberink3)); however, to date, there has been no comprehensive review of the clinical trials investigating the impact of the two major intact milk proteins (casein and whey) and their hydrolysates on BP and vascular function. The specific aim of the present paper was to critically review the growing literature on the effects of milk proteins and their peptides on BP and vascular function from human intervention studies.
High blood pressure as an important, modifiable CVD risk factor
CVD are disorders that affect the heart and blood vessels and are the number one cause of death worldwide. In 2008, CVD were responsible for approximately 17·3 million deaths in the world, which is estimated to increase to 23·6 million by 2030(4). In the last two decades in some Western European countries (including the UK and France), death rates from CVD have decreased. This has been ascribed to a combination of: better health care, higher awareness of risk factors and government policies aimed at improving the lifestyle of the population. However, this is not a consistent finding; in some middle- to low-income European countries (such as Ukraine and Romania), CVD mortality has increased rapidly(5). CVD risk factors can be grouped into two general categories: non-modifiable (for example, sex, age, genetic traits and ethnic origins) and modifiable (for example, hyperlipidaemia, hypertension, lack of exercise, obesity, alcohol and smoking). The latter are related mainly to poor lifestyle and diet(Reference Lovegrove, Jackson and Saarela6). High BP is a controllable risk factor, yet it is responsible for 13 % of annual deaths worldwide, and is one of the five leading global risks for mortality(7). Kearney et al. (Reference Kearney, Whelton and Reynolds8) reported that more than one in four adults worldwide had hypertension in 2000, and it is estimated that this number will increase to approximately 29 % by 2025. Therefore, it is of paramount importance to address this significant public health burden, as its high prevalence has implications, not only for social and economic welfare, but also for the National Health Service (NHS) in the UK(5).
A holistic marker of CVD risk: vascular dysfunction
Vascular dysfunction is now a recognised CVD risk factor that develops due to a number of factors including hypertension. It predicts long-term atherosclerotic disease progression and risk of future cardiovascular events(Reference Schachinger, Britten and Zeiher9). Vascular dysfunction is thought to arise from a combination of abnormalities associated with the vascular system. One of its manifestations is endothelial dysfunction, comprising of disorders such as increased permeability, reduced vasodilation, and activation of thrombotic and inflammatory pathways, which is a pathological condition, and is defined by an imbalance between the production of endothelium-derived relaxants such as NO and prostacyclin and contracting factors such as endothelin-1(Reference Verma and Anderson10). NO is one of the key vasodilators that is responsible for the maintenance of vascular tone and reactivity and has a pivotal role in endothelial function.
Recently, much interest has focused on the early detection and evaluation of endothelial dysfunction. Flow-mediated dilatation, a technique that applies vascular ultrasound to examine the conduit brachial artery, is considered as the ‘gold-standard’ method for the measurement of vascular reactivity. However, this method is highly operator-dependent, which can result in unacceptably high variability(Reference Corretti, Anderson and Benjamin11). In contrast to flow-mediated dilatation, non-invasive laser Doppler imaging and iontophoresis measure the endothelial function of the microcirculation(Reference Turner, Belch and Khan12), which is believed to be the initial site for the development of endothelial dysfunction in ‘at-risk’ populations(Reference Brodsky, Gealekman and Chen13). This technique uses transdermal delivery of endothelium-dependent (acetylcholine) and endothelium-independent (sodium nitroprusside) vasoactive agents by iontophoresis(Reference Morris and Shore14).
Arterial stiffness is also an important risk factor for CVD and an essential determinant of vascular function(Reference Payne and Webb15). Furthermore, it is considered to be an independent predictor of cardiovascular mortality and morbidity(Reference Boutouyrie, Tropeano and Asmar16). Hypertension may result in vascular remodelling, which is a structural change in arteries, and is also considered to be an age-related disorder(Reference Franklin17). According to Stewart et al. (Reference Stewart, Jiang and Millasseau18), arterial stiffness is not explained by high BP, and the reduction of BP may not contribute to improvements. However, it is important to note that any reduction in arterial stiffness would occur over a significant time period, and therefore study of the relationship between hypertension and arterial stiffness would require a long-term trial. Nevertheless, Wilkinson et al. (Reference Wilkinson, Qasem and McEniery19) demonstrated a relationship between endothelial dysfunction and arterial stiffness, suggesting that any treatment aiming to improve endothelial dysfunction may also have a beneficial effect on arterial stiffness.
The ‘gold-standard’ assessment of arterial distensibility and stiffness is the carotid-femoral pulse wave velocity by applanation tonometry(Reference Rhee, Lee and Park20). The principle of this method is that the higher the velocity of pulse waves between two sites, the stiffer the arteries. This method is simple and non-invasive and has high fidelity. It may also be used to measure endothelial function(Reference Swampillai, Doshi and Fraser21), since arterial compliance (the inverse of arterial stiffness) is partly determined by endothelium-dependent vasodilation(Reference Kelly, Millasseau and Ritter22). The augmentation index (AIx), which is an indirect measure of arterial stiffness, can be calculated as the ratio of the magnitude of the reflected pulse wave to the initial wave(Reference Whitworth23).
Impact of milk consumption on cardiovascular health
Milk and dairy products have gained an undeserved and scientifically unfounded reputation in the media for exerting adverse effects on cardiovascular health, primarily because of their high SFA content. The detrimental effect of SFA on CVD risk, mediated to a large extent through elevated serum cholesterol, is well recognised. However, this over-simplified association between milk consumption and CVD development may well be misleading(Reference Givens24). Milk is a complex food and different micronutrients and/or nutrients might counteract the detrimental, cholesterol-raising effects of SFA(Reference Lorenzen and Astrup25). Furthermore, emerging epidemiological evidence suggests that there is an inverse association between high milk consumption and incidence of CVD(Reference Elwood, Pickering and Givens26). One of the possible mechanisms whereby milk consumption has beneficial effects on CVD health is its potential to lower BP. Growing evidence from population studies indicates that increased milk consumption can lower BP, especially in individuals with elevated BP(Reference Beydoun, Gary and Caballero27, Reference Engberink, Hendriksen and Schouten28), and it may result in an improvement in pulse wave velocity and pulse pressure(Reference Crichton, Elias and Dore29).
