Introduction
The US Food and Drug Administration (FDA) was established in 1906 to protect and promote advancements in drugs, biological products, medical devices, food, and cosmetics. It was not until 1976 that the FDA established a risk-based classification system1 to provide assurance of safety and effectiveness for all medical devices. Class I encompasses devices that are minimal in potential harm, such as bandages and examination gloves. Class II devices present moderate risk of harm, including powered wheelchairs and air purifiers. Class III devices sustain or support life, are typically implanted and have a high risk of potential injury such as pacemakers and defibrillators. Most Class II devices are required to be FDA approved and all Class III devices must be FDA approved.
These devices have traditionally been made through processing methods such as casting and machining, favorable for high volume production, low cost, reproducible, and reliable. However, conventional manufacturing comes with limitations especially in the medical field, as no two patients are identical and treatment needs will vary. Three-dimensional printing (3DP) has become the pinnacle for patient-specific medical devices ranging from metal implants such as those used in arthroplasties, ceramic implants used for bone defects, and even bioprinting to produce artificial organs potentially eliminating the need for donors and organ transplants.Reference Bose, Ke, Sahasrabudhe and Bandyopadhyay2–Reference Bandyopadhyay, Bose and Das5 Such a versatile manufacturing approach also allows for the redesigning of implants to aid in clinical success (see Figure 1). Not only can 3DP be used to build physical biomaterials and biomedical devices, the methodology includes 3D modeling, which can also be used for surgical planning. Parts derived from 3DP can be tailored to incorporate other medically relevant necessities such as drug delivery. This article reviews the clinical significance of various 3DP techniques and their use within a multitude of biomedical devices. An outline of the most relevant 3DP processes for biomedical applications is shown in Table I.

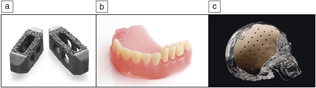
Figure 1. Three-dimensional printed, FDA-approved biomedical devices currently in service. (a) TirboLOX-L titanium lumbar cages for spinal stabilization manufactured by Captiva Spine. Image courtesy of Captiva Spine. (b) DENTCA three-dimensionally printed polymer dentures. Image courtesy of DENTCA. (c) Osteofab craniofacial patient-specific (polymer-based) stabilization device. Image courtesy of Oxford Performance Materials, Inc.
Table I. Different three-dimensional printing methods and their applications in biomaterials and biomedical devices.
Materials and methods for 3D printing in clinical applications
Metals are undoubtedly the most common engineering materials used in load-bearing biomedical applications such as hip and knee arthroplasty, or as fixation devices. Among the many driving points of using 3DP for metallic materials, controlled porosity and compositionally/functionally graded structures are probably the most important.Reference Bose, Ke, Sahasrabudhe and Bandyopadhyay2,Reference Harrysson, Cansizoglu, Marcellin-Little, Cormier and West6 In addition, because most implant materials for knee or hip arthroplasty are either titanium alloys, cobalt-based alloys, or tantalum and other special alloys, making components via additive manufacturing saves machining/casting costs associated with these difficult-to-process materials.
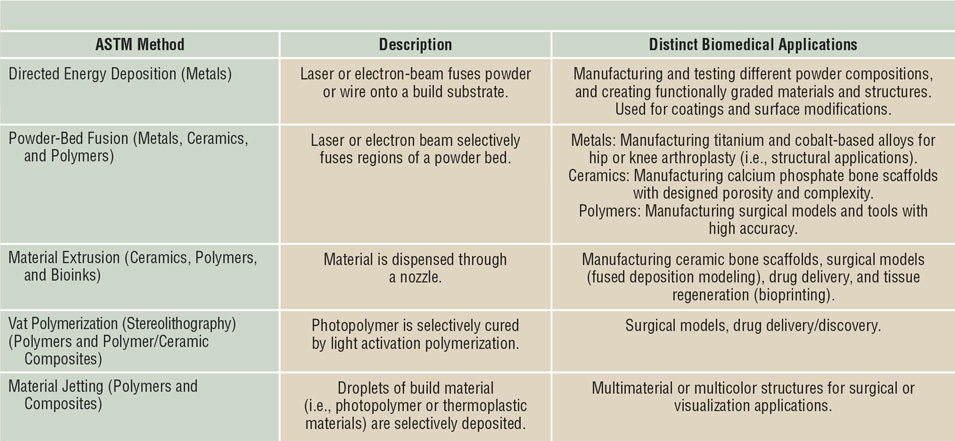
The two main metal-3DP processes used for biomedical applications are directed energy deposition (DED) and powder-bed fusion (PBF), with each maintaining its own specific advantages and disadvantages (see Figure 2a). Discussion of the mechanics of these techniques can be found in Reference Reference Harrysson, Cansizoglu, Marcellin-Little, Cormier and West6. In short, PBF is used to develop single-material components with fine features (such as the porous structures shown in Figure 2b–d, whereas DED is used for changing the composition of a component during printing to optimize specific sections for different operating environments, or providing a surface coating to an existing component.Reference Bandyopadhyay and Traxel7 Because the feedstock is metal powder, leaching of this powder into the body is a major concern as these structures often maintain a surface with partially melted particles. Despite this, manufacturers have used techniques such as chemical etching to remove surface particles and have eliminated the possibility of loose powder leaching from the implant surface.
