The anomalous water solubilities of polyethers have long puzzled researchers. Typically, increasing the oxygen-to-carbon ratio of an organic compound enhances its water solubility. This principle, however, is invalid in the case of polyethers. Polyoxymethylene (POM), a polyether with one carbon atom and one oxygen atom in its repeating unit ([–CH2–O–]n), is entirely insoluble in water. In contrast, poly(ethylene glycol) (PEG), a polyether having two carbon atoms and one oxygen atom in its repeating unit ([–CH2–CH2–O–]n), is infinitely water soluble. The origins of the polyethers’ counterintuitive water solubilities have remained a mystery for decades.
Recently, a research team at the University of Amsterdam in The Netherlands led by Bernd Ensing and Sander Woutersen, together with Johannes Hunger and Mischa Bonn of the Max Planck Institute for Polymer Research in Germany, has shed light on this solubility puzzle for polyethers. Combining spectroscopic and computational techniques, the researchers concluded that the difference in the oxygen charge density dictated the water solubilities of polyethers. This was published in the journal Nature Communications (doi:10.1038/s41467-019-10783-z).
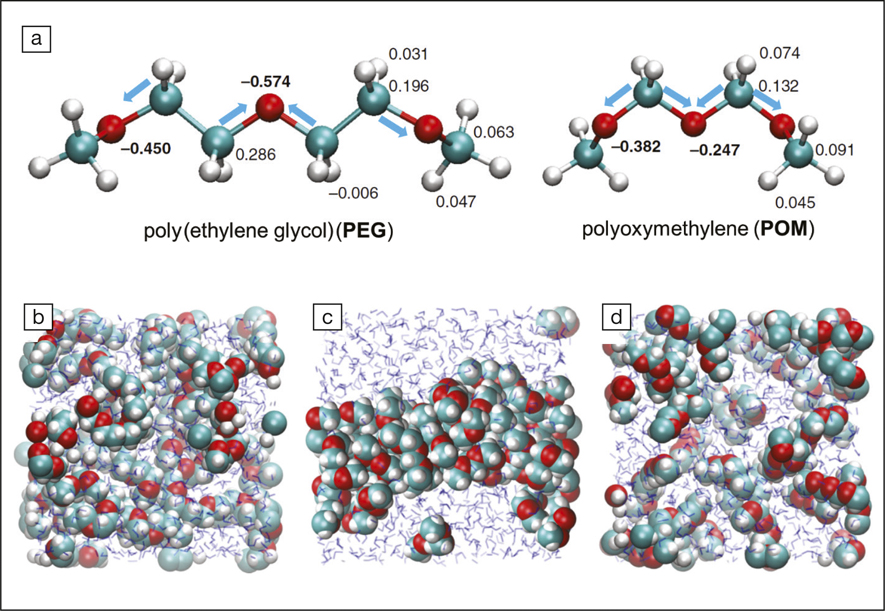
(a) Atomic charges (the numbers) of poly(ethylene glycol) (PEG) (left) and polyoxymethylene (POM) (right). The blue arrows are directions of electron-withdrawing forces. (b-d) Snapshots of the atom positions of (b) PEG molecules, (c) POM molecules, and (d) POM molecules with oxygen charge equal to that of PEG oxygen. Red, blue, and white spheres represent oxygen, carbon, and hydrogen atoms, respectively. The blue V-shaped sticks are water molecules. Credit: Nature Communications.
Computational simulations indicated that the oxygen atoms in PEG were more negatively charged than those in POM. Due to different electronegativities, oxygen atoms pull valence electrons from neighboring carbon atoms. In POM, only one carbon atom segregates two adjacent oxygen atoms. This central carbon atom thus experiences electron-withdrawing force simultaneously exerted by the two side oxygen atoms. The opposite directions weaken the two attractive forces. Whereas in PEG, two carbon atoms divide adjacent oxygen atoms. Each oxygen atom thus attracts electrons from its immediate neighboring carbon atom, with little competition from its oxygen neighbors. Thus, the oxygen atoms in PEG are more negatively charged than those in POM.
This discrepancy in the oxygen charge density influences the extent of water solvation, and consequently, the water solubility. Due to the more negatively charged oxygen atoms, PEG forms stronger hydrogen bonds with water than POM, resulting in excellent water solubility. This conclusion was verified experimentally using infrared and dielectric spectroscopies, as well as theoretically using force-field molecular dynamics. In the theoretical study, PEG molecules uniformly mixed with water molecules after 1 ns, while POM chains still aggregated locally within the same time frame. When the oxygen charge density of POM was increased to the value of PEG, POM started to disperse among the water molecules uniformly, meaning its solubility enhanced considerably.
Qigang Wang of Tongji University, China, highlights the significance of this work: “It is tricky to predict the water solubilities of macromolecules, as they do not always obey the ‘like dissolves like’ principle and empirical rules valid for small molecules. The advanced spectroscopy techniques and the comprehensive molecular simulations demonstrated in this work constitute a novel toolbox to tackle this challenge.”
The conclusion reported in this work is also applicable to explain the water solubilities of other polyethers, for example, dioxane versus trioxane. More generally, this work provides a new theory to predict the water solubilities of oxygen-containing polymers.