Milk contains many bioactive components that have been reported to lower BP; these include Ca, K and milk proteins and/or their bioactive peptides. Bioactive peptides can be released from the intact protein by fermentation with proteolytic starter cultures or hydrolysis by enzymes produced by micro-organisms. Peptides can also be liberated through enzymic hydrolysis during gastrointestinal digestion(Reference Korhonen and Pihlanto30). Bovine milk contains approximately 31–33 g protein per litre, of which about 80 % is casein and 20 % is whey protein. Bioactive peptides are specific protein fragments that have been shown to influence physiological functions and, ultimately, health(Reference Kitts and Weiler31). The activity of these peptides is based on their inherent amino acid composition and sequence. Milk proteins and peptides are reported to possess a wide range of biological properties, and are therefore potential nutraceutical (health-promoting food) ingredients(Reference Hartmann and Meisel32). The health targets of such nutraceuticals include cardiovascular health, bone health, weight management, immune defence, and digestive and dental health(Reference Korhonen and Pihlanto30). Milk peptides may exert their effects on cardiovascular health through angiotensin I-converting enzyme (ACE) inhibition, antioxidant activity and opioid actions(Reference Korhonen and Pihlanto30), which will be discussed in detail below.
Evidence from human interventional trials on the antihypertensive effects of milk proteins
A comprehensive literature search was performed using the electronic databases MEDLINE, the Cochrane Library, EMBASE and Web of Science using the following terms: intervention, randomised controlled trials (RCT), clinical trials, high blood pressure, hypertension, anti-hypert*, vascular function, endothelial function, vascular stiffness, milk protein, milk peptide*, casein, hydrolysate, fermented, sour, humans. Furthermore, hand-searching was performed on the reference lists of both eligible studies and review articles. In addition, Google and Google Scholar were used to confirm that the search was complete. The search period covered studies published in any language until December 2012. The criteria for including studies in the review were: randomised, placebo-controlled trials that examined the effects of milk proteins or peptides on BP and vascular function in men and women aged 18 years or above, with BP and arterial reactivity/stiffness as outcome measures. The intervention products had to contain milk proteins and/or peptides, and be orally administered (at any dose or frequency).
Effects of casein and casein-derived peptides on blood pressure
In the last two decades there has been a growing research focus on the evaluation of the antihypertensive effects of milk proteins and their associated peptides in human subjects. In 1995 Japanese researchers identified two casein-derived LTP that exerted ACE-inhibitory effects and these tripeptides quickly became the most extensively studied milk peptides in relation to their hypotensive actions in animals and humans(Reference Nakamura, Yamamoto and Sakai33). Recently, we have reviewed and performed a meta-analysis on the effects of LTP on BP and observed a significant reduction in both systolic BP (SBP) and diastolic BP (DBP)(Reference Fekete, Givens and Lovegrove34). Therefore these human intervention trials that aimed to evaluate the effects of LTP lie beyond the scope of the present review.
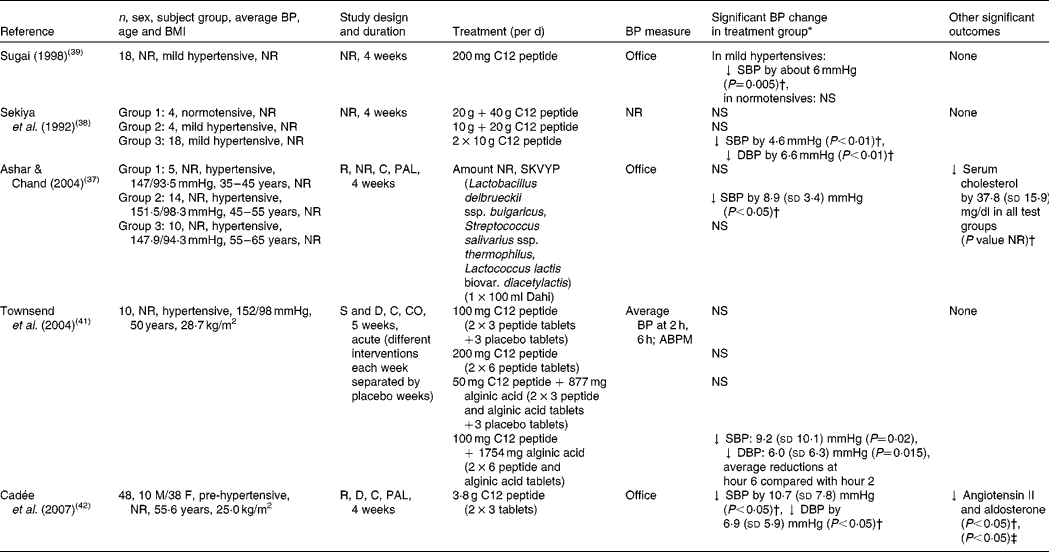
Two human intervention trials have investigated the effects of intact casein on BP (in both acute and chronic settings), which will be discussed within the context of the evidence for whey protein(Reference Pal and Ellis35, Reference Pal and Ellis36). Although a great number of ACE-inhibitory peptides have been isolated from casein, only three (VPP and IPP, serine-lysine-valine-tyrosine-proline (SKVYP), dodeca (C12) peptides) have been investigated in human subjects (Table 1). Ashar & Chand(Reference Ashar and Chand37) undertook a clinical trial to evaluate the antihypertensive effect of a fermented milk, Dahi, containing SKVYP (ACE-inhibitory peptide) prepared by selected lactic acid cultures. In this study, a total of twenty-nine hypertensive subjects were split into three age groups (group 1, 35–45 years; group 2, 45–55 years; group 3, 55–65 years). Only the subjects who consumed the test product showed a statistically significant decline of 8·9 mmHg (P< 0·05) in SBP compared with baseline, which decreased further during the 2-week follow-up period ( − 11·6 mmHg; P< 0·05). No effect was observed on DBP or in the other groups.
Table 1 Human studies investigating the association between casein-derived peptides and blood pressure (BP)

NR, not reported; C12 peptide, dodeca peptide; ↓ , decrease; SBP, systolic blood pressure; DBP, diastolic blood pressure; R, randomised; C, controlled; PAL, parallel; SKVYPT, serine-lysine-valine-tyrosine-proline; S, single-blind; D, double-blind; CO, cross-over; ABPM, 24 h ambulatory blood pressure monitor; M, male; F, female.
* Mean and standard deviation.
† Compared with baseline.
‡ Compared with placebo group at the end of intervention.