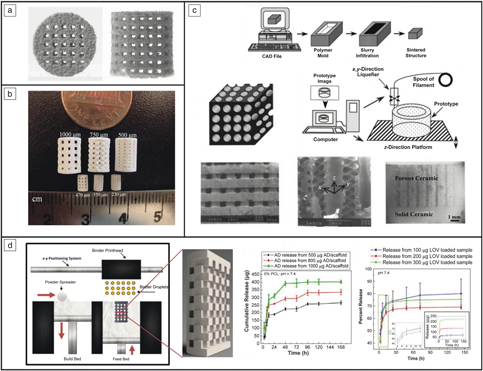
Figure 2. Different metal three-dimensional printing (3DP) types and capabilities. (a) Metal-3DP processes (directed energy deposition [DED]-powder-based, DED-wire-based, powder-bed fusion [PBF], left to right, respectively). Powder-bed-based methods are ideal for developing fine-feature components, whereas directed-energy methods are best suited for compositional and surface changes within single components.Reference Bandyopadhyay and Traxel7 (b) Different porous structure concepts enabled via PBF-based 3DP that are not easily manufactured using traditional methods. Adapted with permission from Reference Reference Zargarian, Esfahanian, Kadkhodapour and Ziaei-Rad22. © 2016 Elsevier. (c) Ti6A14V scaffolds manufactured via PBF for use in in vivo rat studies. Adapted with permission from Reference Reference Van der Stok, Van der Jagt, Amin Yavari, De Haas, Waarsing, Jahr, Van Lieshout, Patka, Verhaar, Zadpoor and Weinans24. © 2013 Wiley. (d) Porous hip stem concepts enabled via PBF-based processing. Adapted with permission from Reference Reference Harrysson, Cansizoglu, Marcellin-Little, Cormier and West6. © 2008 Elsevier.
Among the various applications made possible by the use of 3DP, the concept of “topological design” has significantly grown, where structures are optimized by composition and topology to meet the specific needs of the patient and application, and not limited by the manufacturing process. Methods such as PBF and DED play a large role in this because they enable patient-specific geometry, controlled open-cell and interconnected porosity, and functional gradation from one material to another to increase implant osseointegration.Reference Bose, Ke, Sahasrabudhe and Bandyopadhyay2,Reference Bandyopadhyay and Traxel7,Reference Campoli, Borleffs, Amin Yavari, Wauthle, Weinans and Zadpoor8 Investigations of porous structures produced via PBF show that their properties are largely dependent on the relative density of the final structure, the cell topology, the strut shape, and size distribution, as well as the mechanical properties of the base material (see Table II for some examples of these structures,Reference Campoli, Borleffs, Amin Yavari, Wauthle, Weinans and Zadpoor8,Reference Sahasrabudhe, Soderlind and Bandyopadhyay11–Reference Bose, Sarkar and Banerjee17 as well as Figure 2b–dReference Harrysson, Cansizoglu, Marcellin-Little, Cormier and West6,Reference Sahasrabudhe, Bose and Bandyopadhyay16,Reference Bose, Banerjee, Shivaram, Tarafder and Bandyopadhyay18 ). The inherent challenge with creating these features is that the strut size varies along the length due to the powder-bed support during processing.Reference Van Hooreweder, Lietaert, Neirinck, Lippiatt and Wevers10,Reference Heer and Bandyopadhyay15 More specifically, when producing finer struts (dimensions on the order of micrometers), the powder size (10–50 µm in diameter) prohibits highly smooth surfaces, and partially melted particles are readily observed.Reference Heer and Bandyopadhyay15 While the tendency is to increase the pore size and decrease the relative density to enable a structure with improved osseointegration (bone integration), it is important to strike a balance to maintain the structural properties of the base material.
Table II. Selected literature on porous biomaterials fabricated via three-dimensional printing.
