Article contents
Interface effects on self-forming rechargeable Li/I2-based solid state batteries
Published online by Cambridge University Press: 26 April 2019
Abstract
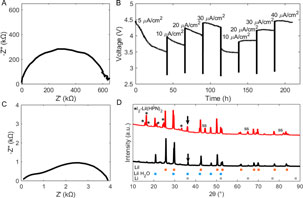
Solid state batteries are an emerging alternative to traditional liquid electrolyte cells that provide potential for safe and high-energy density power sources. This report describes a self-forming, solid state battery based on the Li/I2 couple using an LiI-rich LiI(3-hydroxypropionitrile)2 electrolyte (LiI–LiI(HPN)2). As the negative and positive active materials are generated in situ, the solid electrolyte–current collector interfaces play a critical role in determining the electrochemical response of the battery. Herein, we report the investigation of solid electrolyte–current collector interfaces with a self-forming LiI–LiI(HPN)2 solid electrolyte and the role of varying interface design in reducing resistance during cycling.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 4
- Cited by