Sekiya et al. (Reference Sekiya, Kobayashi and Kita38) and Sugai(Reference Sugai39) examined the effects of a C12 peptide (Phe-Phe-Val-Ala-Pro-Phe-Pro-Glu-Val-Phe-Gly-Lys), as prepared from the hydrolysis of casein with trypsin(Reference Sekiya, Kobayashi and Kita38–Reference Karaki, Doi and Sugano40), on BP. Sekiya et al. (Reference Sekiya, Kobayashi and Kita38) separated the study subjects into three groups: four normotensive (60 g tryptic hydrolysate), four mildly hypertensive (30 g tryptic hydrolysate) and eighteen mildly hypertensive subjects (10 g of tryptic hydrolysate twice per d), respectively, and all subjects consumed the supplements for 4 weeks. Unlike the study of Sekiya et al. (Reference Sekiya, Kobayashi and Kita38), Sugai(Reference Sugai39) conducted a study on eighteen mildly hypertensive participants, who consumed 200 g C12 peptide for 4 weeks. Both studies reported a fall in BP compared with baseline; Sekiya et al. (Reference Sekiya, Kobayashi and Kita38) reported a significant decrease in BP (4·6 mmHg SBP, 6·6 mmHg DBP) in the mildly hypertensive group consuming the 10 g of hydrolysate twice per d (P< 0·01), and Sugai(Reference Sugai39) showed a significant fall in DBP (approximately 5 mmHg; P< 0·001). This stimulated the launch of a soft drink containing the bioactive peptide called Casein DP Peptio Drink (Canebo Co. Ltd) in Japan. Two subsequent clinical trials evaluated the hypotensive effects of the same casein hydrolysate, with the commercial name C12 Peption (DMV International) in Europe(Reference Ashar and Chand37, Reference Townsend, McFadden and Ford41). Townsend et al. (Reference Townsend, McFadden and Ford41) performed an acute, randomised, single-blind, cross-over pilot study in ten hypertensive participants who were assigned randomly to one of the five treatment regimens (100 mg C12; 200 mg C12; 50 mg C12+877 mg alginic acid; 100 mg C12+1754 mg alginic acid; placebo). Significant reductions in BP were only seen in the group consuming the high dose of C12 (100 mg) combined with alginic acid (1754 mg): − 9·2 mmHg in SBP 6 h after oral administration compared with 2 h after ingestion (P= 0·02), and − 6·0 mmHg in DBP 6 h after oral administration compared with 2 h (P= 0·015). Cadée et al. (Reference Cadée, Chang and Chen42) studied the effects of a daily intake of 3·8 g C12 peptide in forty-eight Taiwanese subjects. After 4 weeks, SBP and DBP were reduced by 10·7 mmHg (P< 0·05) and 6·9 mmHg (P< 0·05), respectively, compared with baseline.
It is of note that the trials of Sugai(Reference Sugai39), Sekyia et al. (Reference Sekiya, Kobayashi and Kita38), Ashar & Chand(Reference Ashar and Chand37) and Townsend et al. (Reference Townsend, McFadden and Ford41) were in small groups and may have been underpowered. Their subjects were unevenly and, in most cases, not randomly assigned to the treatment groups. Moreover, Sekyia et al. (Reference Sekiya, Kobayashi and Kita38) and Sugai(Reference Sugai39) did not use control groups. Double-blinding was reported only by Cadée et al. (Reference Cadée, Chang and Chen42) and Townsend et al. (Reference Townsend, McFadden and Ford41). Taken together, the five trials appear to have a high risk of bias, which weakens the strength of evidence supporting their conclusions.
Effects of whey and whey-derived peptides on blood pressure
A limited number of human trials have examined the hypotensive effects of whey protein and its peptides. The results of clinical trials on the effects of whey and associated peptides on BP are shown in Table 2. To date, Pal & Ellis(Reference Pal and Ellis35) are the only research group to compare the effects of the two main intact milk proteins, whey protein (whey protein isolate (WPI), which is the purest intact whey protein obtained from milk, containing about 90–95 g/100 g protein) and casein (sodium caseinate, 90 g/100 g protein) on BP in human subjects. A total of seventy normotensive, overweight subjects were assigned to consume 2 × 27 g/d of either WPI, sodium caseinate or glucose (as a control product) for 12 weeks. Significant reductions in DBP were reported in those who ingested both the whey and casein relative to the control group. Furthermore, whey and casein appeared to be equivalent in their BP-lowering capacity. In 2011, the same research group conducted an acute study(Reference Pal and Ellis36) that compared and evaluated the postprandial effects (6 h) of 45 g WPI with 45 g casein on BP in twenty overweight, postmenopausal women. However, in this case, the proteins had no significant effects on BP. Another study investigated the long-term effects of a protein (30 g WPI/d)-enriched diet, in a 2-year randomised, double-blind, placebo-controlled, parallel trial involving 219 women (between 80–90 years of age)(Reference Hodgson, Zhu and Lewis43). The protein powder was reconstituted with 250 ml water and was consumed daily. Half of the participants in both the treatment and placebo group were taking antihypertensive medication, which did not change over the intervention. While compliance in the treatment group was 88 % by the end of the second year, no significant differences in BP were reported between groups at the end of year 1 and year 2.