Next-generation biomedical concepts include the use of advanced compositions and functionally graded materials that transition from the base material to a different material, optimizing a component for varying local environments. This concept could be used in articulating surface applications where a transition occurs from a material or structure that promotes higher osseointegration to a material that has high hardness and wear resistance. As an example, significant research has been done to see if in situ processing strategies such as nitridation,Reference Balla, Bodhak, Bose and Bandyopadhyay19,Reference Xue, Krishna, Bandyopadhyay and Bose20 reactive-deposition,Reference Hedayati, Ahmadi, Lietaert, Pouran, Li, Weinans, Rans and Zadpoor21,Reference Zargarian, Esfahanian, Kadkhodapour and Ziaei-Rad22 or ceramic-phase deposition/reinforcement,Reference Amin Yavari, Wauthle, Van der Stok, Riemslag, Janssen, Mulier, Kruth, Schrooten, Weinans and Zadpoor23,Reference Van der Stok, Van der Jagt, Amin Yavari, De Haas, Waarsing, Jahr, Van Lieshout, Patka, Verhaar, Zadpoor and Weinans24 can decrease the wear rate and ion release of metallic surfaces during articulation (relative motion between two surfaces). It is envisioned that this concept can be included within a single component that transitions from an area that is porous (and integrated with the host tissue), while simultaneously maintaining an area with an articulating surface.
Among materials used in biomedical applications, ceramics are one of the most difficult to manufacture due to their inherent brittleness and the need for high-temperature densification. Despite the processing difficulties, ceramic implants are of high interest, as bone is a natural ceramic. Naturally, bones and teeth are areas where ceramic implants are widely utilized. Bone is comprised of almost 70% calcium phosphate (CaP)-based ceramic. CaP scaffolds are ideally employed for critical fractures, defects, and voids within bone. There are various forms of CaP that vary in physical, mechanical, and biological properties, especially in dissolution characteristics based on the Ca to P ratio. Three-dimensional printing provides a unique fabrication opportunity to produce complex ceramic parts with interconnected porosity to tailor mechanical performance and biocompatibility. Researchers have shown increased cell–materials interactions due to increased porosity in CaP scaffolds.Reference Bose, Darsell, Kintner, Hosick and Bandyopadhyay25
One popular form of biocompatible and osteoconductive CaP used is hydroxyapatite (HA) with a Ca to P ratio of 1.67. Various 3DP techniques have been employed to fabricate HA-based biomedical parts both for bone and tissue engineering. A nozzle material extrusion-based 3DP technique has a multitude of names, including, but not limited to, fused deposition of ceramics and robocasting. This material extrusion system is one way HA parts have been made for tissue-engineering constructs.Reference Vaezi, Seitz and Yang26,Reference Lin, He, Song, Wang, Gao, Li, Tang, Wang, Bi and Pei27 Another common 3DP method used is powder-bed 3DP equipped with a binder jet printhead with HA embedded into the feedstock (see Figure 3a).Reference Seitz, Rieder, Irsen, Leukers and Tille28 A method similar to powder-bed 3DP is selective laser sintering (SLS), which has also been used to construct HA structures.Reference Shuai, Gao, Nie, Hu, Zhou and Peng29
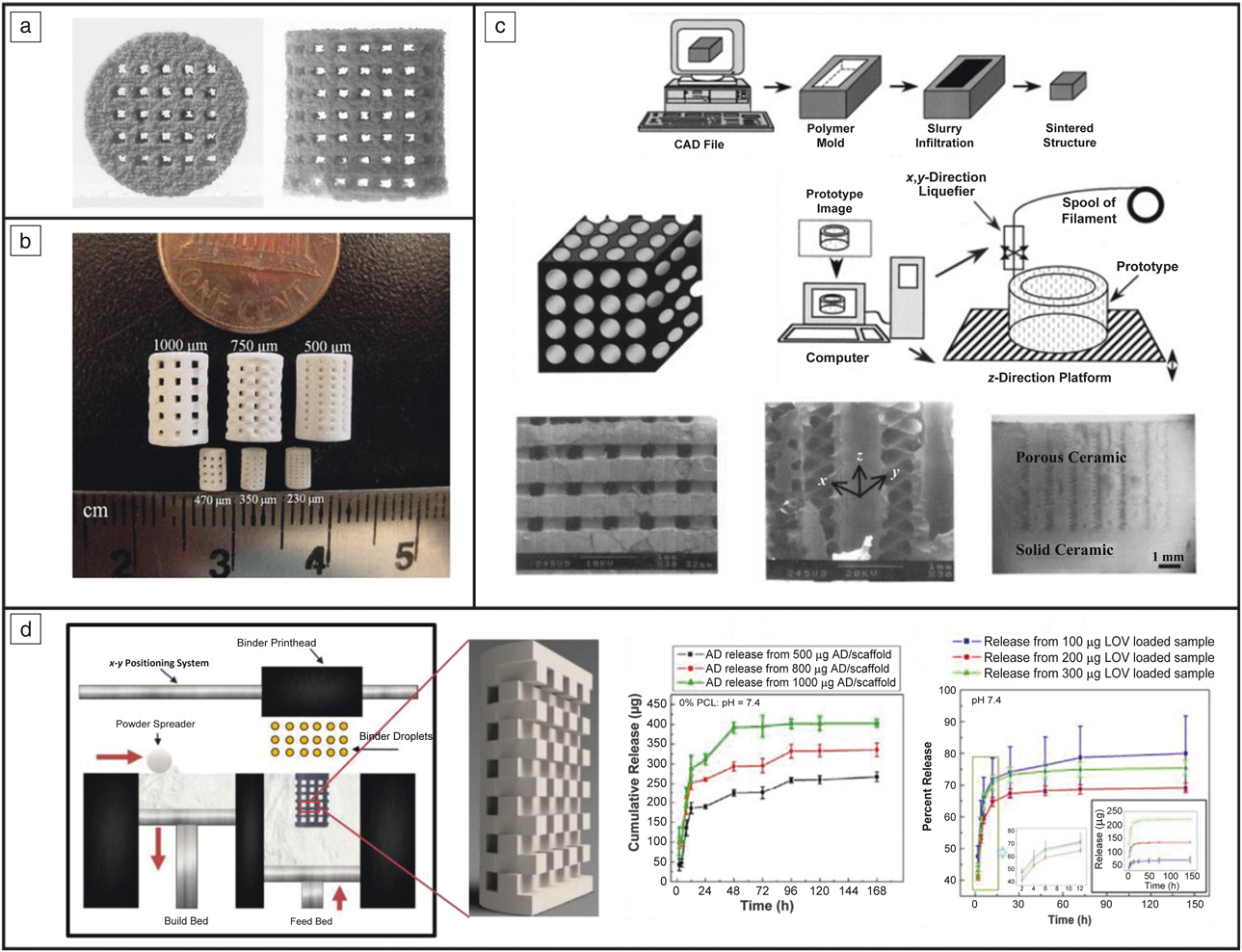
Figure 3. CaP scaffolds produced using three-dimensional printing (3DP). (a) Hydroxyapatite scaffolds fabricated using binder jetting.Reference Seitz, Rieder, Irsen, Leukers and Tille28 (b) Tricalcium phosphate scaffolds manufactured using binder jetting.Reference Tarafder, Balla, Davies, Bandyopadhyay and Bose31 (c) Honeycomb structure made from alumina ceramics for bone grafts.Reference Bose, Suguira and Bandyopadhyay34 (d) Scaffolds produced using 3DP to provide local drug delivery using alendronate (AD) and lovastatin (LOV) for bone-tissue engineering applications.Reference Fielding and Bose33,Reference Tarafder and Bose37,Reference Tarafder, Nansen and Bose38 Note: CAD, computer-aided design.
