Article contents
Leydetite, Fe(UO2)(SO4)2(H2O)11, a new uranyl sulfate mineral from Mas d’Alary, Lodève, France
Published online by Cambridge University Press: 05 July 2018
Abstract
Leydetite, monoclinic Fe(UO2)(SO4)2(H2O)11(IMA 2012–065), is a new supergene uranyl sulfate from Mas d'Alary, Lodève, Hérault, France. It forms yellow to greenish, tabular, transparent to translucent crystals up to 2 mm in size. Crystals have a vitreous lustre. Leydetite has a perfect cleavage on (001). The streak is yellowish white. Mohs hardness is ∼2. The mineral does not fluoresce under long- or short-wavelength UV radiation. Leydetite is colourless in transmitted light, non-pleochroic, biaxial, with α = 1.513(2), γ = 1.522(2) (further optical properties could not be measured). The measured chemical composition of leydetite, FeO 9.28, MgO 0.37, Al2O30.26, CuO 0.14, UO340.19, SO321.91, SiO20.18, H2O 27.67, total 100 wt.%, leads to the empirical formula (based on 21 O a.p.f.u.), (Fe0.93Mg0.07Al0.04Cu0.01)Σ1.05(U1.01O2)(S1.96Si0.02)Σ1.98O8(H2O)11. Leydetite is monoclinic, space group C2/c, with a = 11.3203(3), b = 7.7293(2), c = 21.8145(8) Å, β = 102.402(3)°, V = 1864.18(10) Å3, Z = 4, and Dcalc = 2.55 g cm–3. The six strongest reflections in the X-ray powder diffraction pattern are [dobs in Å (I) (hkl)]: 10.625 (100) (002), 6.277 (1) (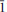 11), 5.321 (66) (004), 3.549 (5) (006), 2.663 (4) (008), 2.131 (2) (0 0 10). The crystal structure has been refined from single-crystal X-ray diffraction data to R1 = 0.0224 for 5211 observed reflections with [I > 3σ(I)]. Leydetite possesses a sheet structure based upon the protasite anion topology. The sheet consists of UO7 bipyramids, which share four of their equatorial vertices with SO4 tetrahedra. Each SO4 tetrahedron, in turn, shares two of its vertices with UO7 bipyramids. The remaining unshared equatorial vertex of the bipyramid is occupied by H2O, which extends hydrogen bonds within the sheet to one of a free vertex of the SO4 tetrahedron. Sheets are stacked perpendicular to the c direction. In the interlayer, Fe2+ ions and H2O groups link to the sheets on either side via a network of hydrogen bonds. Leydetite is isostructural with the synthetic compound Mg(UO2)(SO4)2(H2O)11. The name of the new mineral honours Jean Claude Leydet (born 1961), an amateur mineralogist from Brest (France), who discovered the new mineral.
11), 5.321 (66) (004), 3.549 (5) (006), 2.663 (4) (008), 2.131 (2) (0 0 10). The crystal structure has been refined from single-crystal X-ray diffraction data to R1 = 0.0224 for 5211 observed reflections with [I > 3σ(I)]. Leydetite possesses a sheet structure based upon the protasite anion topology. The sheet consists of UO7 bipyramids, which share four of their equatorial vertices with SO4 tetrahedra. Each SO4 tetrahedron, in turn, shares two of its vertices with UO7 bipyramids. The remaining unshared equatorial vertex of the bipyramid is occupied by H2O, which extends hydrogen bonds within the sheet to one of a free vertex of the SO4 tetrahedron. Sheets are stacked perpendicular to the c direction. In the interlayer, Fe2+ ions and H2O groups link to the sheets on either side via a network of hydrogen bonds. Leydetite is isostructural with the synthetic compound Mg(UO2)(SO4)2(H2O)11. The name of the new mineral honours Jean Claude Leydet (born 1961), an amateur mineralogist from Brest (France), who discovered the new mineral.
Keywords
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2013
References
- 15
- Cited by