Table 2 Randomised controlled trials investigating the association between whey protein and blood pressure (BP)
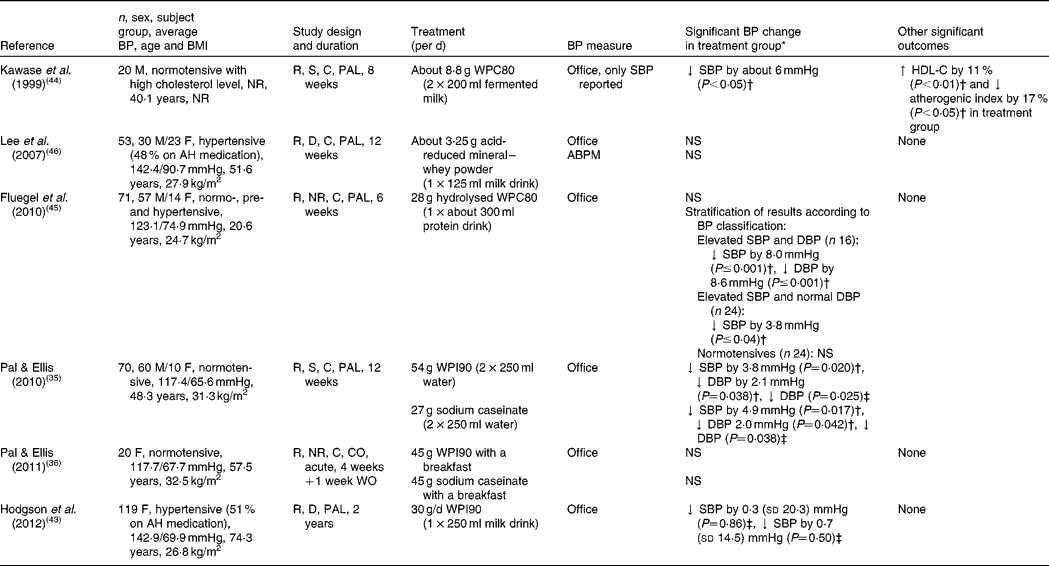
M, male; NR, not reported; R, randomised; S, single-blind; C, controlled; PAL, parallel; WPC80, 80% whey protein concentrate; ↓ , decrease; SBP, systolic blood pressure; HDL-C, HDL-cholesterol; ↑ , increase; F, female; AH, anti-hypertensive; D, double-blind; ABPM, 24 h ambulatory blood pressure monitor; DBP, diastolic blood pressure; WPI90, 90 % whey protein isolate; CO, cross-over; WO, wash-out period.
* Mean and standard deviation.
† Compared with baseline.
‡ Compared with placebo group at the end of intervention.
Kawase et al. (Reference Kawase, Hashimoto and Hosoda44) conducted a single-blind, randomised trial, in which twenty normotensive men were randomised to fermented milk, supplemented with 80 % whey protein concentrate (approximately 8·8 g/d; WPC80) for 8 weeks. In addition to an improvement in serum lipids, they reported a significant decrease in SBP in the test group (approximately 6 mmHg; P< 0·05) compared with the baseline. Interestingly, DBP was not reported in the paper. It is of note that both the test and placebo products contained 0·3 g soyabean peptides/100 g; and the placebo product contained 2 g Na/100 g, while the test product did not. In a study by Fluegel et al. (Reference Fluegel, Shultz and Powers45) normotensive, pre-hypertensive and stage I hypertensive subjects were randomly assigned to consume 28 g/d of either hydrolysed or unhydrolysed WPC80 (as the control product) for 6 weeks. At data analysis, volunteers were stratified according to their baseline BP. Although stage I hypertensives showed a significant reduction in SBP (8 mmHg; P< 0·001) and DBP (8·8 mmHg; P< 0·001) compared with baseline; in pre-hypertensives only DBP (3·8 mmHg; P< 0·04) appeared to decrease significantly. No effect was seen on the BP of normotensives, and it is of note that there were no reports of comparisons with the placebo group.
Lee et al. (Reference Lee, Skurk and Hennig46) evaluated the effects of acid-reduced mineral whey powder on fifty-three hypertensive volunteers (48 % on antihypertensive medication), who consumed 125 ml of a milk drink daily for 12 weeks. Surprisingly, although there were no significant effects on BP in the test group, a significant reduction in BP was observed in the placebo group. When an ambulatory blood pressure monitor (ABPM) was used, no statistically significant effect was reported in either group.
In all six reviewed trials, subjects were randomly allocated to test and placebo products. However, the random sequence generation was only reported by Hodgson et al. (Reference Hodgson, Zhu and Lewis43). Also, only Lee et al. (Reference Lee, Skurk and Hennig46) and Hodgson et al. (Reference Hodgson, Zhu and Lewis43) used double-blinding, whereas Fluegel et al. (Reference Fluegel, Shultz and Powers45) and Pal & Ellis(Reference Pal and Ellis36) failed to report the type of blinding, whilst others used single-blinding. Hodgson et al. (Reference Hodgson, Zhu and Lewis43) and Pal & Ellis(Reference Pal and Ellis35) stated that investigators were also blinded to the outcome assessment. Taken together, the six RCT on whey protein and its peptides appear to be of higher quality than the trials on casein-derived peptides.
Evidence from human interventional trials on the effects of milk proteins on vascular function
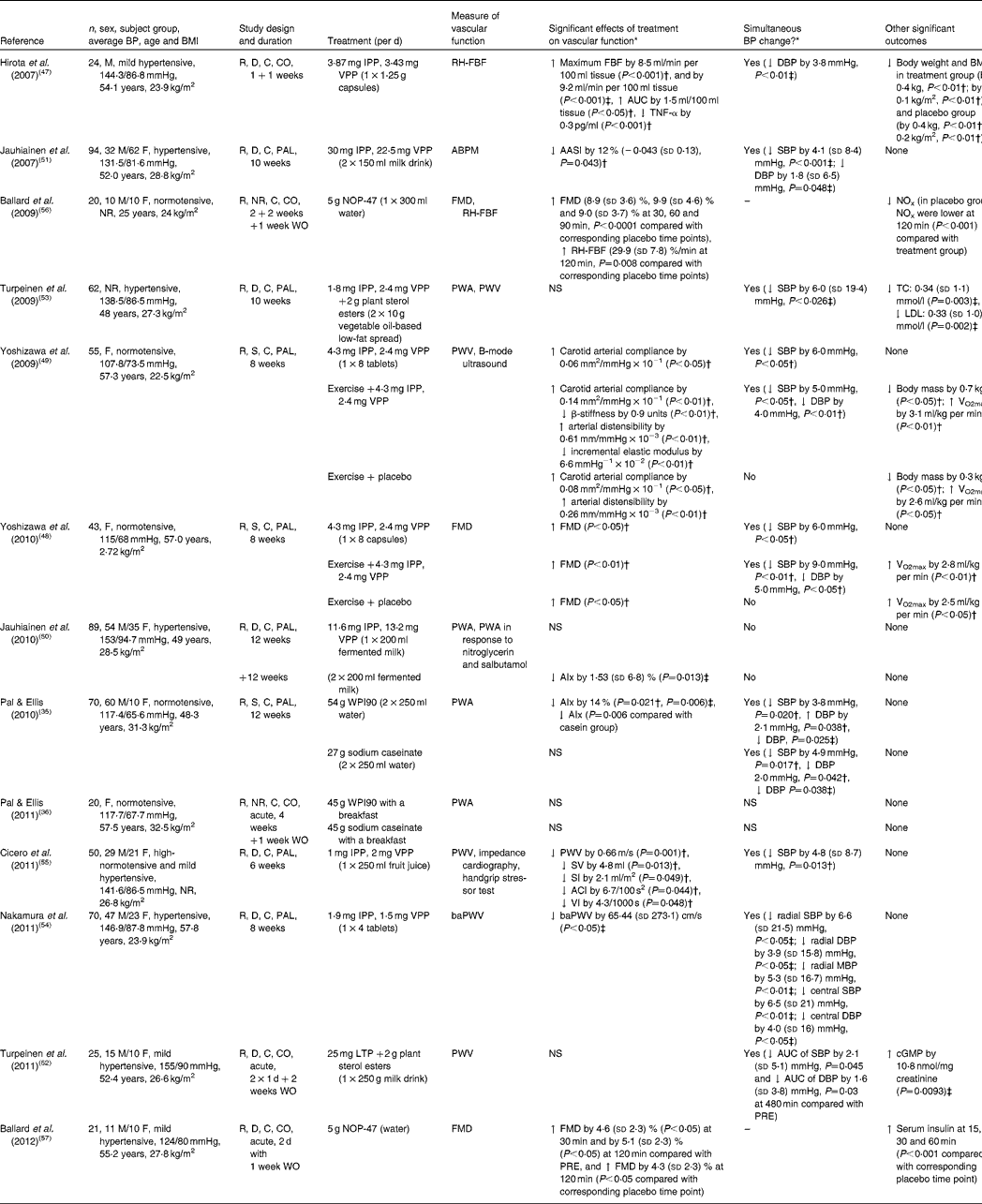
More recently, research focus has shifted from measuring the effects of milk proteins on BP to effects on vascular function. We identified thirteen randomised, placebo-controlled human trials, of which nine(Reference Hirota, Ohki and Kawagishi47–Reference Cicero, Rosticci and Gerocarni55) examined the results of the consumption of casein-derived tripeptides (VPP, IPP) in different forms (for example, milk drink, spread, powder); whilst two of these studies investigated the effects of intact whey protein(Reference Pal and Ellis35, Reference Pal and Ellis36), another two used whey-derived peptide (NOP-47)(Reference Ballard, Bruno and Seip56, Reference Ballard, Kupchak and Volk57). The details of these human trials are shown in Table 3.
