Article contents
Shooting for the moon: using tissue-mimetic hydrogels to gain new insight on cancer biology and screen therapeutics
Published online by Cambridge University Press: 07 September 2017
Abstract
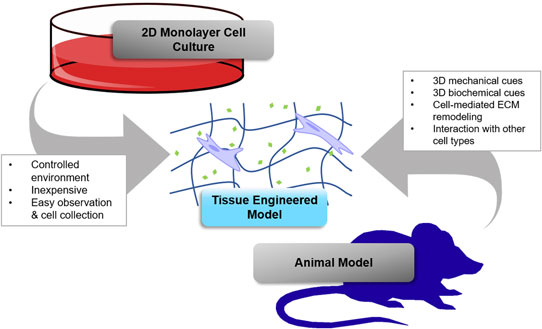
Tissue engineering holds great promise for advancing cancer research and achieving the goals of the Cancer Moonshot by providing better models for basic research and testing novel therapeutics. This paper focuses on the use of hydrogel biomaterials due to their unique ability to entrap cells in three-dimensional (3D) matrix that mimics tissues and can be programmed with physical and chemical cues to recreate key aspects of tumor microenvironments. The chemistry of some commonly used hydrogel platforms is discussed, and important examples of their use in tissue engineering 3D cancer models are highlighted. Challenges and opportunities for future research are also discussed.
- Type
- Biomaterials for 3D Cell Biology Prospective Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 6
- Cited by