Vat polymerization involves photoactive polymers cured using a laser pattern to create complex parts. HA can be mixed with these photoactive polymers and heat treatment can remove the binders followed by densification of the ceramic part.Reference Griffith, Chu, Wagner and Halloran30 Another widely utilized and studied CaP employed for bone-tissue engineering is tricalcium phosphate (TCP), with a Ca to P ratio of 1.5. TCP is known for its biodegradable properties, making it ideal for bioresorbable implants. Dissolution characteristics can be tailored for TCP based on the primary phase (i.e., β-TCP) at low temperatures compared to α and α′, which occur at high temperatures. Binder jet and vat polymerization 3DP techniques have been utilized to produce TCP scaffolds, just as they can be used to make HA scaffolds (Figure 3b).Reference Tarafder, Balla, Davies, Bandyopadhyay and Bose31–Reference Fielding and Bose33
Indirect ceramic implants can also be produced using 3DP. Molds have been fabricated using fused deposition modeling (FDM) where ceramics can be embedded, and the mold is removed via heat treatment. This technique was used in some of the first-generation work related to porous scaffolds for bone-tissue engineering to produce alumina ceramics with controlled porosity in a honeycomb structure (see Figure 3c).Reference Bose, Suguira and Bandyopadhyay34 Bone grafts are used for transplanting bone tissue to repair damaged bone. To enhance effectiveness and cell proliferation, grafts can be customized using 3DP.Reference Kalita, Bose, Bandyopadhyay and Hosick35 Personalization is achievable from computed tomography (CT) scans to produce ceramic bone grafts. Darsell et al. achieved this from a horse’s short pastern bone (knuckle) fabricated via FDM.Reference Darsell, Bose, Hosick and Bandyopadhyay36 All of these methods can be utilized or adapted for use in many other forms of CaPs. Additionally, the addition of dopants, proteins, growth factors, and other drugs can also be embedded within the scaffolds produced via 3DP (see Figure 3d).Reference Tarafder and Bose37,Reference Tarafder, Nansen and Bose38 These ceramic polymer composite systems provide on-site drug delivery, or the presence of dopants aid in healing and recovery. This is seen by the enhanced osteogenesis, angiogenesis, and extracellular matrix formation from an in vivo bilateral rat distal femur model (head of femur bone connecting with the knee) utilizing Fe and Fe-Si doped TCP 3DP scaffolds and in the presence of other dopants and drugs (Figure 4).Reference Bose, Banerjee, Robertson and Vahabzadeh39
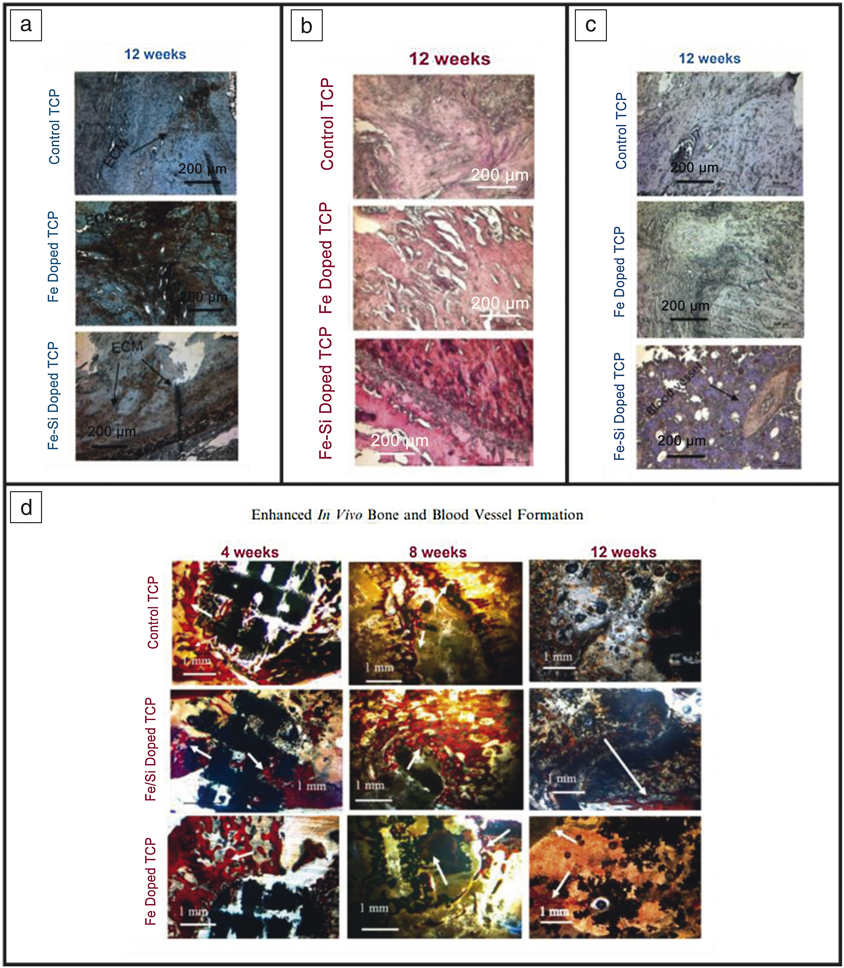
Figure 4. Tricalcium phosphate (TCP) scaffolds produced using three-dimensional printing (3DP) and implanted in a rat distal femur model show enhanced bone and tissue healing with the addition of biologically relevant metallic dopants. (a) Extracellular matrix formation (ECM) via Type I collagen staining shown in brown indicating higher ECM formation with Fe addition. (b) Hematoxylin and Eosin staining showing more and deeper red in Fe-Si doped scaffolds indicating enhanced tissue formation. (c) Blood vessel formation via von Willebrand factor staining shown in purple indicating blood vessel formation in Fe-Si doped scaffolds. (d) Modified Masson–Goldner trichrome staining with red/orange showcasing osteoid tissue and blue/green showcasing mineralized bone. These images portray enhanced early-stage osteoid formation from doped 3DP scaffolds.Reference Bose, Banerjee, Robertson and Vahabzadeh39
Polymers and polymer-based composites have also played a significant role where ceramic materials would be too brittle. These composites provide ductility and higher durability. Different 3DP methods such as stereolithography (SLA), selective laser techniques (SLS), inkjet-based techniques and fused deposition modeling (FDM) have been utilized to fabricate these components for different applications. The most commonly used polymers include poly(caprolactone) (PCL), poly(lactic acid) (PLA), as well as polycarbonates and poly(propylene) fumarate.Reference Childers, Wang, Becker, Fisher and Dean40,Reference Koski, Onuike, Bandyopadhyay and Bose41 Resorbable and nonresorbable polymer materials are often utilized within non-load-bearing applications in tissue engineering, and resorption characteristics are of critical importance.
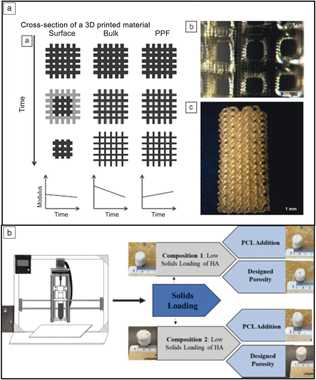
Three-dimensional printing of polymers is also used for various Class I devices. Childers et al. investigated the use of SLA to fabricate polypropylene fumarate scaffolds.Reference Childers, Wang, Becker, Fisher and Dean40 Figure 5a shows a schematic of the degradation characteristics of different materials/pore structures (surface erosion, bulk erosion, erosion characteristics for polypropylene fumarate). Typically, bulk erosion can lead to an immunogenic biological response, and, if not well understood or controlled, is not desired from a design standpoint. In this specific investigation, it was reported that the degradation of polypropylene fumarate resembled a combination of both schemes, providing a material with controlled absorption for future tissue-engineering applications.