Table 3 Randomised controlled trials investigating the association between milk proteins and vascular function

BP, blood pressure; M, male; R, randomised; D, double-blind; C, controlled; CO, cross-over; IPP, isoleucine-proline-proline; VPP, valine-proline-proline; RH-FBF, reactive hyperaemia forearm blood flow; ↑ , increase; ↓ , decrease; DBP, diastolic blood pressure; F, female; PAL, parallel; ABPM, 24 h ambulatory blood pressure monitor; AASI, ambulatory arterial stiffness index; SBP, systolic blood pressure; NR, not reported; WO, wash-out period; FMD, flow-mediated dilatation; NOx, total nitrite/nitrate; PWA, pulse wave analysis; PWV, pulse wave velocity; TC, total cholesterol; S, single-blind; AIx, aorthic augmentation index; WPI90, 90 %whey protein isolate; SV, stroke volume; SI, stroke volume index; ACI, acceleration index; VI, velocity index; baPWV, brachial–ankle pulse wave velocity; MBP, mean blood pressure; LTP, lactotripeptide; PRE, pre-ingestion; cGMP, cyclin guanosine 3′,5-monophosphate.
* Mean and standard deviation.
† Compared with baseline.
‡ Compared with placebo group.
Hirota et al. (Reference Hirota, Ohki and Kawagishi47) investigated the effect of LTP in twenty-four hypertensive men and showed significant improvement in the maximum blood flow during reactive hyperaemia in the treatment group (+8·5 ml/min per 100 ml tissue; P< 0·001) compared with the placebo group after 1 week of 1·25 g LTP consumption. Jauhiainen et al. (Reference Jauhiainen, Ronnback and Vapaatalo51) demonstrated a significant improvement in a calculated index of ambulatory arterial stiffness index (P= 0·043; compared with baseline) in ninety-four hypertensive subjects after 10 weeks of LTP consumption (55·5 mg/d)(Reference Jauhiainen, Ronnback and Vapaatalo51). In a later study, the same researchers fed two doses of LTP (5 and 50 mg LTP/d) to eighty-nine hypertensive subjects for 12+12 weeks(Reference Jauhiainen, Ronnback and Vapaatalo50). Although there was a significant reduction in AIx at the end of the high-dose treatment compared with baseline ( − 1·53 (sd 6·8) %; P= 0·013), there was no significant change in their index of endothelial function. Turpeinen et al. (Reference Turpeinen, Ehlers and Kivimaki52, Reference Turpeinen, Kumpu and Ronnback M53) also failed to report any significant change or differences in arterial stiffness in hypertensive participants in response to the short- and longer-term consumption of a spread containing LTP and plant sterols (short term: 8 h postprandially, n 25, 250 g spread per d(Reference Turpeinen, Ehlers and Kivimaki52); long term: 10 weeks, n 62, 20 g spread per d)(Reference Turpeinen, Kumpu and Ronnback M53). Nevertheless, significant decreases in SBP (P= 0·026), total serum cholesterol (P= 0·003) and LDL (P= 0·002) were reported in the longer-term intervention, relative to the control. Nakamura et al. (Reference Nakamura, Mizutani and Ohki54) investigated the effects of LTP (3·4 mg/d), in the form of tablets, on brachial–ankle pulse wave velocity in seventy subjects with raised BP. They reported a significant reduction in brachial–ankle pulse wave velocity (65·44 (sd 273·1) cm/s; P< 0·05) compared with the placebo group. Cicero et al. (Reference Cicero, Rosticci and Gerocarni55) also reported a significant improvement in pulse wave velocity ( − 0·66 (sd 0·81) m/s; P= 0·001), stroke volume, stroke volume index, acceleration index and velocity index after 6 weeks of treatment with LTP (3 mg/d) in fifty subjects, though these changes were relative to baseline. Yoshizawa et al. (Reference Yoshizawa, Maeda and Miyaki48, Reference Yoshizawa, Maeda and Miyaki49) investigated the combined effect of exercise and LTP consumption on endothelium-dependent dilatation and carotid arterial compliance in postmenopausal women. They found significant improvements in both flow-mediated dilatation and arterial compliance in response to LTP, LTP + exercise, and exercise + placebo groups, but not in the placebo group, and concluded that whilst LTP had a beneficial effect on vascular function, the addition of exercise produced a synergistic effect.
