No CrossRef data available.
Article contents
Transparent carbon nanotube electrodes for electric cell–substrate impedance sensing
Published online by Cambridge University Press: 30 August 2019
Abstract
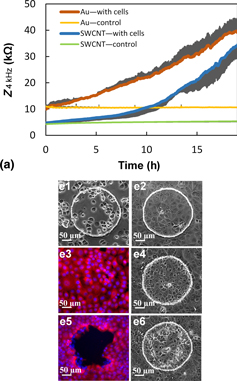
Electric cell–substrate impedance sensing is widely used to study cell behavior such as adhesion, migration, and cell toxicity. However, a simultaneous optical imaging of cells is limited by inefficient transmission of visible light through the gold electrodes. To overcome this limitation, we fabricated carbon nanotube (CNT) electrodes with high electrical conductivity as well as optical transmittance. The impedimetric monitoring of cell proliferation and migration by gold and CNT electrodes were compared and analyzed. Taking advantage of the optical transparency of CNTs, we demonstrated a simultaneous electronic and optical monitoring of MCF7 cells, with acquisition of high-resolution images.
- Type
- Research Letters
- Information
- Copyright
- Copyright © The Author(s) 2019