Figure 5. Examples of polymers and polymer-ceramic composites used for biomedical applications. (a) Degradation mechanisms for different types of polymer scaffolds in tissue engineering. Subfigures display the relationships between time and degradation characteristics, as well as the surface topography of the scaffolds.Reference Childers, Wang, Becker, Fisher and Dean40 (b) Solid freeform fabrication of polymer-ceramic composites by varying the solids loading of hydroxyapatite (HA) and polycaprolactone (PCL).Reference Koski, Onuike, Bandyopadhyay and Bose41
Extrusion-based freeform fabrication technology is another method that can directly manufacture polymer-ceramic composites via variation of the feedstock composition. Koski et al. demonstrated this process by incorporating starch (natural polymer) in HA-based slurries.Reference Koski, Onuike, Bandyopadhyay and Bose41 A schematic of the mechanism is shown in Figure 5b, where a slurry is loaded into a piston and extruded via a motor-screw setup. In the study, starch and PCL percentage, as well as designed porosity, were investigated to increase the mechanical strength of as-processed scaffolds and cell viability. The authors report increasing the strength of the scaffolds by a factor >2 with the incorporation of starch due to a reinforcing mechanism, highlighting the unique capabilities of this technique for manufacturing polymer-ceramic composites.Reference Koski, Onuike, Bandyopadhyay and Bose41 We next showcase clinical applications for using 3DP alongside some of these materials, including surgical planning, bioprinting, and drug delivery.
Three-dimensional printing in surgical planning
Three-dimensional printing has played a significant role in surgery by providing doctors a “third eye” for visualization and surgical preparation.Reference Javaid and Haleem42,Reference Reddy, Vasamsetty, Kumar Malyala and Alwala43 With 3DP, practitioners can readily create models of body parts or organs for educational purposes, as well as for surgical planning and practice. Most studies report the ability of 3DP to produce anatomical models, surgical guides, implants, and molds for surgical aids.Reference Martelli, Serrano, Van Den Brink, Pineau, Prognon, Borget and El Batti44 Despite the ability to create unique models, the biggest challenge that remains is the ability to rapidly go from a CT scan to a 3D printed part in a short period of time. Data transfer is a large issue within hospitals, and because of this, efforts to increase the efficiency of information transfer from scanner to printer are continuously under development.
FDM is a commonly used process for quick-fabrication of models, but for more advanced applications, the standard machines are of the SLA and SLS type, for creating fine microscale features and details.Reference Garg and Mehta45,Reference Malik, Darwood, Shaunak, Kulatilake, El-Hilly, Mulki and Baskaradas46 Other techniques are also employed depending on the need to add functionality such as color and controlled porosity, including poly-jet or multi-jet additive techniques that are currently used in different hospitals.Reference Leng, McGee, Morris, Alexander, Kuhlmann, Vrieze, McCollough and Matsumoto47
A prime example of this functionality is in the field of maxillofacial surgery, where doctors have historically been challenged to find ways to practice performing surgeries. Surgeons in this field are required to perform many hours of practice on wax molds or cadaver models, where incorporating 3DP for making the models saves significant time and cost in the development process. An example of this is shown in Figure 6a where a patient having a cleft defect in the upper lip was provided with an implant.Reference Malyala, Ravi Kumar, Kankanala, Vasamsetty and Alwala48 By performing a CT scan, forming a 3D-computer-aided design (CAD) model, and printing the patient’s jaw structure, the surgeons were able to fine-tune the structure of the implant before performing the surgery. This saves significant troubleshooting time and effort during surgery, which results in better surgical outcomes as well as lower overall costs for hospitals.
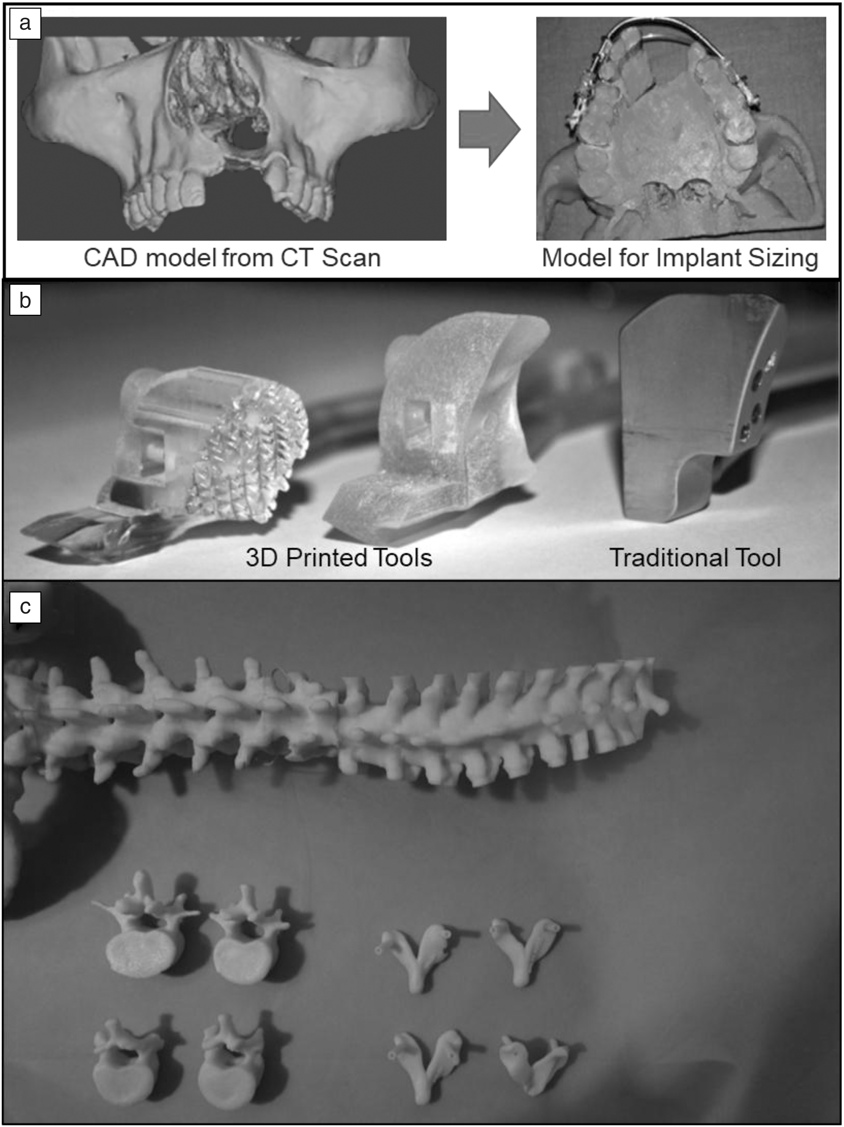
Figure 6. Examples of three-dimensional printing (3DP) in surgical planning and implementation. (a) Cleft computer-aided design (CAD) model used to aid surgeons in implant procedure. (b) Bone-drilling guides enabled via 3DP. Adapted with permission from Reference Reference Malik, Darwood, Shaunak, Kulatilake, El-Hilly, Mulki and Baskaradas46. © 2015 Elsevier. (c) Pedicle screw guides 3D-printed to ensure accuracy and screw location during spinal surgery. Reprinted with permission from References Reference Malyala, Ravi Kumar, Kankanala, Vasamsetty and Alwala48 and Reference Garg and Mehta45. © 2018 Elsevier.