To the best of our knowledge, Pal & Ellis(Reference Pal and Ellis35, Reference Pal and Ellis36) are the only group to date to investigate the effects of the two main intact proteins of milk (casein and whey) on BP and vascular function in postmenopausal, obese women. In a 12-week chronic study, they found that only whey protein lowered the AIx compared with the placebo (P= 0·006) and casein (P= 0·006) groups(Reference Pal and Ellis35). In addition, they conducted a postprandial trial measuring not only BP, but also arterial stiffness(Reference Pal and Ellis36). A breakfast meal in conjunction with one of the three drinks (45 g of whey isolate, sodium caseinate or glucose) was administered to twenty overweight and obese postmenopausal women in a three-way cross-over study design, but could show no effect on vascular function. However, it should be emphasised that during the chronic study, 27 g of WPI was administered twice per d, whereas in their acute study 45 g of WPI was given in one dose with a breakfast meal. The different administrative protocols may have affected the physiological effects of the WPI. Ballard et al. (Reference Ballard, Bruno and Seip56, Reference Ballard, Kupchak and Volk57) examined the acute effects of a whey protein hydrolysate (NOP-47) in a preliminary study, which involved young healthy adults(Reference Ballard, Bruno and Seip56) and older subjects with confirmed impaired vascular function(Reference Ballard, Kupchak and Volk57). Significant improvements in endothelium-dependent dilatation were reported in both studies after test product ingestion compared with placebo. However, the acute effect was determined over a period of only 120 min after a single dose.
Interestingly, Hirota et al. (Reference Hirota, Ohki and Kawagishi47) and Jauhiainen et al. (Reference Jauhiainen, Ronnback and Vapaatalo50) reported improvement in vascular endothelial function and AIx, respectively, after LTP ingestion, but neither could show any effect of LTP on BP. In contrast, significant reductions in BP (either SBP or both SBP and DBP) were reported in the earlier studies of Jauhiainen et al. (Reference Jauhiainen, Ronnback and Vapaatalo51), Cicero et al. (Reference Cicero, Rosticci and Gerocarni55), Yoshizawa et al. (Reference Yoshizawa, Maeda and Miyaki48, Reference Yoshizawa, Maeda and Miyaki49), Nakamura et al. (Reference Nakamura, Mizutani and Ohki54) and Turpeinen et al. (Reference Turpeinen, Ehlers and Kivimaki52, Reference Turpeinen, Kumpu and Ronnback M53) after feeding LTP, and intact whey and casein proteins(Reference Pal and Ellis35). However, in a number of these studies(Reference Yoshizawa, Maeda and Miyaki48, Reference Yoshizawa, Maeda and Miyaki49, Reference Cicero, Rosticci and Gerocarni55), the significant results were expressed relative to baseline only.
Although in all thirteen reviewed RCT, subjects were randomised into test and control groups, only Cicero et al. (Reference Cicero, Rosticci and Gerocarni55) reported the method of random sequence generation. Likewise, the blinding of the investigator responsible for data analysis was only reported in the chronic study of Pal & Ellis(Reference Pal and Ellis35). Blinding of subjects was not stated in the trial of Ballard et al. (Reference Ballard, Bruno and Seip56) and Pal & Ellis(Reference Pal and Ellis36). Taken together, potential biases in the reviewed trials were moderate, which might influence the strength of the evidence base.
Discussion
Although an emerging body of evidence supports the beneficial role of the casein-derived LTP consumption in lowering BP, especially in Japanese populations(Reference Fekete, Givens and Lovegrove34), the results of human studies investigating the potential anti-hypertensive effects of other casein-derived peptides, intact casein and whey and whey-derived peptides seem to be inconsistent, and firm conclusions cannot be drawn. Moreover, whilst there are several RCT involving LTP, the number of human studies that have studied the effects of casein, whey and their peptides are limited.
The degree of heterogeneity in the studies described here has precluded the use of meta-analysis as a means of summating the totality of evidence to link milk proteins with BP. An example of this would be the differing ways in which BP change was reported between studies, as highlighted by Boelsma & Kloek(Reference Boelsma and Kloek2). The change in BP was often expressed relative to baseline, rather than to control, which is prone to confounding due to the ‘white-coat effect’. This describes a phenomenon in which stress caused by the clinical environment, which may decrease with time as familiarisation with the environment develops, causes a significant impact on BP measures. However, if data are compared with the placebo or control group (which does not contain the tested bioactive component), information on the differential effect of the peptides on BP can be drawn. Hence, it is of paramount importance to compare results with control rather than baseline values. Furthermore, in order to eliminate or reduce the adverse impact of the ‘white-coat effect’, a run-in period in which participants take the placebo or visit the clinical unit to familiarise themselves with the measurement techniques can be effectively employed. Only eight of the identified and reviewed studies involved a run-in period(Reference Pal and Ellis35, Reference Pal and Ellis36, Reference Cadée, Chang and Chen42, Reference Lee, Skurk and Hennig46, Reference Jauhiainen, Ronnback and Vapaatalo50, Reference Turpeinen, Kumpu and Ronnback M53–Reference Cicero, Rosticci and Gerocarni55).
The method of BP measurement might also be a potential confounder. Many factors can contribute to the variability in BP, independently of the intervention, such as the central nervous system(Reference Mancia, Ferrari and Gregorini58). Office BP measurements were performed by sphygmomanometer in the majority of studies, with only a few using 24 h ABPM(Reference Townsend, McFadden and Ford41, Reference Lee, Skurk and Hennig46, Reference Jauhiainen, Ronnback and Vapaatalo51). However, there is evidence that 24 h ABPM is a better predictor of CVD risk, since it measures BP more accurately, and has been reported to be more reproducible and can help to eliminate the ‘white-coat effect’(Reference Mancia, De Backer and Dominiczak59).
