Article contents
Breakthrough additive technology for improving the performance of high-power lithium ion batteries
Published online by Cambridge University Press: 01 November 2011
Abstract
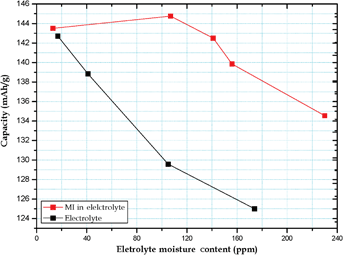
We report a breakthrough in the field of electrolyte additives for use in lithium ion batteries. Batteries containing maleimide (0.1 wt%) as an electrolyte additive absorbed moisture (H2O) from a high-humidity atmosphere. When compared with batteries without the maleimide and absorbed moisture, the capacity of batteries with the “binary additive” showed improvements of 7.4% and 5.2% in a 0.1C/0.1C cycle test, and 394% and 174% in high-power 3C rate tests conducted at room temperature and 55 °C, respectively. Thus, this innovative additive formation can effectively reduce the requirement for anhydrous conditions during the fabrication and operation of lithium ion batteries.
- Type
- Rapid Communications
- Information
- Copyright
- Copyright © Materials Research Society 2011
References
- 5
- Cited by