Elsewhere, 3DP has also been used to make patient-specific tooling that is implemented during a surgical procedure. These devices are used during the surgery, but are subsequently removed before the end of the procedure. An example of this (shown in Figure 6b) is a bone-drilling procedure that utilizes a guide piece that is set on the patient throughout the procedure.Reference Malik, Darwood, Shaunak, Kulatilake, El-Hilly, Mulki and Baskaradas46 These patient-specific devices ensure the best alignment with the host geometry and ensure higher implant performance and longevity.
Another example comes from the field of spine surgery (Figure 6c) where pedicle screw guides were created from a patient’s spine geometry. In this case, doctors were able to scan a patient’s bone geometry, print tooling that conformed to the patient’s geometry, and test out the tool before the surgery. Such tools enable doctors to optimize their surgical procedure and decrease the amount of time required for the surgery.Reference Garg and Mehta45 With 3DP, these guides can conform to the anatomy of the patient, enabling a much safer and stabilized drilling procedure. While specialized for each patient, 3DP does add additional time to the surgery preparation due to sizing and print time, therefore, its use must play an integral role in the overall surgery to make it economic for surgeons and practitioners.Reference Garg and Mehta45
Three-dimensional printing for coatings and surface modifications
Three-dimensional printing is known for producing biomedical parts and devices, but 3DP can also be used for coatings or surface modifications. These modifications can help minimize biocorrosion or enhance implant-tissue integration. Most 3DP techniques can be used for surface modifications such as binder jetting to create support layers or directed energy deposition, specifically laser metal deposition, to create melt pools on the surface. Deposition-based 3DP can be utilized to create surface patterns to change surface topography, increase surface roughness, alter surface energies, and adjust crystallinity. Metal implants, in particular, have naturally smooth surfaces with high surface energies. Surface modifications can help facilitate better cell adhesion by introducing roughness. Another surface property necessary for high implant integration is porosity. Three-dimensional printing can be strategically used to build more complex parts with an outermost layer designed with appropriate porosity and interconnectivity. DED-based 3DP has been utilized to produce gradient structures with a metal/ceramic composite outer layer to increase implant integration with host bone tissue with good interfacial strength between build layers.Reference Balla, Das, Bose, Janaki Ram and Manna49,Reference Roy, Balla, Bandyopadhyay and Bose50 Metal implants are not the only medical devices that can undergo surface modifications. Ceramic implants can also benefit from surface modifications through polymeric coatings. These coatings can be designed for application specificity and deposited using 3DP techniques.
Bioprinting
Bioprinting is a rapidly expanding field that is driven by the desire to manufacture artificial tissues comprised of a scaffold and cellular media to operate within a biologically relevant microenvironment.Reference Bose, Ke, Sahasrabudhe and Bandyopadhyay2,Reference Li, Chen, Fan and Zhou51–Reference Zhang, Jin, Yin, Xu, Xiong, Christensen, Ringeisen, Chrisey and Huang53 Some emerging applications include the use of bioprinting to study bacterial growth and disease progression,Reference Poldervaart, Wang, Van der Stok, Weinans, Leeuwenburgh, Oner, Dhert and Alblas54–Reference Gudapati, Dey and Ozbolat56 as well as in the development of scaffolds and organs for implantation in the body.Reference Zhu, Shin, van Kempen, Li, Ponraj, Nasajpour, Mandla, Hu, Liu, Leijten, Lin, Hussain, Zhang, Tamayol and Khademhosseini57 This process has also enabled more ethical methods to explore disease and cellular growth.Reference Li, Jiang, Li, Chen, Gao, Yao and Sun52
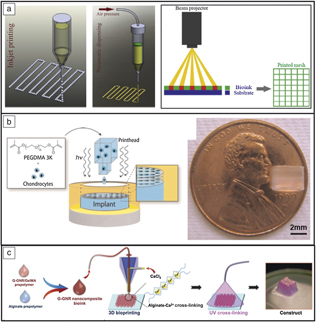