It is well known that body weight and BMI have a key role to play in BP management(Reference Drøyvold, Midthjell and Nilsen60), yet exercise levels and changes in body weight and BMI were rarely reported in the reviewed trials. Only Hirota et al. (Reference Hirota, Ohki and Kawagishi47) reported a significant decrease in BMI and body weight in both test and placebo groups; however, the change seemed to be somewhat smaller in the test group. Fluegel et al. (Reference Fluegel, Shultz and Powers45), Pal & Ellis(Reference Pal and Ellis35, Reference Pal and Ellis36), Hodgson et al. (Reference Hodgson, Zhu and Lewis43) and Yoshizawa et al. (Reference Yoshizawa, Maeda and Miyaki48, Reference Yoshizawa, Maeda and Miyaki49) stated baseline and end-of-treatment BMI/weight and exercise level data, but, however, failed to report any statistically significant changes.
In most studies the test products were sour or fermented milk(Reference Ashar and Chand37, Reference Sekiya, Kobayashi and Kita38, Reference Sugai39, Reference Hodgson, Zhu and Lewis43, Reference Kawase, Hashimoto and Hosoda44, Reference Lee, Skurk and Hennig46, Reference Jauhiainen, Ronnback and Vapaatalo50–Reference Turpeinen, Ehlers and Kivimaki52), tablets or capsules(Reference Townsend, McFadden and Ford41, Reference Cadée, Chang and Chen42, Reference Hirota, Ohki and Kawagishi47–Reference Yoshizawa, Maeda and Miyaki49, Reference Nakamura, Mizutani and Ohki54), fruit juice(Reference Cicero, Rosticci and Gerocarni55), or spread containing peptides(Reference Turpeinen, Kumpu and Ronnback M53). However, Ballard et al. (Reference Ballard, Bruno and Seip56, Reference Ballard, Kupchak and Volk57), Fluegel et al. (Reference Fluegel, Shultz and Powers45) and Pal & Ellis(Reference Pal and Ellis35, Reference Pal and Ellis36) requested that their subjects consumed the protein hydrolysate and isolate mixed with water. Food vehicles may influence the physiological effects of the bioactive components, as other constituents within the food matrices can exert synergistic or antagonistic effects. Furthermore, additional bioactive peptides may enhance absorption of Ca and K, which may have independent hypotensive effects(Reference Sato, Noguchi and Naito61). The choice of an appropriate control product is of paramount importance in order to evaluate the effect of the active ingredient. While some studies provided detailed information on nutrient composition, and evidence of closely matched test and control products, others revealed differences in both the energy and carbohydrate content between the test and control(Reference Jauhiainen, Ronnback and Vapaatalo50, Reference Jauhiainen, Ronnback and Vapaatalo51, Reference Turpeinen, Kumpu and Ronnback M53). In a few cases, a higher Ca(Reference Lee, Skurk and Hennig46, Reference Jauhiainen, Ronnback and Vapaatalo50, Reference Jauhiainen, Ronnback and Vapaatalo51) or K(Reference Jauhiainen, Ronnback and Vapaatalo50, Reference Jauhiainen, Ronnback and Vapaatalo51) content of the test product could have influenced its BP-lowering effects. The control products in some trials had a higher protein content than treatment products(Reference Kawase, Hashimoto and Hosoda44, Reference Turpeinen, Kumpu and Ronnback M53). Fluegel et al. (Reference Fluegel, Shultz and Powers45) used unhydrolysed WPC80 as a control as it closly mimicked the distinctive mouth-feel and appearance of the test product (hydrolysed WPC80). In contrast, Pal & Ellis(Reference Pal and Ellis35, Reference Pal and Ellis36) used glucose as the control; this was despite existing evidence to show that sugar consumption has been reported to have detrimental effects on hypertension and gene expression, which may render glucose a less valuable control(Reference Chess, Lei and Hoit62–Reference Sharma, Okere and Barrows64).
A growing number of human trials have investigated the impact of milk proteins and peptides on vascular function. The majority of these trials have studied the effects of LTP and only a few have examined the effects of whey, whey-derived peptides and casein-derived peptides other than LTP. In most studies, a range of different methods was used to assess vascular function. While some studies evaluated only one parameter of vascular function(Reference Hirota, Ohki and Kawagishi47, Reference Jauhiainen, Ronnback and Vapaatalo51), other trials assessed not only endothelial function and arterial stiffness, but also inflammatory markers(Reference Pal and Ellis35, Reference Pal and Ellis36, Reference Ballard, Bruno and Seip56, Reference Ballard, Kupchak and Volk57). Although the results of these human studies suggest some benefit on vascular reactivity after consuming milk protein or peptide, the evidence still has insufficient strength for reliable conclusions and dietary recommendations. Further human trials must assess the impact of milk proteins on a range of measures of vascular function before we can fully understand their potential benefit to cardiovascular health.
Putative mechanisms underlying the impact of milk peptides on hypertension and vascular function
There is evidence to suggest that the BP-lowering effects of milk-derived proteins and peptides are mediated through the inhibition of ACE (as a direct pathway in BP regulation) or via non-ACE-inhibition routes (as indirect pathways in BP regulation). The most thoroughly investigated hypotensive mechanism by which milk proteins and their associated peptides may ameliorate BP is through ACE inhibition. The principle of this mechanism is based on the inhibition of the enzyme ACE, the formation of different vasoconstrictor substances (for example, angiotensin II, endothelin-1) and the reduced degradation and inhibition of vasodilatory compounds (for example, bradykinin). Thus, ACE inhibition can exert a favourable effect not only on BP, but also on vascular function. Studies in vitro with the rat model (for reviews, see Murray & Fitzgerald(Reference Murray and FitzGerald65) and Saito(Reference Saito66)) observed ACE-inhibitory activity and identified IC50 values (concentrations of peptides that are required to exert 50 % reduction in ACE) of a large number of peptides isolated from the enzymic digest of milk proteins(Reference Korhonen and Pihlanto30). Whey-derived hydrolysate is reported to exert the highest ACE-inhibitory activity, but has failed to show BP-lowering effects in spontaneously hypertensive rats; however, peptides with low ACE-inhibitory activity triggered significant BP-lowering(Reference Costa, Gontijo and Netto67). Thus, the results of the in vitro or animal studies appear to be inconsistent, supporting other potential mechanisms of action. There has also been inconsistency in the outcomes from human studies.
Sipola et al. (Reference Sipola, Finckenberg and Korpela68) demonstrated that the administration of α-lactorphin or β-lactorphin to spontaneously hypertensive rats induced an endothelium-dependent relaxation of mesenteric arteries, which was suppressed by a NO synthase inhibitor. This suggested that NO may be involved in this process, playing an important role in endothelial function and hence in BP regulation. Furthermore, Hirota et al. (Reference Hirota, Ohki and Kawagishi47) found that LTP-induced endothelium-dependent relaxation of isolated aortic rings was suppressed by NO synthase inhibitors, bradykinin B2 receptor antagonists and K+ channel inhibitors.
