Published online by Cambridge University Press: 05 July 2018
Karpovite, ideally Tl2VO(SO4)2(H2O), was found in a fumarole of the 1st cinder cone of the North Breach of the Great Fissure Tolbachik volcano eruption (1975–1976), Kamchatka Peninsula, Russia. Karpovite occurs as bundles of white, needle-like crystals associated with shcherbinaite, pauflerite, bobjonesite, markhininite, evdokimovite and microcrystalline Mg, Al, Fe and Na sulfates. Karpovite is monoclinic, P21, a = 4.6524(4), b = 11.0757(9), c = 9.3876(7) Å , β = 98.353(2)º, V = 478.60(7) Å3, Z = 2 (from single-crystal diffraction data). The eight strongest lines of the X-ray powder diffraction pattern are (I/d/hkl): 64/4.289/012, 81/4.253/110, 38/3.683/111, 47/3.557/022, 100/3.438/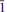 21, 52/2.982/013, 59/2.945/112, 54/2.354/132. The chemical composition determined by the electron microprobe analysis is (wt.%) Tl2O 61.43, VO2 11.53, SO3 23.55, H2O 2.61, total 99.12. The empirical formula (calculated on the basis of 10 O a.p.f.u.) is Tl2.00V0.96S2.03O9(H2O). The simplified formula of karpovite is Tl2VO(SO4)2(H2O), which requires Tl2O 61.93, VO2 12.09, SO3 23.34, H2O 2.62 total 100.00 wt.%. The crystal structure was solved by direct methods and refined to R1 = 0.026 for 4196 independent observed reflections. The structure contains two symmetrically independent Tl+ sites, one V4+ site and two S6+ sites. VO5H2O octahedra and SO4 tetrahedra link together by sharing corners to form kröhnkite-type stripes parallel to the a axis with their planes oriented parallel to (021) and (02
21, 52/2.982/013, 59/2.945/112, 54/2.354/132. The chemical composition determined by the electron microprobe analysis is (wt.%) Tl2O 61.43, VO2 11.53, SO3 23.55, H2O 2.61, total 99.12. The empirical formula (calculated on the basis of 10 O a.p.f.u.) is Tl2.00V0.96S2.03O9(H2O). The simplified formula of karpovite is Tl2VO(SO4)2(H2O), which requires Tl2O 61.93, VO2 12.09, SO3 23.34, H2O 2.62 total 100.00 wt.%. The crystal structure was solved by direct methods and refined to R1 = 0.026 for 4196 independent observed reflections. The structure contains two symmetrically independent Tl+ sites, one V4+ site and two S6+ sites. VO5H2O octahedra and SO4 tetrahedra link together by sharing corners to form kröhnkite-type stripes parallel to the a axis with their planes oriented parallel to (021) and (02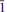 ). Tl+ cations are located between the chains, linked into a three-dimensional structure. The new mineral is named in honour of Professor Gennadii Alexandrovich Karpov (b. 1938), volcanologist at the Institute of Volcanology, Russian Academy of Sciences, Petropavlovsk-Kamchatskii, Kamchatka Peninsula, Russia.
). Tl+ cations are located between the chains, linked into a three-dimensional structure. The new mineral is named in honour of Professor Gennadii Alexandrovich Karpov (b. 1938), volcanologist at the Institute of Volcanology, Russian Academy of Sciences, Petropavlovsk-Kamchatskii, Kamchatka Peninsula, Russia.