Undoubtedly the largest barrier to widespread implementation is maintaining viability of cells throughout the printing process to create fully functional constructs. Different methods are typically utilized such as inkjet-based processing (cellular and acellular), pressure-assisted extrusion, and laser-assisted deposition (Figure 7a). In short, inkjet-based methods are known for producing low-viscosity fine droplets onto a substrate or culture dish; pressure-assisted methods can produce higher-viscosity pastes or slurries (typically due to the expense of cell viability due to shear forces); and laser-assisted methods are known for processing hydrogel-based materials.Reference Li, Chen, Fan and Zhou51 While bioprinting is promising for full transplantation, full vascularization, and functionality, acceptance into the human body remains a formidable challenge. Most current studies use previously determined biocompatible materials and growth factors to control cell proliferation and bone regeneration locally, as opposed to the fabrication of entire organs.Reference Derakhshanfar, Mbeleck, Xu, Zhang, Zhong and Xing58
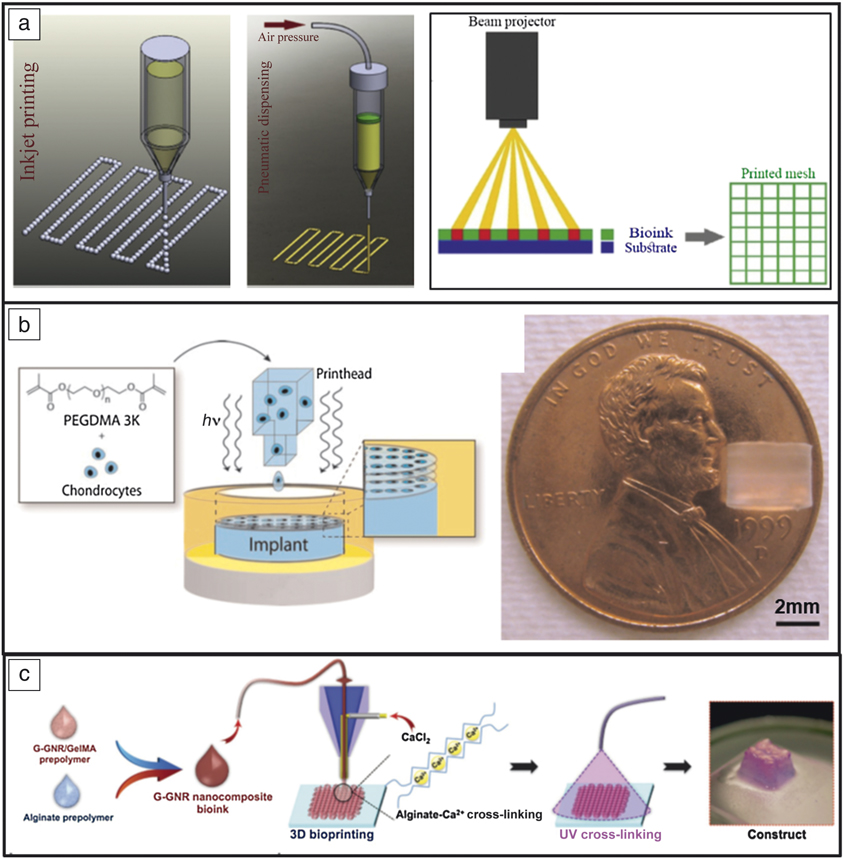
Figure 7. Examples of bioprinting. (a) Typical methods of fabrication: inkjet, pressure-assisted, and laser- or beam-assisted. Reprinted with permission from Reference Reference Li, Chen, Fan and Zhou51. © 2016 Springer. (b) Bioprinting to study the effects of different growth factors on drug behavior. With three-dimensional printing, chondrocytes can be more effectively distributed within poly(ethylene glycol) (PEG) scaffolds than with post-printing incorporation. Reprinted with permission from Reference Reference Cui, Breitenkamp, Lotz and D’Lima55. © 2012 Wiley. (c) Gold-nanoparticle-assisted bioprinting to aid cardiac tissue regeneration. Reprinted with permission from Reference Reference Zhu, Shin, van Kempen, Li, Ponraj, Nasajpour, Mandla, Hu, Liu, Leijten, Lin, Hussain, Zhang, Tamayol and Khademhosseini57. © 2017 Wiley.
Bioprinting, specifically, inkjet processing, has become an established method for studying drug delivery because of the control the user has on the placement of the cells, various growth factors, and final geometry. Cui et al. used this method to study the synergistic effects of fibroblast growth factor-2 and transforming growth factor-beta1 in tissue-engineering applications.Reference Cui, Breitenkamp, Lotz and D’Lima55 Advantages of bioprinting for this application were reported as the ability to accurately distribute chondrocytes within the 3DP hydrogel (PEG) as opposed to post-printing, which leads to an uneven distribution within the hydrogel (see Figure 7b). Because of this, cell viability reached 90%, a significant increase from previous results of 40% using a traditional casting technique. Although full-scale organ printing is not currently feasible, bioprinting still maintains applications in tissue engineering, where scaffolds or biomimetic constructs can be used to aid in cell proliferation and tissue regeneration. Bioprinting has been used to lay a gold-based nanostructure to aid in cardiac tissue regeneration by incorporation of gold nanorods into a gelatin-based ink.Reference Zhu, Shin, van Kempen, Li, Ponraj, Nasajpour, Mandla, Hu, Liu, Leijten, Lin, Hussain, Zhang, Tamayol and Khademhosseini57 The advantage of using this technique was increased electrical conduction between cells (and subsequent improvement in the cardiac construct tissue) (Figure 7c).
Drug delivery using 3DP
Three-dimensional printing has dawned a new era for pharmaceutical manufacturing. These products can be complex, personalized, and produced for immediate use. Drug-release kinetics can be tailored by the composition of the materials, shape, and techniques. For example, a highly porous product could break down orally to provide drug release, whereas a product produced with a pH-controlled outer layer would disintegrate further along the digestion tract. For each application, the medicine can be improved, and the drug effectiveness enhanced with a reduction in side effects. Each drug administration could be personalized for dosing, differentiating between children, adults and the elderly, as well as body weight and anatomy. Assorted drugs can also be incorporated into different release mechanism layers allowing for single administration, but provide multiple drugs and drug doses at appropriate times, eliminating the risk of failed medication instruction and burden on patients. Three-dimensional printing provides the added benefit of removing the need for additional machining while also providing the convenience of printing immediately at the point of care, notably during emergencies. Printing only when necessary will also eradicate the issue of drugs with limited shelf lives.