Another indirect route to attenuate BP may be through immunomodulatory pathways. Rutherfurd-Marwick & Moughan(Reference Rutherfurd-Markwick and Moughan69) suggested that milk-derived peptides may activate the maturation and proliferation of T cells and natural killer cells, thus playing an important role in immunomodulation(Reference Rutherfurd-Markwick and Moughan69). According to Meisel(Reference Meisel70), the remarkable relationship between the immune system and opioid peptides is supported by the fact that opioid μ receptors for endorphins can be found on T lymphocytes and human phagocytic leucocytes.
The inhibition of the sympathetic nervous system through opioid activity might be another potential mechanism of action. In a recent study, Usinger et al. (Reference Usinger, Ibsen and Linneberg71) reported a significant decrease in sympathetic activity after consumption of fermented milk for 8 weeks in borderline hypertensive subjects. However, simultaneous BP lowering was not observed in this trial. There is evidence that some milk protein hydrolysates, for instance α- and β-casomorphins, act as opioid agonists(Reference Loukas, Varoucha and Zioudrou72), and may exert an analgesic effect on the nervous system(Reference Chang, Cuatrecasas and Wei73, Reference Matthies, Stark and Hartrodt74). This is supported by the study of Nurminen et al. (Reference Nurminen, Sipola and Kaarto75), in which an opioid receptor antagonist (naloxone) was employed and researchers found an absence of any effect on BP. This suggests that BP lowering was mediated by the opioid receptors. Phosphopeptides of milk proteins may enhance the solubility of different minerals such as Ca, thus improving their absorption in the intestine(Reference Sato, Noguchi and Naito61). Although oral supplementation of Ca has not been shown to decrease hypertension(Reference Dickinson, Nicolson and Cook76), dietary Ca intake might have a favourable impact on raised BP(Reference Hatton and McCarron77).
There is growing evidence that oxidative stress and reduced antioxidant status are involved in the pathogenesis of hypertension(Reference de Champlain, Wu and Girouard78, Reference Rodrigo, Prat and Passalacqua79). Several studies reported antioxidant effects of both casein and whey hydrolysates produced by different enzymes(Reference Rival, Boeriu and Wichers80, Reference Hernandez-Ledesma, Davalos and Bartolome81). Many tripeptides (other than LTP, for example, Xaa–Xaa–Trp/Tyr, Tyr–His–Tyr) that show high scavenging activity and strong synergistic effects with phenolic antioxidants have been identified in milk(Reference Pihlanto82). A tripeptide, Xaa–Xaa–Cys(SH), has been shown to possess a high peroxynitrite-scavenging activity(Reference Pihlanto82), which is an important compound in the health of vascular function(Reference Szabó, Ischiropoulos and Radi83).
Bioavailability
During gastrointestinal digestion, peptides and amino acids are released from the ingested protein. To be effective in humans, milk peptides, which have been shown to exert beneficial effects on BP and vascular function in vitro and in animal models, must first be absorbed. A peptide is bioavailable if it is resistant to further digestion, stable while being transported across the gut mucosa and intestinal wall, and resistant to serum peptidases in the circulation(Reference Vermeirssen, Van Camp and Verstraete84). In addition, the peptide must reach its target site or organ in an active form(Reference Vermeirssen, Van Camp and Verstraete84). Evidence supports the idea that short-chain peptides are absorbed intact via different intestinal transporters (for example, carrier-mediated, paracellular diffusion or specific transporter) and induce systemic effects(Reference Masuda, Nakamura and Takano85, Reference Foltz, Cerstiaens and van Meensel86). Conversely, Wuerzner et al. (Reference Wuerzner, Peyrard and Blanchard87) reported that VPP and IPP were poorly absorbed and eliminated from the human body quickly, preventing ACE-inhibitory activity. The phenomenon of effective ACE-inhibitory activity of some peptides in vitro does not always translate into significant effects in animal models or human studies. Discrepancy between the ACE-inhibitory activity of tripeptides in vitro and in vivo experiments may be due to the bioavailability of these peptides. However, there have been several reports of potent ACE-inhibitory activity in peptides produced from the digestion of protein using a combination of enzymes(Reference Vermeirssen, Van Camp and Decroos88–Reference Murakami, Tonouchi and Takahashi90). Interestingly, Foltz et al. (Reference Foltz, Cerstiaens and van Meensel86) found significant concentrations of IPP in the blood of subjects consuming the placebo product, a WPI, which did not contain this tripeptide. This implies that IPP was produced during digestion. Information on the in vivo kinetics of bioactive peptides derived from milk proteins is very inconsistent, yet an essential factor for understanding the action of milk proteins. More research is required to elucidate their bioavailability in the human body.
Conclusions and future recommendations
There has been increasing interest in producing functional foods to improve cardiovascular health, as CVD remains one of the major public health burdens. Consumption of milk and dairy products is associated with beneficial cardiovascular effects. One hypothesised mechanism is through the hypotensive effect of milk proteins and peptides. The current available literature provides some evidence for the beneficial effects of milk proteins and peptides on BP and vascular function, but sufficiently powered RCT will be required to substantiate these effects. In contrast to the pharmacological treatment of hypertension, food-derived proteins have not been shown to cause side-effects, making them safe to consume by individuals with a variety of other disease conditions.
Robust, well-designed, RCT conducted in ‘at-risk’ population will be required to confirm the potential beneficial impacts of milk-derived proteins and peptides on BP and vascular function. Future studies should consider investigating both acute and chronic effects of proteins, applying 24 h ABPM and more detailed cardiovascular reactivity tests to assess the impacts on both endothelial function and arterial compliance. Comparison of the two major intact milk proteins could provide valuable information on which is the more efficacious in attenuating BP and/or improving vascular function. Existing evidence for the mechanisms by which milk-derived proteins and peptides may make an impact on BP and vascular function is both limited and inconsistent; further study is clearly warranted.
Acknowledgements
The present review received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. A. A. F. is supported by a UK Biotechnology and Biological Sciences Research Council studentship.
A. A. F. drafted the manuscript. All authors contributed to and approved the final version of the manuscript.
The authors declare no conflicts of interest.