Published online by Cambridge University Press: 06 November 2017
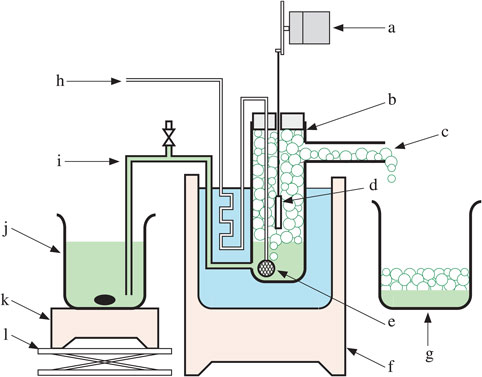
Nickel thin films were prepared by electroless plating in a foam of electrolyte that was generated by bubbling nitrogen gas into a hypophosphite-based electroless plating solution to which was added a surfactant of sulfuric acid monododecyl ester sodium salt. Although the film growth rate in the foam was considerably lower than that in the conventional liquid, film growth was enhanced by inducing a flow in the foam. Compared with films deposited in liquid, the films deposited in foam had a smaller number of pinholes, smaller crystallite size, and superior corrosion resistance. The ferroxyl indicator test showed that the area of corrosion can be reduced to less than 1/20 by depositing the film in foam instead of liquid.