Common 3DP techniques currently used for pharmaceuticals include binder jet printing, material jetting, and all types of extrusion (Figure 8a).Reference Norman, Madurawe, Moore, Khan and Khairuzzaman59 One form of drug delivery system currently utilized is hydrogels, which swell upon different stimuli. Swelling is induced by pH changes, solution variation, time, and polymer composition (Figure 8b–c).Reference Melchels, Domingos, Klein, Malda, Bartolo and Hutmacher60,Reference Gupta, Vermani and Garg61 Other 3DP approaches can be used indirectly such as SLA to produce hydrogels that can provide drug delivery; syringe-based 3DP for use during surgery, and the creation of molds. Another way to consider using 3DP for drug delivery is manufacturing the devices first and then drug loading them post production. Drug kinetics can be tailored using a variety of biodegradable polymers such as PCL or poly(ethylene glycol) (PEG). These polymers in conjunction with a biorelevant drug can provide targeted drug delivery if loaded into a device or scaffold contrived from 3DP with desired porosity, complexity, and structure.

Figure 8. Examples of three-dimensional printing drug delivery methods. (a) Binder jet printing and material jetting for drug delivery applications. Reprinted with permission from Reference Reference Norman, Madurawe, Moore, Khan and Khairuzzaman59. © 2017 Elsevier. (b) Hydrogel printing with layering of dye-containing alginate. Reprinted with permission from Reference Reference Melchels, Domingos, Klein, Malda, Bartolo and Hutmacher60. © 2012 Elsevier. (c) Hydrogel response mechanism based on various stimuli. Reprinted with permission from Reference Reference Gupta, Vermani and Garg61. © 2002 Elsevier.
Current challenges and future trends
Many challenges pervade the field of 3DP materials and devices that lead to many future trends within the industry. As manufacturers continue to develop new products that implement 3DP, a broader understanding of the effects of processing parameters and material composition needs to be developed to ensure reliability and reproducibility of products from different machines of the same type. For metallic implants, loose metal powder release will remain an issue and can be mitigated by the use of careful post-processing methods. Such approaches need to be optimized for different powder compositions and processing environments. The use of different post-processing tools for metallic devices to enhance surface finish is also expanding.
Three-dimensional printing is a layer-by-layer deposition technique. With time, better process monitoring devices need to evolve that can detect defects as the part is being manufactured and can correct this during that layer deposition, as opposed to finding out after the build is complete. For metal 3DP processes, temperature variations at different locations during the build or variations in powder particle size are other factors that needs special attention. For ceramic-based 3DP, batch-to-batch variation in starting powder particle size or surface area can make a significant difference in part quality. Local humidity or the sphericity of powders can also make a difference in part quality. From both a processing and post-processing operation point of view, a sterile environment in manufacturing is important for parts that will be used in vivo.
Finally, 3DP is anticipated to do a great deal more than what it is being used for today. Parts can be produced with different chemistries, varying porosity along with different shapes in one operation. For such structures to become reality, multimaterial CAD along with finite element analysis capability will be useful. It is envisioned that with the help of 3DP, more complex surgeries will be performed with a higher success rate at various hospitals, more patient-matched devices will be used to treat complex health issues, and the availability of a variety of artificial tissues will make tissue engineering a more common healthcare intervention procedure than before.
Summary
This article has explored the clinical significance of 3DP biomaterials and biomedical devices. Patient-specific devices can be fabricated from metals, ceramics, polymers, and composites using various 3DP techniques. The methodology of 3DP also provides state-of-the-art patient-treatment options, including surgical planning, bioprinting for futuristic synthetic organ donations, and smart, targeted drug delivery. Although each aspect of 3DP biomaterials and biomedical devices still bear challenges, progress in this field has overcome immense hurdles to make many possibilities a reality. As the field advances, these challenges can be overcome and medical care is expected to flourish in ways never thought possible, thanks to 3DP.
Acknowledgments
This article is based on the scientific progress presented and discussed at the MRS/Kavli Future of Materials Workshop on “3D Printing of Biomedical Materials and Devices.” This workshop, sponsored by MRS Bulletin and the Kavli Foundation, was held after the 2017 MRS Fall Meeting in Boston, Mass. We would like to acknowledge financial support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Nos. R01 AR066361–01A1 and R01 AR067306–01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

Susmita Bose is a Herman and Brita Lindholm Endowed Chair Professor in the School of Mechanical and Materials Engineering at Washington State University. Her research interests focus on the interface of chemistry, materials science, mechanical engineering, and biology, involving three-dimensional printed bone scaffolds, implant materials, and drug delivery, including natural medicinal compounds. She is a Fellow of the AAAS, MRS, NAI, ASM, AIMBE, and ACERS, has advised more than 40 PhD and MS students, and has published more than 250 technical articles, including more than 200 journal articles, seven edited books, and 11 issued patents. Bose can be reached by email at sbose@wsu.edu.

Kellen Traxel is a doctoral candidate and research assistant in the W.M. Keck Biomedical Materials Research Laboratory at Washington State University (WSU) in mechanical engineering. He received his MS degree in materials science and engineering in 2018, and his BS degree in mechanical engineering in 2017, both from WSU. His research focuses on creating site-specific properties in metals and composites for full-scale application optimization. He has published several articles in Additive Manufacturing, received NASA Space Grants three times for his research in manufacturing metal-ceramic composites, as well as travel grants from the National Science Foundation and Metal Powder Industries Foundation to present at international conferences. Traxel can be reached by email at kellen.traxel@wsu.edu.

Ashley Vu is a doctoral candidate in the School of Mechanical and Materials Engineering and an instructor for the Voiland College of Engineering and Architecture at Washington State University (WSU). She received her BS degree in mechanical engineering and her MS degree in materials science and engineering, both from WSU. Her research interests include calcium phosphate bone-tissue engineering scaffold fabrication, induction plasma spray deposition of bioceramics, and implant modifications for drug delivery. Vu can be reached by email at ashley.vu@wsu.edu.

Amit Bandyopadhyay is a Herman and Brita Lindholm Endowed Chair Professor in the School of Mechanical and Materials Engineering at Washington State University. He has published more than 300 technical papers, has 19 issued patents, and has edited 10 books. He has supervised 17 doctoral and 30 master’s students in mechanical engineering, materials science and engineering, and physics. His research focuses on additive manufacturing or three-dimensional printing of advanced materials, with special emphasis on biomaterials and structural materials. Bandyopadhyay can be reached by email at amitband@wsu.edu.