Article contents
Omsite, (Ni, Cu)2Fe3+(OH)6[Sb(OH)6], a new member of the cualstibite group from Oms, France
Published online by Cambridge University Press: 05 July 2018
Abstract
Omsite (IMA 2012-025) is a new mineral from the Correc d'en Llinassos, Oms, Pyrénées-Orientales Department, France. It occurs as bright yellow to amber yellow discoidal tablets, flattened on {001}, which form rosettes typically 50–100 μm in diameter. Omsite generally crystallizes on siderite without associated supergene minerals; it occurs less commonly with glaukosphaerite. Crystals have a vitreous to resinous lustre, and are transparent to translucent. Omsite is not fluorescent in either short-wave or long-wave ultraviolet light. It has an estimated hardness of 3 on the Mohs' scale, is brittle with an irregular fracture, and has one poor cleavage on {001}. The calculated density is 3.378 g cm–3. Crystals are uniaxial (–), with indices of refraction of ω = 1.728(3) and ε = 1.66(1), measured in white light. Pleochroism is ω = orange-yellow, ε = pale orange-yellow; ω > ε. The empirical formula [based on 12 (OH + Cl) p.f.u.] is (Ni1.0992+Cu0.6652+Mg0.107Fe0.0453+)Σ 1.916Fe1.0003+(Sb0.9475+As0.072Na0.029)Σ1.048OH11.967Cl0.033. Omsite crystallizes in space group P , with unit-cell parameters a = 5.3506(8), c = 19.5802(15) Å, V = 485.46(10) Å3 and Z = 2 determined by single crystal X-ray diffraction. The five strongest lines in the X-ray powder diffraction pattern [d in Å, (Irel), (hkl)] are as follows: 4.901, (100), (004); 4.575, (83), (011); 2.3539, (81), (11
, with unit-cell parameters a = 5.3506(8), c = 19.5802(15) Å, V = 485.46(10) Å3 and Z = 2 determined by single crystal X-ray diffraction. The five strongest lines in the X-ray powder diffraction pattern [d in Å, (Irel), (hkl)] are as follows: 4.901, (100), (004); 4.575, (83), (011); 2.3539, (81), (11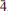 ); 1.8079, (48), (11
); 1.8079, (48), (11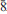 ); 3.781, (34), (103). The crystal structure was solved to R1 = 0.0896 for 356 observed reflections [Fo>4σFo] and 0.1018 for all the 469 unique reflections. Omsite is a layered double hydroxide (LDH) mineral, with a topology consistent with members of the hydrotalcite supergroup and cualstibite group.
); 3.781, (34), (103). The crystal structure was solved to R1 = 0.0896 for 356 observed reflections [Fo>4σFo] and 0.1018 for all the 469 unique reflections. Omsite is a layered double hydroxide (LDH) mineral, with a topology consistent with members of the hydrotalcite supergroup and cualstibite group.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2016
References
- 14
- Cited by




